Abstract
The nucleolus, the site of ribosomal ribonucleic acid (rRNA) transcription and assembly, is an important player in the cellular response to stress. Altered nucleolar function and morphology, including decreased nucleolar volume, has been observed in Parkinson’s disease; thus the nucleolus represents a potential indicator of neurodegeneration in the disease. This study determined the effects of a partial unilateral intrastriatal 6-hydroxydopamine (6-OHDA) lesion, which models the dopaminergic loss found in Parkinson’s disease, on the nucleoli of dopaminergic cells in the substantia nigra pars compacta (SNpc). Adult male Long-Evans rats underwent unilateral intrastriatal infusion of 6-OHDA (12.5 μg). Lesions were verified by amphetamine-stimulated rotation 7 days later, and rats were euthanized 14 days after infusion. Coronal sections (50μm) were stained for tyrosine hydroxylase-silver nucleolar (TH-AgNOR) stain using MultiBrain Technology (NeuroScience Associates), which resulted in clearly defined nucleoli and neuronal outlines. Stereological methods were used to compare dopaminergic morphology between lesioned and intact hemispheres in each rat. In cells exhibiting a definable nucleolus, nucleolar volume was decreased by 16% on the ipsilateral side. The ipsilateral SNpc also exhibited an 18% decrease in SNpc planimetric volume, a 46% decrease in total TH-positive neuron number, and an 11% decrease in neuronal body volume (all P<0.05 by paired t-test). These findings suggest that the 6-OHDA lesion alters nucleolar morphology and that these changes are similar to those occurring in Parkinson’s disease.
Keywords: silver nucleolar stain, 6-hydroxydopamine, stereology, substantia nigra pars compacta, neuronal morphology, nucleolar volume
Introduction
The nucleolus is a likely participant in the neurodegenerative process of Parkinson’s disease. In addition to ribosomal ribonucleic acid (rRNA) transcription and assembly, the nucleolus is involved in directing the cellular response to stress [3], thus implicating it in the process of neurodegeneration. Notably, altered postmortem nucleolar size and nucleolar damage have been observed in Parkinson’s, as well as in several other neurodegenerative diseases [13, 20, 24]. Despite the importance of the nucleolus in neuronal function, the exact mechanisms of its dysfunction in degenerating neurons are still poorly understood, justifying the need for better knowledge of the roles of this influential organelle in neurodegenerative disease.
The partial unilateral intrastriatal 6-hydroxydopamine (6-OHDA) model of Parkinson’s disease destroys SNpc neurons by neurotoxic oxidative stress mechanisms [9, 17]. In contrast to more extensive dopaminergic ablation models, this method causes moderate SNpc neuronal loss resembling that found in early Parkinson’s [2, 6, 9, 15, 17]. In addition to decreases in neuronal number, morphological changes to dopaminergic neurons and their nucleoli after 6-OHDA may also reflect the processes occurring in Parkinson’s disease [10, 13, 19]. Nucleolar function has been associated with nucleolar size; [3] hence a decrease in nucleolar volume in the lesioned SNpc could reflect a loss of nucleolar function after 6-OHDA lesion, and thereby model the processes involved in Parkinson’s disease. Design-based stereology is a well-established method for obtaining robust data, and has been extensively used to determine neuronal number and morphology of the SNpc in Parkinson’s disease and in animal models [1, 4, 7, 8, 15, 25, 26]. Accordingly, this study employed the tyrosine hydroxylase-silver nucleolar (TH-AgNOR stain), which combines TH staining for catecholaminergic neurons with silver-binding nucleolar AgNOR staining [12], and stereological analysis techniques to determine the effects of a partial unilateral 6-OHDA model on the SNpc TH-positive neuronal and nucleolar morphology in adult male Long-Evans rats.
Materials and methods
All experiments were performed in compliance with the NIH Guide for the Care and Use of Animals and were approved by the University of Kansas Medical Center Institutional Care and Use Committee.
