Abstract
CD4+Foxp3+ regulatory T cells (Treg) are critical regulators of immune homeostasis and self-tolerance. Whereas thymic-derived or natural Treg (nTreg) stably express Foxp3, adaptive or induced Treg (iTreg) generated from peripheral CD4 T cells are susceptible to inflammation-induced reversion to pathogenic effector T cells (Teff). Building upon our previous observations that T cell-expressed receptors for C3a (C3aR) and C5a (C5aR) drive Th1 maturation, we tested the impact of C3aR/C5aR signaling on induction and stability of alloreactive iTreg. We observed that genetic deficiency or pharmacological blockade of C3aR/C5aR signaling augments murine and human iTreg generation, stabilizes Foxp3 expression, resists iTreg conversion to IFNγ/TNFα-producing Teff, and as a consequence, limits the clinical expression of graft-versus-host disease. Together the findings highlight the expansive role of complement as a crucial modulator of T cell alloimmunity and demonstrate proof-of-concept that targeting C3a/C3aR and C5a/C5aR interactions could facilitate iTreg-mediated tolerance to alloantigens in humans.
Introduction
CD4+Foxp3+ thymic-derived nTreg are critical regulators of self-tolerance (1) while Treg induced in the periphery (iTreg) shield against mucosal injury (2). Both protect against pathogenic allo-immunity and facilitate tolerance induction in murine models of solid organ and hematopoietic cell transplantation (3–6). In contrast to the stable phenotype of nTreg (7), iTreg are susceptible to inflammation-induced reprogramming into pathogenic effector T cells (Teff) (8), thereby limiting their therapeutic potential.
We previously demonstrated that complement activation products C3a and C5a bind their respective G-protein coupled receptors, C3aR and C5aR, expressed on T cells to provide costimulatory signals that enhance Teff activation (9) and limit nTreg function (10). Herein we test whether and how T cell expressed C3aR/C5aR impact generation and stability of alloreactive iTreg. Our findings indicate that blocking C3a/C3aR and C5a/C5aR ligations favor induction and stability of murine and human iTreg. Together the results identify a previously unrecognized set of pharmacological targets that could be exploited to facilitate induction of immune tolerance to alloantigens in the context of human solid organ or hematopoietic cell transplants.
Materials and Methods
Mice
Wild-type (WT), CD45.1, and rag1−/− (H-2b), BALB/c, C.B.-17 scid (H-2d), NOD-scid IL2Rγ−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6 C3ar1−/−C5ar1−/− mice expressing the Foxp3GFP reporter and B6 Daf1−/− mice (10) were developed and maintained through intercrossing at Mount Sinai. Study protocols were approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine, New York, NY, USA.
Reagents
We purchased recombinant human C3a and C5a from R&D Systems (Minneapolis, MN USA), rat IgG2bκ and blocking anti-C3a and anti-C5a mAb from BD Biosciences (Franklin Lakes, NJ USA), C3aR antagonist SB290157 and AKT inhibitor VIII from Calbiochem (San Diego, CA USA), and C5aR peptide antagonist [Ac-Phe-cyclo(Orn-Pro-dCha-Trp-Arg)] from GenScript (Piscataway, NJ USA).
Flow cytometry
Cell surface and intracellular staining was performed as described (10), data were collected on a FACSCanto II (BD Biosciences) and analyzed using FloJo software (Tree Star, Ashland, OR USA).
Cell isolation
T cell depletion from bone marrow suspensions and isolation of murine splenic and LN CD4+ T cells and CD11c+ DCs was accomplished using magnetic beads (Miltenyi Biotec, Auburn, CA USA). CD4+Foxp3GFP+ cells were isolated using a MoFlo Legacy Cell Sorter from Beckman Coulter (Indianapolis, IN USA). Human CD4+CD25− T cells and CD14+ monocytes were obtained from PBMC of healthy controls (under Mount Sinai IRB consent) using appropriate kits (Miltenyi).
