Abstract
In previous studies, we found that the consumption of legumes decreased bone turnover in ovariectomized rats. The purpose of the present study is to determine whether the protective effects on bone mineral density (BMD) and the microarchitecture of a diet containing legumes are comparable. In addition, we aim to determine their protective actions in bones by studying bone specific gene expression. Forty-two Sprague-Dawley rats are being divided into six groups during the 12 week study: 1) rats that underwent sham operations (Sham), 2) ovariectomized rats fed an AIN-93M diet (OVX), 3) ovariectomized rats fed an AIN-93M diet with soybeans (OVX-S), 4) ovariectomized rats fed an AIN-93M diet with mung beans (OVX-M), 5) ovariectomized rats fed an AIN-93M diet with cowpeas (OVX-C), and 6) ovariectomized rats fed an AIN-93M diet with azuki beans (OVX-A). Consumption of legumes significantly increased BMD of the spine and femur and bone volume of the femur compared to the OVX. Serum calcium and phosphate ratio, osteocalcin, expression of osteoprotegerin (OPG), and the receptor activator of nuclear factor κB ligand (RANKL) ratio increased significantly, while urinary excretion of calcium and deoxypyridinoline and expression of TNF-α and IL-6 were significantly reduced in OVX rats fed legumes, compared to OVX rats that were not fed legumes. This study demonstrates that consumption of legumes has a beneficial effect on bone through modulation of OPG and RANKL expression in ovariectomized rats and that legume consumption can help compensate for an estrogen-deficiency by preventing bone loss induced by ovarian hormone deficiency.
Keywords: Bone mass density, bone-specific gene expression, cytokines, legumes, ovariectomized rats
Introduction
Osteoporosis is a major public health threat worldwide. The Korean National Health and Nutrition Examination Survey IV indicate that the prevalence of osteoporosis is 39% in women over 50 years old [1]. Ovarian hormone deficiency associated with menopause results in an increased rate of bone turnover and causes an imbalance between resorption and formation, thereby accelerating bone losses [2,3]. Estrogen deficiency leads to an up-regulation of receptor activator of nuclear factor κB ligand (RANKL) on bone marrow cells, which is an important determinant of increased bone resorption [4], whereas estrogen itself stimulates osteoprotegerin (OPG) production in osteoblasts and thus exerts anti-resorptive effects on bone [5]. Thus, estrogen replacement therapy is very effective in reversing the impact of menopause on bone density, but increases the risk of endometrial cancer, breast cancer, ovarian cancer, venous thrombosis, and dementia [6-8]. To avoid complications of taking estrogen, alternative means to decrease bone loss with proven efficacy and safety should be developed for prevention and treatment of postmenopausal osteoporosis.
Selective estrogen receptor modulators (SERMs), a group of chemically diverse non-steroidal compounds that bind to and interact with estrogen receptors, have refocused interest on the treatment of osteoporosis [9,10]. Soy isoflavones have been characterized as naturally occurring SERMs with similar beneficial effects on bone [11]. However, it is unclear whether the bone protective effects of soy are derived from soy isoflavones, soy protein, or a combination of the two. Previous studies have focused only on the effect of soy isoflavones on bone metabolism [12-15] and neglected to study legumes containing isoflavones.
Legumes have played an important role in the traditional diets of many regions over the years, particularly in the Asian countries. Legumes are known for their protein and soluble fiber content, and more recently have been recognized as a unique dietary source of a group of phytochemicals called isoflavones [16]. We previously reported that consumption of intact beans such as soybeans, mung beans, cowpeas, and azuki beans, improves bone formation markers in ovariectomized rats [17]. The purpose of the present study is to determine the effects of legumes on bone mineral density (BMD) and the microarchitecture, and their protective actions in bone by studying bone specific gene expression.
Materials and Methods
Animals and diets
The study protocol for all animal experiments was approved by the Institutional Animal Care and Use Committee of Hanyang University (HY-IACUC-10-034). Nine-week old female Sprague-Dawley rats (Jung Ang Lab. Animal Inc., Seoul, Korea) were housed individually in ventilated cages within an air-conditioned room maintained at 22 ± 2℃ with a 12-h light-dark cycle. All rats were provided laboratory food pellets and fresh tap water ad libitum.
After one week of adaptation to this environment, 35 rats underwent bilateral ovariectomy while seven rats were subjected to sham operations. During recovery from surgery, all rats were fed an AIN-93M diet for two weeks before the experimental diet started. After two weeks, the ovariectomized rats were randomly divided into five groups (n = 7 per group) as follows: ovariectomized rats fed an AIN-93M diet (OVX), ovariectomized rats fed an AIN-93M diet with soybeans (OVX-S), ovariectomized rats fed an AIN-93M diet with mung beans (OVX-M), ovariectomized rats fed an AIN-93M diet with cowpeas (OVX-C), and ovariectomized rats fed an AIN-93M diet with azuki beans (OVX-A). Rats that underwent the sham operation (Sham) continued to be fed an AIN-93M diet. Rats were fed these experimental diets for 12 weeks.