Animals, 6-OHDA lesion procedures and lesion validation
Animal husbandry protocol and experimental surgical and lesion validation procedures are previously described in detail [12]. Briefly, male Long-Evans rats (90–100 days old) (n=11) were given single-site unilateral intrastriatal microinjections with 6-OHDA (12.5 μg in 5 μl). Amphetamine-stimulated rotation was assessed 7 days later. After an additional 7 days to allow clearance of drug [>10 t1/2s, tissue t1/2 = 5–9 hrs for the elimination phase [18], rats were euthanized by transcardial perfusion under pentobarbital anesthesia. Brains were extracted and fixed as previously described [12].
Histological analysis
TH-AgNOR staining
Brains were sent to NeuroScience Associates (Knoxville, TN) for embedding, sectioning and staining. Brains were prepared using MultiBrain® Technology, in which multiple brains are embedded within gelatin blocks, providing uniform exposure of the brains to sectioning and staining conditions. Free-floating 50 μm sections were stained with a modified TH-AgNOR stain as previously described [27], and mounted on gelatinized (subbed) glass slides.
Stereology
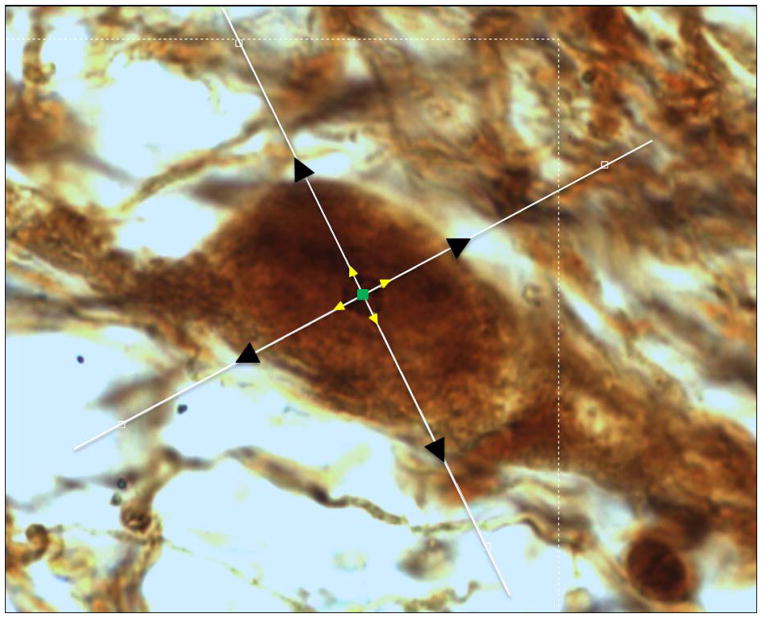
Every sixth section containing SNpc was selected for stereological quantitation (Bregma −4.70 to −6.30mm). TH-AgNOR-stained cells were quantified using the Microbrightfield Stereoinvestigator software package combined with a Nikon Eclipse TE2000-U microscope coupled to a Heidenhein linear encoder unit and a QImaging Retiga-2000R color digital video camera. Using an atlas [22], the borders of the entire SNpc were carefully outlined in rostrocaudal sections at 4X magnification to exclude the pars reticulata, pars lateralis, pars medialis, and the ventral tegmental area (VTA). Series characteristically contained between 5–7 slides per animal. The variations in brain positioning in the MultiBrain® embedding process provide a randomized sectioning start for each brain quantified. Cells were counted and volumes measured at 100X magnification using the simultaneous application of the optical fractionator and a double nucleator method. The nucleus was used as the unique marker for each neuron. The double nucleator was then employed by automatic software placement of four randomly oriented crossed rays centered at the counting point, and four discriminately placed markers on the outside borders of the nucleolus and the outline of the cell body, respectively (fig. 2). In some neurons (mean 20.1±1.5% on the ipsilateral side and 17.4±2.3% on the contralateral side, with no significant difference between the two by paired t-test [p=0.3]) the nucleolus was visible within the nucleus, but dark staining of the nucleus made it impossible to accurately distinguish the borders of the nucleolus for placement of the nucleator probe. These neurons were included in the total cell count, but were not included in the volumetric analyses because it was not possible to employ the double nucleator techinique. The mean measured thickness was 31.2 ± 0.4 μm. A maximum coefficient of error of 0.15 (m=1) was accepted for all results [11].