Cell stimulation
B6 anti-BALB/c allo-iTregs were generated by culturing 2×105 Tconv with 5×104 CD11c+ DCs (BALB/c) + IL-2 and TGFβ for 5d. Stability was assessed by restimulating 5×104 allo-iTreg with 5×104 CD11c+ DCs + IL-2 for 5d. Human iTreg were generated by culturing 2×105 Tconv with 5×104 monocyte-derived DCs with anti-CD3, IL-2, and TGFβ for 5d. To generate DCs, CD14+ monocytes were matured for 5d with GM-CSF and IL-4 followed by LPS stimulation for 48h. Human iTreg stability was assessed by restimulating 5×104 CD25+ iTreg with 5×104 DCs + anti-CD3 and IL-2 for 3d. C3aR-A, C5aR-A, or vehicle control were added to cultures as indicated.
In vivo models
Homeostatic proliferation and in vivo iTreg conversion was tested by transfer of 2×104 naïve Foxp3GFPneg CD62LhiCD44lo Tconv in rag1−/− mice. Short term acute GVHD experiments were done in lethally irradiated (850 rad) BALB/c hosts receiving 2.5×105 sorted allo-iTreg + 1×106 CD45.1+ Tconv and 2×106 T cell depleted CD45.1+ bone marrow cells. In GVHD outcome studies, 4wk old C.B.-17 scid hosts received 5×105 CD45.1+ Tconv ± 2.5×105 sorted allo-iTreg. Human xenogenic GVHD experiments were performed in NOD scid γcnull mice given 2×107 human PBMCs (i.v.) from the same donor. Animals received CTLA4Ig (Abatacept, Bristol Meyers Squibb, 7 × 100mg i.p. every 48h) and/or C5aR peptide antagonist (1 mg/kg/d by osmotic pump from Alzet, Cupertino, CA USA, up to d 28 and daily i.p injections thereafter).
In vitro suppression assays
Murine suppression assays were performed as described (10): 2×105 CFSE-labeled CD45.1+ Tconv (B6) were mixed with 5×104 APCs (BALB/c) in 96-well plates ± titrated numbers of CD45.2+ Foxp3GFP+ iTreg and CSFE dilution was assessed on d 5. Human suppression assays were performed similarly with 2×105 CFSE-labeled PBMC anti-CD3 ± CD25+ isolated iTreg.
Immunoblots
Immunoblots for Foxo1 and phospho-Foxo1 was detected using antibodies from Cell Signaling Technologies (Beverly, MA USA) as described (10).
siRNA knockdown
We obtained DAF or control scrambled siRNA from Thermo Scientific (Waltham, MA USA). DCs were transfected using the NEON electroporation system (Life Technologies) providing a single pulse of 1600mV for 20 mSec.
Statistical analyses
Results are shown as means ± s.d. or s.e.m. as indicated. Comparisons of replicate datasets were performed using two-tailed unpaired Student’s t test. Human data involving multiple donors were analyzed using two-tailed paired t test. p < 0.05 was considered statistically significant.