The experimental diets were isocaloric and the energy ratio of carbohydrate:protein:lipid was 65:15:20. The Korean Rural Development Administration provided all of the legumes, which were soaked in water for 24 hours, cooked under pressure for 15 minutes, thoroughly dried, and ground into powder. Diets were prepared by replacing 300 g of the casein and corn starch in the AIN-93M diet with powdered soybean, mung bean, cowpea, or azuki bean (Table 1). During the experimental period, body weight was measured once a week, and food intake was measured daily.
Table 1.
Composition of the experimental diets1)
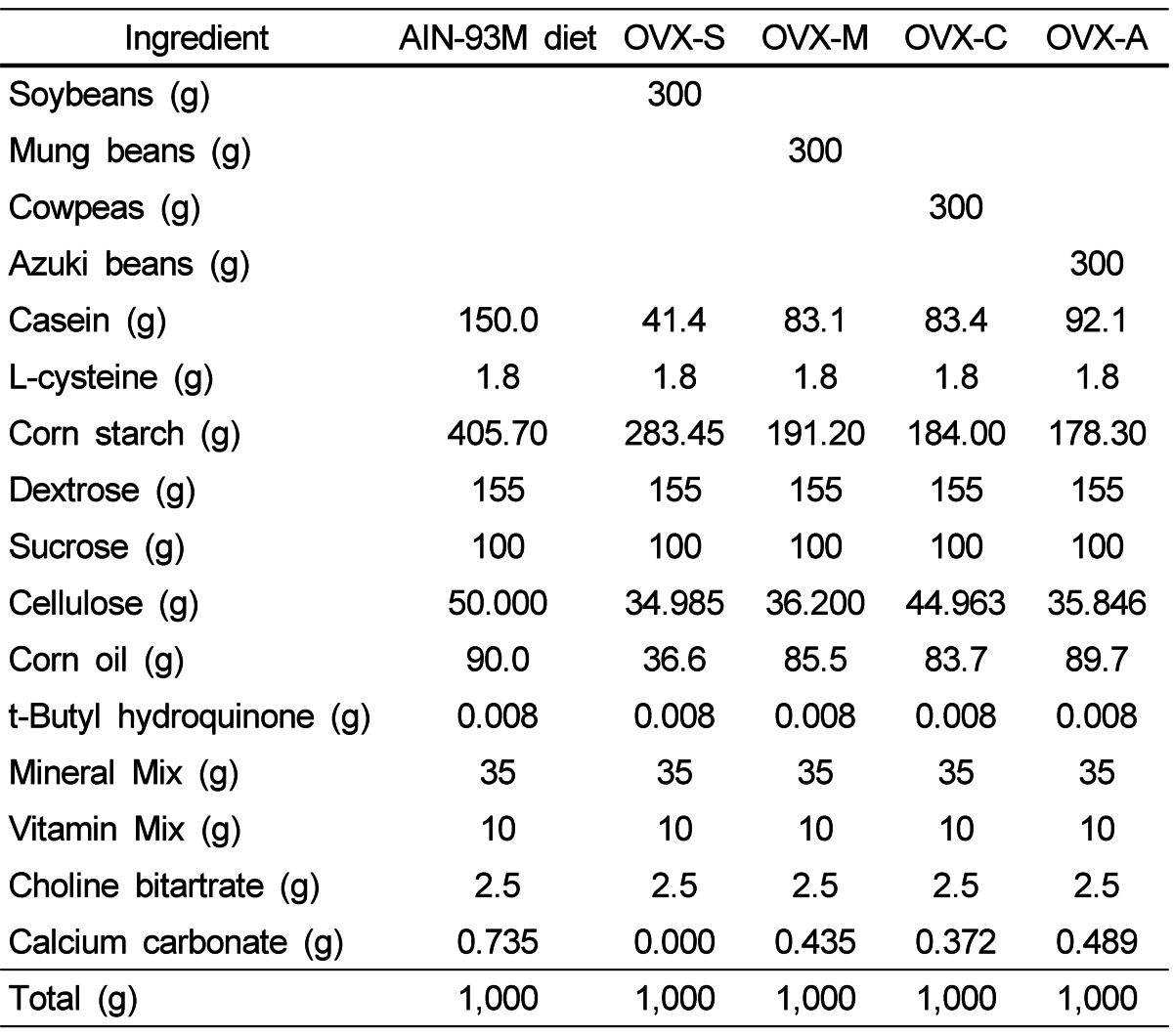
1)AIN-93M diet (Sham, OVX), soybean (OVX-S), mung bean (OVX-M), cowpea (OVX-C), or azuki bean (OVX-A)
Sample collection
Rats were housed in individual metabolic cages for 24 hours during the last week of the experiment in order to collect urine samples. At the end of the experiment, all rats were fasted overnight and euthanized the next day via exsanguination under anesthesia with an intraperitoneal injection of tiletamine (25 mg/kg), zolazepam (25 mg/kg), and xylazine (10 mg/kg). Blood was collected in serum separation tubes from the abdominal aorta and centrifuged at 3,000 rpm for 15 min (HA 1000-3, Hanil Sciences Industrial CO. Ltd., Incheon, Korea).