Fig. 2. The double nucleator technique.
Markers are placed on the nucleolus center and where the nucleator rays intersect with the borders of the nucleolus and the neuronal body outline.
Data analysis
Data are presented as the mean ± S.E.M. The effects of 6-OHDA between the ipsilateral and contralateral hemispheres were tested for statistical significance using paired t-tests (SigmaPlot v.11.0). Within-subject changes in any parameter were expressed as a percentage relative to the contralateral side. Correlation between d-amphetamine-stimulated rotational behavior and percent of neuronal loss was determined by linear regression and significance was tested by the Pearson product-moment correlation (SigmaPlot, v.11.0).
Results
Amphetamine-stimulated rotation
The mean number of amphetamine-stimulated rotations completed by lesioned rats during the 60-min observation period was 154± 47. The relationship between rotation and percent of SNpc neuronal was poorly correlated (r2 = 0.135).
Stereological Analysis
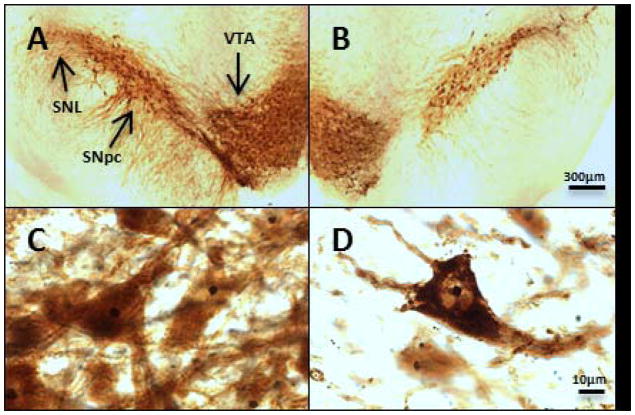
Low-and high-power photomicrographs show the morphology of the ipsilateral and contralateral SNPC TH-positive neurons. Low (4X) magnification demonstrates the loss of TH-positive neurons typical of neurodegeneration of the lesioned SNpc (fig. 1A and B). High-power (100X) magnification (fig. 1C and D) shows the outlines of nucleolar bodies, which are heavily pigmented compared to the surrounding cytoplasm.
Fig. 1. High-power and low-power micrographs of TH-AgNOR stained sections of contralateral and ipsilateral substantia nigra.
4X images of TH-AgNOR-stained sections of contralateral and ipsilateral SN qualitatively show depletion of the lesioned dopaminergic regions (1A and B). 100X images show the outlines of the contralateral and ipsilateral neurons and the AgNOR-stained nucleoli (1C and D). Representative sections from the same rat are shown. Abbreviations: SNL- substantia nigra lateralis, SNpc- substantia nigra pars compacta, VTA- ventral tegmental area
TH-AgNOR-stained SNpc region volume
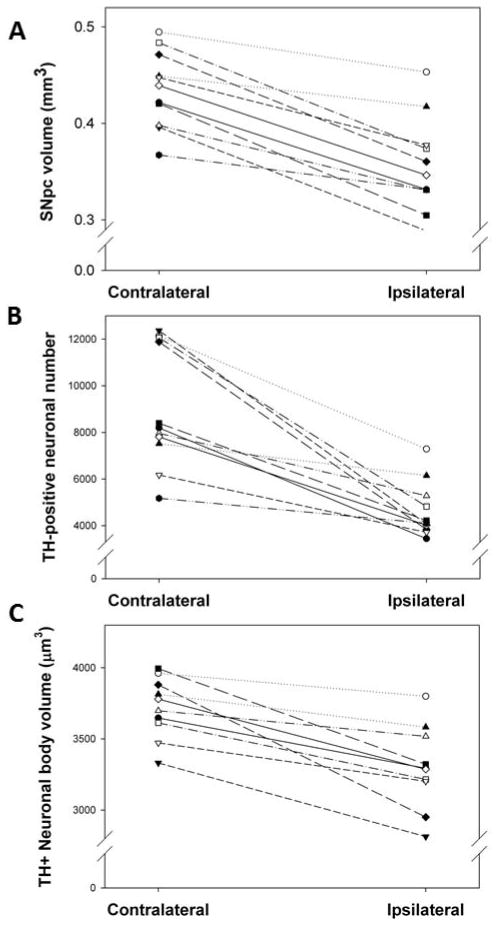
Regional volume of the ipsilateral SNpc was smaller in all rats compared to the contralateral SNpc (fig. 3A). The mean within-subject decrease in planimetric volume between the ipsilateral and contralateral SNpc was 18.3 ± 2.2% (P<0.05).