Results and Discussion
Absent C3aR/C5aR signaling on CD4+ T cells augments iTreg generation
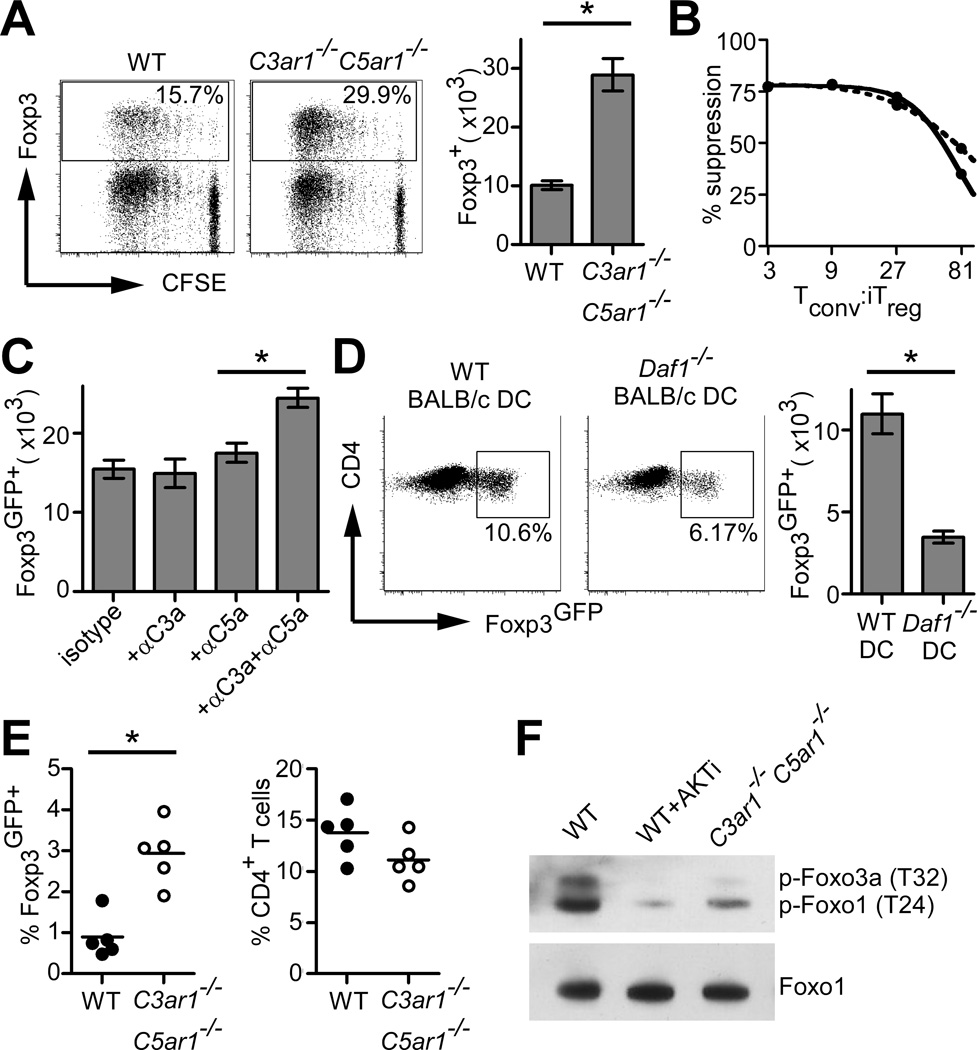
To address whether C3aR/C5aR signaling impacts induction and/or function of CD4+Foxp3+ alloantigen-specific iTreg (allo-iTreg) we stimulated flow-sorted naïve, conventional T cells (Tconv) from B6 WT or C3ar1−/−C5ar1−/− Foxp3GFP reporter mice (10) with allogeneic BALB/c splenic DCs, IL-2, and TGFβ. These assays showed higher percentages and absolute numbers of Foxp3GFP+ cells in the cultures initiated with C3ar1−/−C5ar1−/− CD4+ T cells (Fig. 1A). In vitro suppression assays demonstrated equivalent suppressive capacities (Fig. 1B). Blocking anti-C3a/C5a antibodies similarly enhanced allo-iTreg generation (Fig. 1C). Conversely, we generated iTreg using DCs deficient in decay accelerating factor (DAF/CD55), a cell surface regulator of the C3 convertase (9). DAF deficiency lifts restraint on complement activation resulting in augmented local, immune cell derived C3a and C5a (10, 11). The assays revealed that DAF deficient DCs diminished iTreg generation (Fig. 1D).
FIGURE 1. C3aR/C5aR signaling on CD4+ T cells modulates allo-iTreg generation.