Subcutaneous fat and tissue from the uterus were harvested, rinsed with physiological saline, and weighed. The bilateral femur and tibia were dissected and any adherent soft tissues were removed from the bone, and then the length of each femur was measured with a ruler. Serum, tissue, and the left femur and tibia were stored at -80℃ until further analysis. The right femur and tibia were fixed in a tube with 4% paraformaldehyde for micro-computed tomography (CT) analysis.
Measurement of BMD and micro-CT
Measurement of BMD was executed on the last day of the experiment. After the rats were anesthetized, the BMD of the right femur, tibia, and lumbar spine were measured using dual-energy X-ray absorptiometry (DEXA, GE Lunar, Madison, WI, USA) in units of milligrams per square centimeters (mg/cm2). The right femur was scanned using micro-CT (Skyscan 1076, Skyscan Co., Antwerp, Belgium). The femur was placed on a holder between the x-ray source and the charge-coupled device (CCD) camera (Skyscan 1076, Skyscan Co., Antwerp, Belgium) and, while in the field of view, was rotated around the vertical axis at intervals of 0.9° for 180°. The beam was projected onto a phosphorus screen that converted the x-rays into visible light detectable by a CCD camera. The data were then digitized by the frame grabber and transmitted to a computer equipped with topographic reconstruction software. Serial two-dimensional cross-sectional 1,968 × 1,968 pixel images were acquired through micro-CT. From the two-dimensional images (pixel: 35 × 35 µm), a three-dimensional structural image was reconstructed with voxels 35 × 35 × 35 µm in size. Bone morphometric parameters including the total bone volumes of the femur and femoral trabecular bone volumes were determined [18].
Bone turnover markers in serum and urine
Concentrations of calcium, phosphorus, and creatinine in serum or urine were measured using a commercial kit (Quanti Chrom™ Bioassay Systems, Hayward, CA, USA) [16]. Serum alkaline phosphatase (ALP) and osteocalcin were measured using a commercial ALPkit (Asan Pharm, Seoul, Korea) and Rat-MID™ Osteocalcin EIA kit (Immunodiagnostic systems Inc., Stoughton, MA, USA), respectively. Urinary deoxypyridinoline (DPD) was measured using the METRA™ DPD EIA kit (Quidel Corporation, San Diego, CA, USA). All experiments were performed according to the manufacturer's instructions and the results were analyzed using a microplate reader (iMark Microplate Reader, BioRad, Richmond, CA, USA). Values for DPD were adjusted for volume by estimating urinary creatinine using a kit (Quanti Chrom™ Bioassay Systems, Hayward, CA, USA) and the concentrations were presented as nmol DPD per mmol creatinine.
Protein extraction and western blot analysis
Frozen left femora and tibiae were grounded by using a mortar and pestle was cooled with dry ice. Bone powder was extracted for 1h at 4℃ with lysis buffer (10 mM Tris-HCl pH 7.4, 0.1M EDTA, 0.5% Triton X-100, 10 mM NaCl, protease inhibitor cocktails, and sterile solution). The protein extract with Folch solution (chloroform:methanol = 2:1) was centrifuged at 3,000 rpm for 10 min and the supernatants were collected. Protein concentration in the supernatant was determined using Bio-Rad protein reagent (BioRad, Richmond, CA, USA) with bovine serum albumin as the standard.
Equal amounts of protein (30 µg) were loaded on 10% polyacrylamide gels, then transferred to polyvinylidine fluoride membranes, and blocked for 1 hour at room temperature with 5% skim milk in Tris-buffered saline containing Tween20 (TBST) and then incubated with tumor necrosis factor-alpha (TNF-α; 1:500), interleukin-6 (IL-6; 1:300), interleukin-1 β (IL-1 β; 1:1,000), OPG (1:1,000), or RANKL (1:500) with 5% skim milk in TBST overnight at 4℃. All antibodies for western blot analysis were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). After several washes with TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000), which was either anti-rabbit IgG or anti-goat IgG, for 1 hour at room temperature. Immunoreactive bands were visualized with enhanced chemiluminescence and were captured on X-ray film. The relative and normalized protein expression was calculated by β-action (1:1,000, BD Transduction Laboratories, NJ, USA).