Fig. 3. Effects of unilateral intrastriatal 6-OHDA lesion on TH-AgNOR-stained planimetric volume (A), neuron number (B) and neuron volume (C) in the SNpc.
All rats showed decreased SNpc planimetric volume between the ipsilateral and contralateral hemispheres. The mean within-subject decrease was 18.3 ± 2.2% (P<0.05 by paired t-test, n=11). All rats showed decreased neuronal number between the ipsilateral and contralateral SNpc. The mean within-subject decrease was 45.7% ± 5.1% (P<0.05 by paired t-test, n=11). All rats showed decreased neuronal volume between the ipsilateral and contralateral SNpc. The mean within-subject decrease was 11.2 ± 3.1% (P<0.05 by paired t-test, n=11).
TH-AgNOR-stained SNpc neuron number
The mean total number of TH-positive SNpc neurons was 4631 ± 355 on the ipsilateral and 9062 ± 781 on the contralateral sides (fig. 3B), which is comparable with other stereological studies [1, 4, 7, 10, 15]. All rats had decreased TH-positive cells between the ipsilateral and contralateral SNpc, with a mean within-subject decrease of 45.7% ± 5.1% (P<0.05).
TH-AgNOR-stained SNpc neuron volume
Average neuronal body volume was smaller in all rats in the ipsilateral SNpc compared to the contralateral SNpc (fig. 3C), with a mean within-subject decrease of 11.2 ± 3.1% (P<0.05).
TH-AgNOR-stained SNpc nucleolar volume
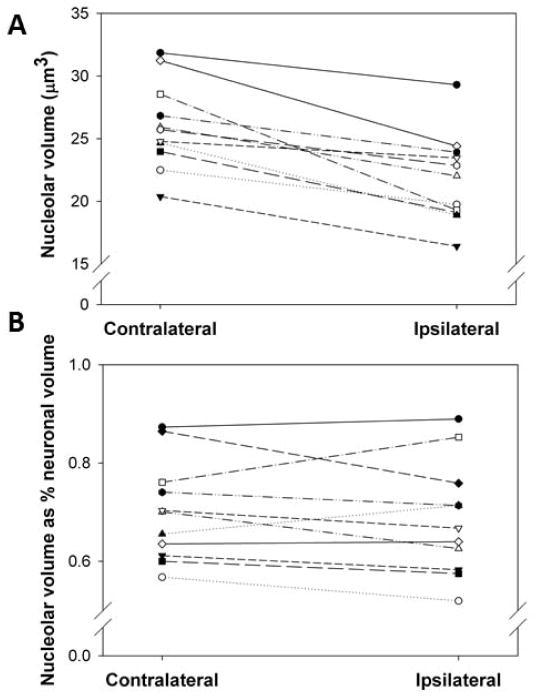
Average nucleolar volume was smaller in all rats in the ipsilateral SNpc compared to the contralateral SNpc, with a mean within-subject decrease of 16.4 ± 2.4% (P<0.05) (fig. 4A). However, the ratio of nucleolar volume to neuronal volume between the ipsilateral and contralateral SNpc was smaller in only 6 of the 11 rats and there no significant difference between sides (fig. 4B).