(A) Representative flow plots (left and middle, 12,000 events shown in each plot) and total number (right) of Foxp3+ cells generated from CFSE-labeled CD4+ WT or C3ar1−/−C5ar1−/− Tconv. (B) Suppression capacities of WT (solid line) or C3ar1−/−C5ar1−/− (dashed line) allo-iTreg. (C) Total Foxp3GFP+ cells generated as in (A) ± blocking anti-C3a/C5a mAb. (D) Percentages (left/middle) and total numbers (right) of Foxp3+ cells generated as in (A) using allogeneic WT or Daf1−/− DCs. (E) Splenic frequencies of Foxp3GFP+ (left) and total CD4+ cells (right) 14d after adoptive transfer of naive WT or C3ar1−/−C5ar1−/− CD4+ cells into syngeneic rag1−/− mice. (F) Immunoblot for pFoxo1 and total Foxo1 from stimulated iTreg cell lysates. Results are each representative of 3 independent experiments. Error bars indicate mean ± s.d. *p<0.05
We observed 3-fold higher frequencies of Foxp3GFP+ iTreg following adoptive transfer of naïve C3ar−/−C5ar−/− CD4+ T cells into rag1−/− recipients (Fig. 1E), verifying that absent C3aR/C5aR signaling augments iTreg generation in vivo.
We previously linked C3aR/C5aR signaling to PI-3Kγ-dependent AKT phosphorylation in Tconv and nTreg (9, 10, 12). Others showed that pAKT inhibits iTreg generation (13), in part through phosphorylating the transcription factors Foxo1/3a which prevents nuclear transfer required to induce Foxp3 (14). We observed reduced AKT-dependent pFoxo1 in cultures of C3ar1−/−C5ar1−/− iTreg vs. WT (Fig. 1F), thereby linking C3aR/C5aR signaling to known molecular mechanisms required for iTreg induction.
Absence or blockade of C3aR/C5aR enhances allo-iTreg stability
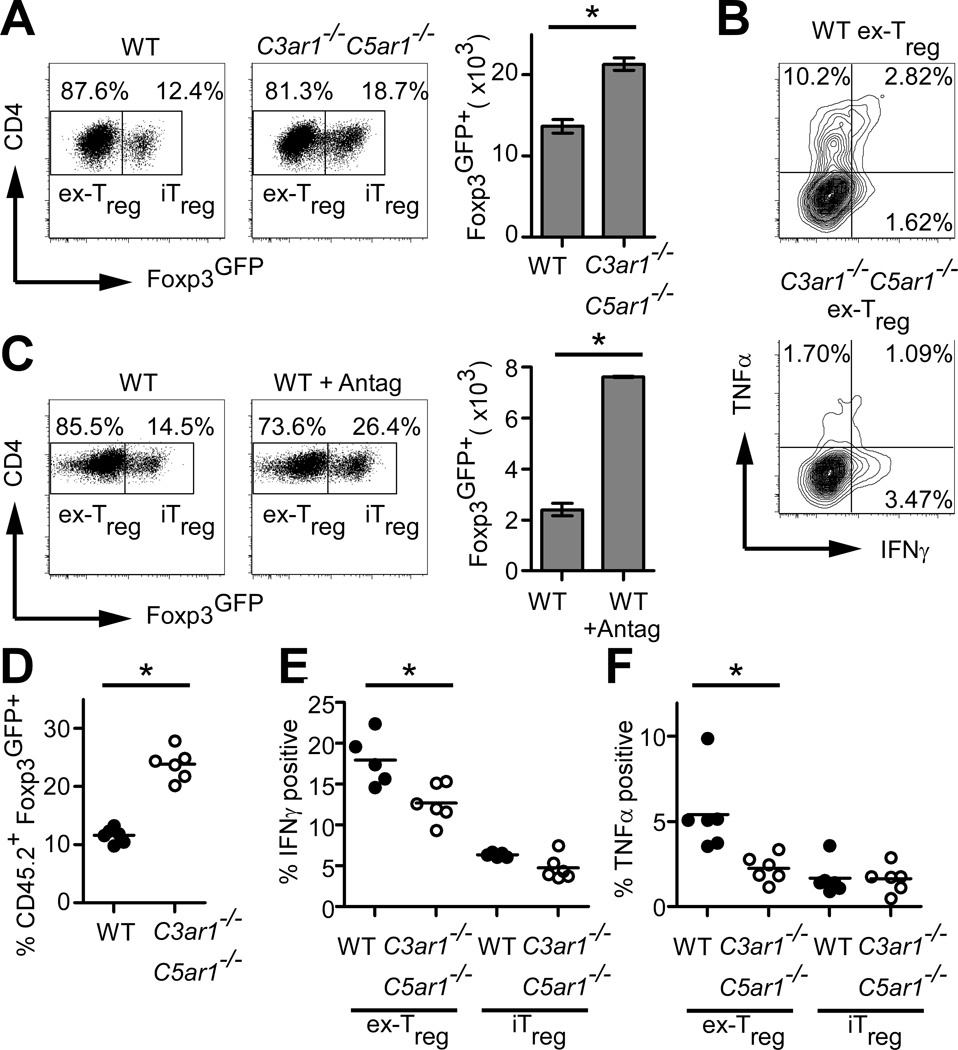
iTreg are susceptible to Foxp3 loss and can acquire effector phenotypes (8), prompting us to test the effects of absent C3aR/C5aR on iTreg stability. Flow-sorted Foxp3GFP+ allo-iTreg (>98% purity) were re-stimulated in secondary MLRs in the absence of TGFβ and examined for Foxp3GFP expression, revealing higher