Statistical analysis
Data were analyzed using the SPSS-PC+ statistical software package for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as mean ± standard error of the mean (SEM). Differences between experimental groups were analyzed using one-way analysis of variance (ANOVA), followed by Duncan's multiple-range test. Differences with P < 0.05 were considered to be statistically significant.
Results
Food intakes, bodyweight, and organ weight
Dietary intakes and initial body weights were not significantly different between groups (Table 2). Final body weight and subcutaneous fat weights were significantly higher, while uterus weight was significantly lower in the OVX group compared to the Sham group. Consumption of any type of legume was associated with significant decreases of final body weight compared to the OVX group. Subcutaneous fat decreased significantly in rats that consumed soy as compared to rats in the OVX, but excepting soybean, any other legumes showed no significant differences with OVX group.
Table 2.
Dietary intake, body weight and organ weight1)
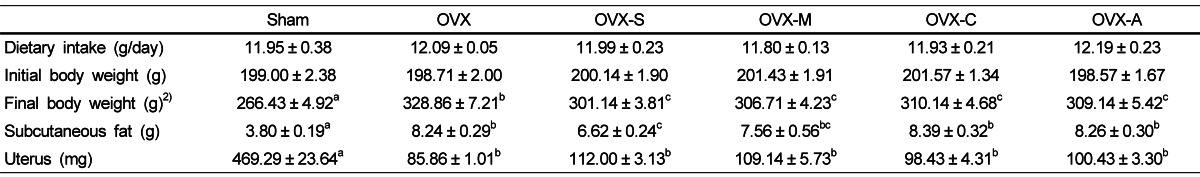
1)Values are expressed as mean ± SEM, n = 7; Sham: rats that underwent a sham operation and were fed an AIN-93M diet; OVX: ovariectomized rats fed an AIN-93M diet; OVX-S: ovariectomized rats fed an AIN-93M diet with soybeans; OVX-M: ovariectomized rats fed an AIN-93M diet with mung beans; OVX-C: ovariectomized rats fed an AIN-93M diet with cowpeas; OVX-A: ovariectomized rats fed an AIN-93M diet with azuki beans.
2)Values with different superscripts within a row are significantly different at P<0.05 by ANOVA with Duncan's multiple-range test.
Bone mineral density and bone volume
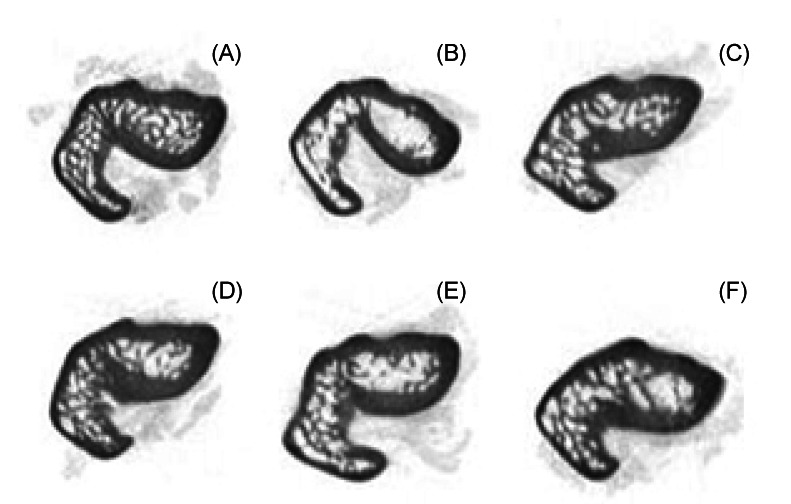
BMD of the spine, femur, and tibia, and bone volume of the femur and trabecular were significantly lower in the OVX group compared to the Sham group (Table 3). When compared to the OVX group, the consumption of any type of legume significantly increased BMD of the spine and femur but not the tibia. Micro-CT analysis of the femur indicated that consumption of any type of legume significantly increased total bone volume as compared with the OVX group, but trabecular bone volumes were not significantly improved by consumption of any type of legume (Table 3). There were no significant differences in femur length among the groups. Visual inspection of the micro-CT data showed that, in general, rats in the Sham and rats that consumed any type of legume had greater bone volume than rats in the OVX that did not consume legumes (Fig. 1).
Table 3.
Bone mineral density, bone microarchitecture, and bone length1)
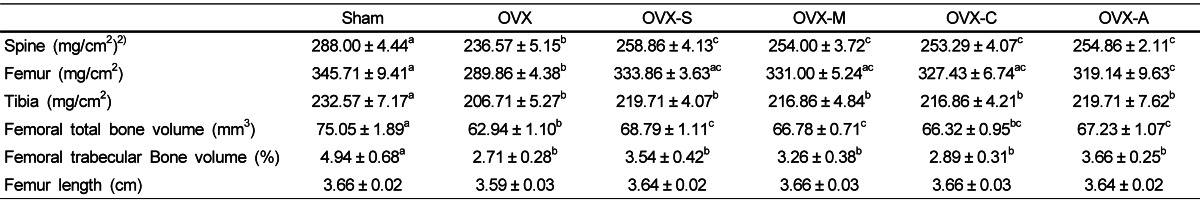
1)Values are expressed as mean ± SEM, n = 7; Sham: rats that underwent a sham operation and were fed an AIN-93M diet; OVX: ovariectomized rats fed an AIN-93M diet; OVX-S: ovariectomized rats fed an AIN-93M diet with soybeans; OVX-M: ovariectomized rats fed an AIN-93M diet with mung beans; OVX-C: ovariectomized rats fed an AIN-93M diet with cowpeas; OVX-A: ovariectomized rats fed an AIN-93M diet with azuki beans.