Fig. 4. Effects of unilateral intrastriatal 6-OHDA lesion on TH-AgNOR-stained nucleolar volume in the SNpc.
All rats showed decreased nucleolar volume between the ipsilateral and contralateral SNpc (4A). The mean within-subject decrease was 16.4 ± 2.4% (P<0.05 by paired t-test, n=11). The ratio of nucleolar volume to neuronal volume in the ipsilateral SNpc was smaller in only 6 of the 11 rats the contralateral SNpc and there was no significant difference between sides (4B).
Discussion
This study determined the effects of unilateral 6-OHDA neurotoxic lesion on SNpc neuronal number, soma volume and nucleolar morphology using stereology and TH-AgNOR staining. A functional dopaminergic deficit was confirmed in the ipsilateral striatum by amphetamine-stimulated rotations. The single-site injection of 6-OHDA (12.5 μg) resulted in an 18% decrease in the planimetric volume of the TH-positive ipsilateral SN and a 46% depletion of SNpc TH-positive neurons (Figs. 3A and 3B). Appearance of clinical signs in PD has been associated with SNpc neuronal losses of around 50% [21] thus, cell loss of this magnitude reflects a clinically relevant model of moderate Parkinson’s. Neuronal volume loss was 11% with a corresponding 16% decrease in nucleolar volume on the ipsilateral side. Overall, these findings suggest that the partial unilateral 6-OHDA lesion achieved a moderate depletion of SNpc neurons comparable to the threshold findings in parkinsonian human brains.
Using the TH-AgNOR stain, the present data show a 16% decrease in nucleolar volume accompanied by an 11% decrease in neuronal body volume (fig. 3C). These decreases in nucleolar and neuronal size support morphological findings observed in postmortem Parkinson’s brains [20, 25]. This supports the validity of the 6-OHDA lesioned rat as model of the early stages of parkinsonian neurodegeneration, at least with respect to these parameters.
Altered morphology is an informative tool in documenting the changes caused by the neurodegenerative process to neuronal and nucleolar volume. Quantitation of morphological changes is an important first step in establishing differences between neurons, which can then be further explored with complementary functional or biochemical assays to determine the mechanisms of change. The volumetric changes that occurred in this study could be due to either neurodegeneration on the ipsilateral side, or to compensation-related hypertrophy on the contralateral side, since striatal sprouting after unilateral brain lesions has been proposed as a compensatory mechanism [5, 8]. In future studies, it would be interesting to determine the neuronal and nucleolar volume in additional unlesioned rats for comparison; however, another study using a unilateral intrastriatal 6-OHDA (8μg) and solvent-injected control animals using stereological analysis found no difference in contralateral TH-positive SNpc soma volumes between the 6-OHDA- and solvent-injected animals [10]. Nucleolar size is associated with nucleolar activity [3], thus the 16% change in nucleolar volume in the ipsilateral hemisphere (fig. 4A) may reflect a change in nucleolar function and provide a valuable index of neuronal damage. Other changes to nucleolar morphology, such as nucleolar segregation and disruption, are associated with various cellular stressors, including DNA damage, hypoxia, viral infection, and nutrient stress [3]. Likewise, the stressors potentially involved in the etiology of Parkinson’s could also cause altered nucleolar morphology. Altered nucleolar morphology has been reported in clinical Parkinson’s disease, as the volume of nucleoli was decreased in post-mortem Parkinson’s brains by 16% [20]. Since neuronal and nucleolar function are closely interrelated, however, it may be more relevant to consider neuronal and nucleolar volume together than separately. Indeed, in our study there was no difference in the percentage of the nucleolus to total neuronal volume in the ipsilateral and contralateral SNpc (fig. 4B), suggesting a concomitant decrease of both neuronal body and nucleolus that may indicate their interdependent morphology. Although the morphological relationship between the nucleolar and neuronal body volumes in clinical Parkinson’s is not currently known, stereological assessment of neuronal and nucleolar volumes in postmortem brains from Alzheimer’s patients showed both neuronal and nucleolar atrophy in the CA1 region of the hippocampus [14], implicating decreased nucleolar volume in neurodegenerative disease.