percentages and total numbers of Foxp3GFP+ cells in cultures containing the C3ar1−/−C5ar1−/− cells (Fig. 2A). Absent C3aR/C5aR signaling also prevented conversion of former (ex-) iTreg to IFNγ/TNFα-secreting effectors (Fig. 2B). No IL-17A was detected (not shown). Blocking C3aR/C5aR signaling on WT cells using receptor antagonists had similar effects (Fig. 2C).
FIGURE 2. Absent C3aR/C5aR signaling enhances allo-iTreg stability.
(A) Percentage (left/middle) and number (right) of Foxp3GFP+ cells from sorted allo-iTreg 5d after restimulation with allogeneic DCs + IL-2. (B) Representative intracellular IFNγ/TNFα cytokine staining of cultures gated on ex-Treg. (C) Percentage (left/middle) and number (right) of Foxp3GFP+ cells stimulated as in (A) ± C3aR-A/C5aR-A antagonists (Antag). (D–F) Sorted CD45.2 allo-iTregs were injected into lethally irradiated BALB/c mice with B6CD45.1 bone marrow transplant + Tconv. Foxp3 expression in splenic CD45.2 (D) and intracellular IFNγ (E) and TNFα (F) in CD45.2 Foxp3GFPneg (ex-Treg), and Foxp3GFP+ (iTreg) populations are shown. Results are each representative of at least 3 independent experiments. Error bars indicate mean ± s.d. *p<0.05
To address whether the enhanced stability was associated with reduced epigenetic methylation of the CNS2 region within the Foxp3 promoter (15, 16), we performed bisulfite sequencing in the WT and C3ar1−/−C5ar1−/− allo-iTreg. These assays showed similar methylation of the CNS2 regions in the WT and C3ar1−/−C5ar1−/− allo-iTreg (Supplemental Fig. 1).
We assessed the impact of C3aR/C5aR signaling on iTreg stability in vivo by injecting B6 CD45.2 WT or C3ar1−/−C5ar1−/− allo-iTreg into lethally irradiated BALB/c hosts with B6 bone marrow and congenic CD45.1 Tconv, and examined Foxp3GFP expression 5d later (design/gating in Supplementary Fig. 2). These assays showed more Foxp3GFP+ cells (Fig 2D) and fewer IFNγ/TNFα-producing ex-Treg (Fig 2E–F) in recipients of C3ar1−/−C5ar1−/− allo-iTreg.