2)Values with different superscripts within a row are significantly different at P<0.05 by ANOVA with Duncan's multiple-range test.
Fig. 1.
Morphometric analysis of femora by micro-CT. (A), rats that underwent a sham operation and fed an AIN-93M diet; (B), ovariectomized rats fed a AIN-93M diet; (C), ovariectomized rats fed an AIN-93M diet with soybeans; (D), ovariectomized rats fed an AIN-93M diet with mung beans; (E), ovariectomized rats fed an AIN-93M diet with cowpeas; (F), ovariectomized rats fed an AIN-93M diet with azuki beans.
Bone turnover markers
When comparing the OVX group to the Sham group, the calcium/phosphate ratio and concentration of serum calcium were significantly lower; but serum ALP, urinary calcium excretion and urinary DPD/creatinine were significantly higher, suggesting that the ovariectomy increased bone turnover (Table 4). Rats that consumed any types of legume had significantly increased serum OC concentrations and calcium/phosphate ratios, but had decreased urinary calcium and DPD excretion compared to rats in the OVX group. Serum concentrations of calcium tend to increase in rats that consumed beans, but significantly increases only in rats that consumed soybeans. However, there was no significant differences in serum ALP concentrations or serum phosphorus in rats that consumed beans compared to rats in the OVX group.
Table 4.
Bone remodeling biomarkers in serum and urine1)
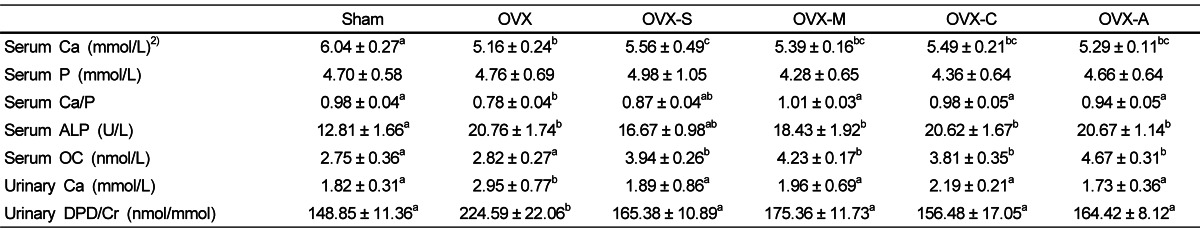
1)Values are expressed as mean ± SEM, n = 7; Sham: rats that underwent a sham operation and were fed an AIN-93M diet; OVX: ovariectomized rats fed an AIN-93M diet; OVX-S: ovariectomized rats fed an AIN-93M diet with soybeans; OVX-M: ovariectomized rats fed an AIN-93M diet with mung beans; OVX-C: ovariectomized rats fed an AIN-93M diet with cowpeas; OVX-A: ovariectomized rats fed an AIN-93M diet with azuki beans. Ca, calcium; P, phosphate; ALP, alkaline phosphatase; OC, osteocalcin; DPD, deoxypyridinoline; Cr, creatinine.
2)Values with different superscripts within a row are significantly different at P<0.05 by ANOVA with Duncan's multiple-range test.
Expression of cytokines and bone-specific genes
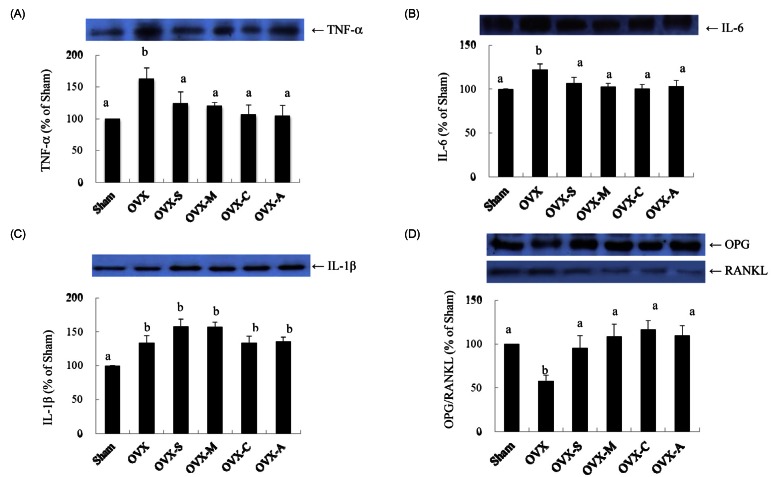
After the ovariectomies, the expression of TNF-α, IL-6, and IL-1β was significantly elevated, while the OPG/RANKL ratio was significantly decreased (Fig. 2). Consumption of any types of legume resulted in significantly decreased expressions of TNF-α and IL-6 and increased expression of the OPG/RANKL ratio compared to the OVX group. However, the expression of IL-1β was not significantly impacted by the consumption of any types of legume.