How nucleolar morphology reflects the neurodegeneration in Parkinson’s disease is still uncertain, although there are several potential mechanisms for nucleolar damage and consequent morphology changes in Parkinson’s. Greater nucleolar damage to postmortem Parkinson’s neurons, assessed by loss of nucleolar integrity, has been found relative to controls [24]. Oxidative damage to RNA has been a suspected factor in a number of neurodegenerative diseases, including Parkinson’s [16], and may play a role in nucleolar damage. For example, in mice, 1,2,3,6-tetrahydro-1-methyl-4-phenylpyridine hydrochloride (MPTP) treatment induced nucleolar damage marked by nucleophosmin staining localization to the cytoplasm and inhibited mammalian target of rapamycin (mTOR) signaling, a regulator of rRNA synthesis. [24]. The transcription initiation factor 1A (TIF-1A) regulates the nucleolus-specific RNA polymerase 1 (Pol1), and TIF-1A ablation in mouse embryonic fibroblasts results in nucleolar disruption and in upregulation of tumor-suppressing protein p53 [28]. Adult mice with selective dopaminergic neuron ablation of TIF-1A demonstrated progressive loss of SN neurons and locomotor deficits [24]. Nucleolar damage in Parkinson’s could also be precipitated by DNA damage, as the DNA-topoisomerase-2 inhibitor etoposide inhibited Pol1 and induced nucleolar stress indicated by staining for B23/nucleophosmin [23]. Future studies must determine specifically how nucleolar damage, and corresponding nucleolar morphology changes, contribute to the pathophysiology of Parkinson’s disease and whether nucleolar morphology may prove useful as an indicator of disease progression.
In conclusion, TH-AgNOR staining combined with stereological assessment indicated altered nucleolar morphology of SNpc dopaminergic neurons in rats after a partial unilateral intrastriatal 6-OHDA. The observed decreased nucleolar volume suggests that this organelle plays a critical role in neurodegenerative processes and possibly also in the early clinical course of the Parkinson’s disease.
Highlights.
Unilateral intrastriatal 6-OHDA lesions in rats were analyzed with stereology and TH-AgNOR stain
SNpc TH-AgNOR+ nucleolar volume was decreased by 16%
There was no change in the ratio of nucleolar volume to neuronal volume in lesioned SNpc neurons
SNpc TH+ planimetric volume, neuronal number and body volume were decreased after 6-OHDA lesions
Acknowledgments
The authors thank Drs. Robert Switzer and Stan Benkovic of NeuroScience Associates for preparing the AgNOR stained sections and facilitating our use of this staining technique in this study. Supported by NIH Grants NS067422 (BL), RR016475 (BL), HD02528 (BL, JAS), the Institute for Advancing Medical Innovation (MH-S), the Mabel A. Woodyard Fellowship in Neurodegenerative Disorders (MH-S), the University of Kansas Endowment (MH-S), and the University of Kansas Medical Center Institute for Neurological Discoveries (MH-S).
Abbreviations
- rRNA
ribosomal ribonucleic acid
- 6-OHDA
6-hydroxydopamine
- NOR
nucleolar organizing region
- AgNOR
silver nucleolar
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TH-AgNOR
tyrosine hydroxylase - silver nucleolar
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle Healy-Stoffel, Email: mstoffel@kumc.edu.
S. Omar Ahmad, Email: sahmad13@slu.edu.
John A. Stanford, Email: jstandford@kumc.edu.
Beth Levant, Email: blevant@kumc.edu.
References
- 1.Ahmad S, Park J, Radel J, Levant B. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci Lett. 2008;438:303–307. doi: 10.1016/j.neulet.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethel-Brown CS, Zhang H, Fowler SC, Chertoff ME, Watson GS, Stanford JA. Within-session analysis of amphetamine-elicited rotation behavior reveals differences between young adult and middle-aged F344/BN rats with partial unilateral striatal dopamine depletion. Pharmacol Biochem Behav. 2010;96:423–428. doi: 10.1016/j.pbb.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho GA, Nikkhah G. Subthalamic nucleus lesions are neuroprotective against terminal 6-OHDA-induced striatal lesions and restore postural balancing reactions. Exp Neurol. 2001;171:405–417. doi: 10.1006/exnr.2001.7742. [DOI] [PubMed] [Google Scholar]
- 5.Cheng HW, Tong J, McNeill TH. Lesion-induced axon sprouting in the deafferented striatum of adult rat. Neurosci Lett. 1998;242:69–72. doi: 10.1016/s0304-3940(98)00050-0. [DOI] [PubMed] [Google Scholar]
- 6.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 7.Eriksen N, Stark AK, Pakkenberg B. Age and Parkinson’s disease-related neuronal death in the substantia nigra pars compacta. J Neural Transm Suppl. 2009:203–213. doi: 10.1007/978-3-211-92660-4_16. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 9.Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997;50:55–66. doi: 10.1007/978-3-7091-6842-4_7. [DOI] [PubMed] [Google Scholar]
- 10.Gomide V, Bibancos T, Chadi G. Dopamine cell morphology and glial cell hypertrophy and process branching in the nigrostriatal system after striatal 6-OHDA analyzed by specific sterological tools. Int J Neurosci. 2005;115:557–582. doi: 10.1080/00207450590521118. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 12.Healy-Stoffel M, Ahmad SO, Stanford JA, Levant B. A novel use of combined tyrosine hydroxylase and silver nucleolar staining to determine the effects of a unilateral intrastriatal 6-hydroxydopamine lesion in the substantia nigra: A stereological study. J Neurosci Methods. 2012 doi: 10.1016/j.jneumeth.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetman M, Pietrzak M. Emerging roles of the neuronal nucleolus. Trends Neurosci. 2012;35:305–314. doi: 10.1016/j.tins.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- 16.Kong Q, Lin CL. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci. 2010;67:1817–1829. doi: 10.1007/s00018-010-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]
- 18.Kuhn CM, Schanberg SM. Metabolism of amphetamine after acute and chronic administration to the rat. J Pharmacol Exp Ther. 1978;207:544–554. [PubMed] [Google Scholar]
- 19.Lee CS, Sauer H, Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 20.Mann DM, Yates PO. Pathogenesis of Parkinson’s disease. Arch Neurol. 1982;39:545–549. doi: 10.1001/archneur.1982.00510210015004. [DOI] [PubMed] [Google Scholar]
- 21.Marsden CD. Parkinson’s disease. Lancet. 1990;335:948–952. doi: 10.1016/0140-6736(90)91006-v. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego, California, USA: 1998. [Google Scholar]
- 23.Pietrzak M, Smith SC, Geralds JT, Hagg T, Gomes C, Hetman M. Nucleolar disruption and apoptosis are distinct neuronal responses to etoposide-induced DNA damage. J Neurochem. 2011;117:1033–1046. doi: 10.1111/j.1471-4159.2011.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, Stotz S, Neumann M, Yuan X, Grummt I, Schütz G, Parlato R. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31:453–460. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 27.Switzer RC, III, Baun J, Tipton B, Segovia C, Ahmad SO, Benkovic SA. Modification of AgNOR staining to reveal the nucleolus in thick sections specified for stereological assessment of dopaminergic neurotoxicity in substantia nigra, pars compacta. Society for Neuroscience. 2011:Abstract, 2011. [Google Scholar]
- 28.Yuan X, Zhou Y, Casanova E, Chai M, Kiss E, Gröne HJ, Schütz G, Grummt I. Genetic inactivation of the transcription factor TIF-IA leads to nucleolar disruption, cell cycle arrest, and p53-mediated apoptosis. Mol Cell. 2005;19:77–87. doi: 10.1016/j.molcel.2005.05.023. [DOI] [PubMed] [Google Scholar]