Fig. 2.
Expression of tumor necrosis factor-alpha (TNF-α; A), interleukin-6 (IL-6; B), interleukin-1β (IL-1β; C) and osteoprotegerin/receptor activator of nuclear factor κB ligand (OPG/RANKL; D) in the femur and tibia. Sham: rats that underwent a sham operation and were fed an AIN-93M diet; OVX: ovariectomized rats fed an AIN-93M diet; OVX-S: ovariectomized rats fed an AIN-93M diet with soybeans; OVX-M: ovariectomized rats fed an AIN-93M diet with mung beans; OVX-C: ovariectomized rats fed an AIN-93M diet with cowpeas; OVX-A: ovariectomized rats fed an AIN-93M diet with azuki beans. Data are expressed relative to the Sham group level, which was set at 100%. Values are expressed as mean ± SEM (n = 7).
Discussion
The present study showed that a diet containing soybeans, mung beans, cowpeas, or azuki beans significantly increased the BMD of the femur and spine, and bone volume of the femur in ovariectomized rats. These effects were mediated by OPG and RANKL expression. Previous studies have consistently shown that soy protein [13,19-21], soy extract [15], and isoflavones [14,22] are effective in decreasing bone loss induced by ovariectomy of rats. Byun and Lee [23] also reported that dietary soybean and sword bean intake significantly increased BMD of the femur in ovariectomized rats.
After an ovariectomy, the rate of bone turnover is known to increase [19,24-25]. The results of the present study consistently showed that ovariectomized rats that were not fed legumes had a decrease in serum ALP and OC, which are indirect markers of bone formation; with an increase in urinary DPD, an indirect marker of bone resorption. This suggests that the rate of bone turnover was increased. Ovariectomized rats fed soybeans, mung beans, cowpeas, and azuki beans showed analogous changes in these indexes with further elevations in serum ALP and OC activities but a reduction in urinary DPD concentrations. In summary, the consumption of beans did not appear to ameliorate the ovariectomy-induced increase in bone turnover, yet the femoral bone density of rats that consumed legumes was similar to that of rats that underwent the sham operation, suggesting that consumption of legumes has a positive effect on bone mass. Previous studies also reported that soy isoflavones and extract reduce bone turn over in ovariectomized rats [15,20] by stimulating proliferation and differentiation of osteoblasts through protection from apoptosis [26-28]. Picherit et al. [12] suggested that isoflavones reduced bone turnover by antiosteoclastic activity in a dose-dependent manner, and several human studies support the hypothesis that dietary soy blunted bone resorption [29,30].
To compare the effects of bean consumption on bone at the molecular level, this study determines the expression of OPG and RANKL, bone-specific genes that are involved in the production of osteoblasts and osteoclasts [31,32]. Estrogen deficiency in postmenopausal women leads to an up-regulation of RANKL on bone marrow cells, which is an important determinant of increased bone resorption, whereas estrogen itself stimulates OPG production in osteoblasts and thus exerts anti-resorptive effects on bone [5]. Zhang et al. [15] demonstrated that in ovariectomized rats, soy extract increased OPG mRNA expression and genistein decreased RANKL mRNA expression but increased OPG/RANKL ratio. The present study reports that consumption of soybean, mung bean, cowpea, and azuki bean increased the expression of OPG/RANKL. These results are in agreement with clinical studies in which lower levels of serum-soluble RANKL were found in postmenopausal women who received genistein treatment [33,34].
RANKL and OPG are members of the TNF subfamily, and have emerged as essential mediators of pro-inflammatory cytokines on osteoclastogenesis [5,35]. Apart from its direct effects on OPG synthesis, estrogen may indirectly block RANKL activity by limiting the availability of the major RANKL stimulators TNF-α, IL-1β, and IL-6 [5,28,35]. Both TNF-α and IL-1 have potent effects on osteoclast function by inhibiting collagen synthesis and stimulating proliferation of osteoblasts [35,36]. Serum inflammatory cytokines, TNF-α, IL-1β, and IL-6 are higher in postmenopausal women than in premenopausal women [35], and are associated with bone masses in healthy postmenopausal women [37]. We consistently observed that consumption of any type of bean significantly reduced TNF-α and IL-6 expression, suggesting that dietary intake of beans increases OPG/RANKL expression by modulating cytokine production.
The positive effects of soy on BMD may enhance intestinal calcium absorption [19,38] and reduce urinary loss of calcium [21]. Arjmandi et al. [19] indicated that a diet rich in soy, regardless of isoflavone content, significantly increased femoral bone density, which was related to stimulation of duodenal calcium transport. In fact, content of isoflavones in soybean, mung bean, cowpea, and azuki bean was reported to be varied from 0.02 mg/100 g to 12.5 mg/100 g, but we found a similar effect of legumes on bone. The enhanced intestinal absorption of calcium along with modulation of parathyroid hormone and renal function may provide partial explanations for the beneficial effects of soybean protein on bone health [13]. In a previous study [17], we found that when rats were fed calcium-deficient diets with soybean, mung bean, cowpea, and azuki bean, bone formation markers were increased but serum calcium concentrations and bone mass did not. On the other hand, in the present study, rats fed calcium-adequate diets with soybeans, mung beans, cowpeas, or azuki beans had increased serum calcium and phosphate ratios, bone formation markers and bone mass, with a decrease in urinary calcium excretion. Although intestinal calcium absorption was not directly measured, these results suggest that the protective effect of soybeans, mung beans, cowpeas, and azuki beans on bone health could be partly due to improved calcium balance.
Ovariectomized rats gained significantly more body weight than the Sham group in this study. Increased weight gain due to an ovariectomy, despite similar food consumption, has been well documented [13,25]. Both estrogen administration and the soybean diet prevented this ovariectomy-induced body weight gain, suggesting that soy isoflavones might serve as a source of proestrogenic compounds.
A limitation of this study is that the content of isoflavone and other phytoestrogens in the beans were not measured. It should also be noted that there are some limitations when applying findings from the rat model to postmenopausal osteoporosis in humans. In conclusion, soybean, mung bean, cowpea, and azuki bean consumption may have beneficial effects on the bone through modulation of OPG and RANKL expression in ovariectomized rats, and appear to be an estrogen substitute in terms of preventing bone loss induced by ovarian hormone deficiency. Further research is needed to determine the components of soy that promote bone formation in humans, as well as to explore bone-specific gene expression (other than OPG and RANKL) related to bean consumption.
Footnotes
This work was supported by a Korea Research Foundation grant funded by the Korean Government (2012R1A1A2040553).
References
- 1.Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention. The fourth Korea National Health and Nutrition Examination Survey (KNHANES 4) Cheongwon: Korea Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 2.Gallagher JC. The pathogenesis of osteoporosis. Bone Miner. 1990;9:215–227. doi: 10.1016/0169-6009(90)90039-i. [DOI] [PubMed] [Google Scholar]
- 3.Stěpán JJ, Pospíchal J, Presl J, Pacovský V. Bone loss and biochemical indices of bone remodeling in surgically induced postmenopausal women. Bone. 1987;8:279–284. doi: 10.1016/8756-3282(87)90002-0. [DOI] [PubMed] [Google Scholar]
- 4.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 6.Lacey JV, Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 7.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 8.Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, Thomas JA, Umbach D. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol. 2006;77:485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 10.Cosman F, Lindsay R. Selective estrogen receptor modulators: clinical spectrum. Endocr Rev. 1999;20:418–434. doi: 10.1210/edrv.20.3.0371. [DOI] [PubMed] [Google Scholar]
- 11.Setchell KD. Soy isoflavones--benefits and risks from nature's selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20:354S–362S. doi: 10.1080/07315724.2001.10719168. [DOI] [PubMed] [Google Scholar]
- 12.Picherit C, Bennetau-Pelissero C, Chanteranne B, Lebecque P, Davicco MJ, Barlet JP, Coxam V. Soybean isoflavones dose-dependently reduce bone turnover but do not reverse established osteopenia in adult ovariectomized rats. J Nutr. 2001;131:723–728. doi: 10.1093/jn/131.3.723. [DOI] [PubMed] [Google Scholar]
- 13.Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996;126:161–167. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Jo HJ, Choi MJ. Effects of isoflavone supplementation on the bone mineral density of growing female rats. Nutr Res Pract. 2008;2:68–73. doi: 10.4162/nrp.2008.2.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Li Q, Wan HY, Helferich WG, Wong MS. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr. 2009;139:2230–2236. doi: 10.3945/jn.109.108399. [DOI] [PubMed] [Google Scholar]
- 16.Chen KI, Erh MH, Su NW, Liu WH, Chou CC, Cheng KC. Soyfoods and soybean products: from traditional use to modern applications. Appl Microbiol Biotechnol. 2012;96:9–22. doi: 10.1007/s00253-012-4330-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Jin N, Paik DJ, Kim DY, Chung IM, Park Y. Consumption of legumes improves certain bone markers in ovariectomized rats. Nutr Res. 2011;31:397–403. doi: 10.1016/j.nutres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Waarsing JH, Day JS, Weinans H. An improved segmentation method for in vivo microCT imaging. J Bone Miner Res. 2004;19:1640–1650. doi: 10.1359/JBMR.040705. [DOI] [PubMed] [Google Scholar]
- 19.Arjmandi BH, Birnbaum R, Goyal NV, Getlinger MJ, Juma S, Alekel L, Hasler CM, Drum ML, Hollis BW, Kukreja SC. Bone-sparing effect of soy protein in ovarian hormone-deficient rats is related to its isoflavone content. Am J Clin Nutr. 1998;68:1364S–1368S. doi: 10.1093/ajcn/68.6.1364S. [DOI] [PubMed] [Google Scholar]
- 20.Blum SC, Heaton SN, Bowman BM, Hegsted M, Miller SC. Dietary soy protein maintains some indices of bone mineral density and bone formation in aged ovariectomized rats. J Nutr. 2003;133:1244–1249. doi: 10.1093/jn/133.5.1244. [DOI] [PubMed] [Google Scholar]
- 21.Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, Wastney M, Weaver CM. Comparative effect of soy protein, soy isoflavones, and 17beta-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res. 2005;20:828–839. doi: 10.1359/JBMR.041236. [DOI] [PubMed] [Google Scholar]
- 22.Fanti P, Monier-Faugere MC, Geng Z, Schmidt J, Morris PE, Cohen D, Malluche HH. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos Int. 1998;8:274–281. doi: 10.1007/s001980050065. [DOI] [PubMed] [Google Scholar]
- 23.Byun JS, Lee SS. Effect of soybeans and sword beans on bone metabolism in a rat model of osteoporosis. Ann Nutr Metab. 2010;56:106–112. doi: 10.1159/000277663. [DOI] [PubMed] [Google Scholar]
- 24.Wronski TJ, Cintrón M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988;123:681–686. doi: 10.1210/endo-123-2-681. [DOI] [PubMed] [Google Scholar]
- 25.Kalu DN, Liu CC, Salerno E, Hollis B, Echon R, Ray M. Skeletal response of ovariectomized rats to low and high doses of 17 beta-estradiol. Bone Miner. 1991;14:175–187. doi: 10.1016/0169-6009(91)90021-q. [DOI] [PubMed] [Google Scholar]
- 26.Choi EM, Suh KS, Kim YS, Choue RW, Koo SJ. Soybean ethanol extract increases the function of osteoblastic MC3T3-E1 cells. Phytochemistry. 2001;56:733–739. doi: 10.1016/s0031-9422(00)00484-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Chen X, Anderson JJ. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutr Res. 2001;21:1287–1298. [Google Scholar]
- 28.Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. 2002;295:417–422. doi: 10.1016/s0006-291x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- 29.Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: a four-week study. J Am Coll Nutr. 2002;21:97–102. doi: 10.1080/07315724.2002.10719200. [DOI] [PubMed] [Google Scholar]
- 30.Scheiber MD, Liu JH, Subbiah MT, Rebar RW, Setchell KD. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause. 2001;8:384–392. doi: 10.1097/00042192-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 32.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 33.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D'Anna R, Cannata ML, Corrado F, Cancellieri F, Faraci M, Marini R, Adamo EB, Wilson S, Squadrito F. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008;23:715–720. doi: 10.1359/jbmr.080201. [DOI] [PubMed] [Google Scholar]
- 34.Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, Minutoli L, Atteritano M, Levy RM, D'Anna R, Frisina N, Mazzaferro S, Cancellieri F, Cannata ML, Corrado F, Frisina A, Adamo V, Lubrano C, Sansotta C, Marini R, Adamo EB, Squadrito F. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787–4796. doi: 10.1210/jc.2008-1087. [DOI] [PubMed] [Google Scholar]
- 35.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 36.Ragab AA, Nalepka JL, Bi Y, Greenfield EM. Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am J Physiol Cell Physiol. 2002;283:C679–C687. doi: 10.1152/ajpcell.00421.2001. [DOI] [PubMed] [Google Scholar]
- 37.Gertz ER, Silverman NE, Wise KS, Hanson KB, Alekel DL, Stewart JW, Perry CD, Bhupathiraju SN, Kohut ML, Van Loan MD. Contribution of serum inflammatory markers to changes in bone mineral content and density in postmenopausal women: a 1-year investigation. J Clin Densitom. 2010;13:277–282. doi: 10.1016/j.jocd.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omi N, Aoi S, Murata K, Ezawa I. Evaluation of the effect of soybean milk and soybean milk peptide on bone metabolism in the rat model with ovariectomized osteoporosis. J Nutr Sci Vitaminol (Tokyo) 1994;40:201–211. doi: 10.3177/jnsv.40.201. [DOI] [PubMed] [Google Scholar]