C3ar1−/−C5ar1−/− allo-iTreg better suppress GVHD onset
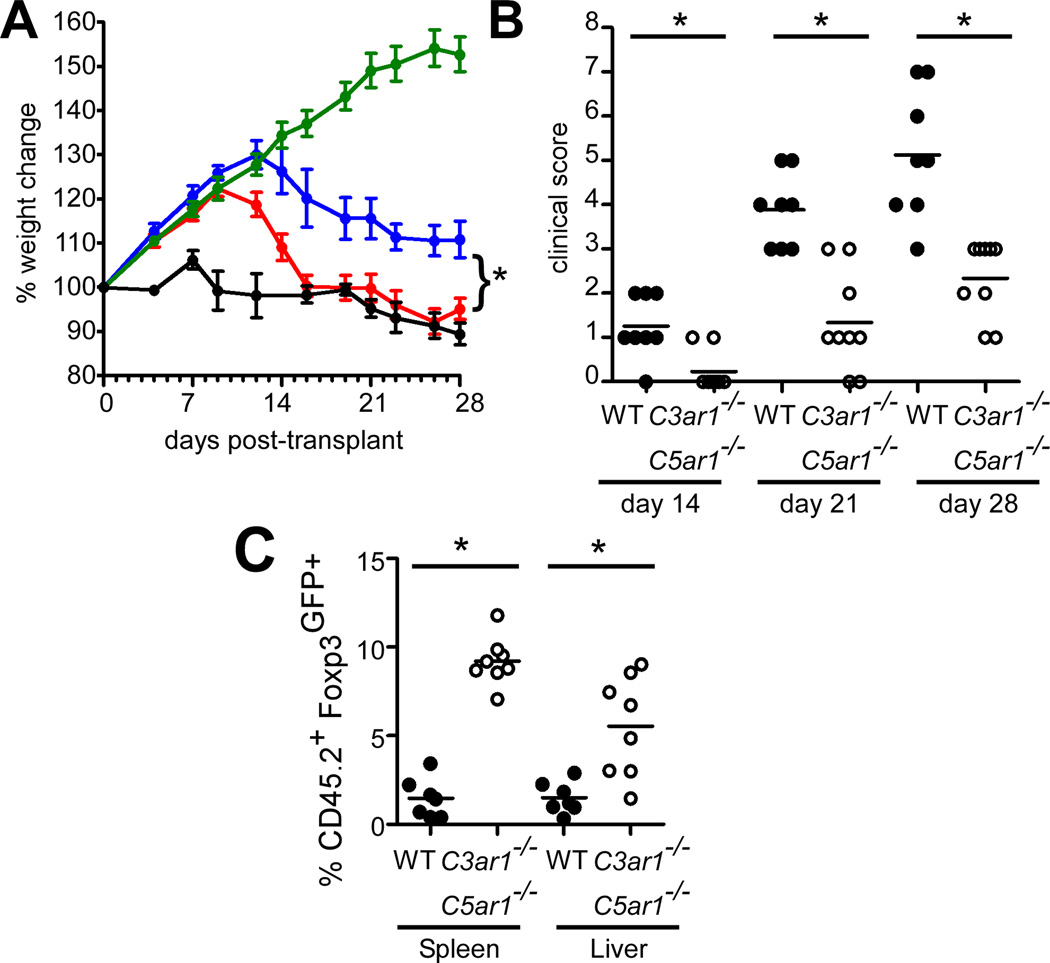
To test the functional significance of the observed enhanced iTreg stability resulting from C3aR/C5aR deficiency, we used a model of acute GVHD in which Tconv ± iTreg are adoptively transferred into 4 week old allogeneic SCID recipients (17). Adoptive transfer of Tconv alone induced GVHD manifested in part as preventing the weight gain that normally occurs as the animal age to maturity (Fig 3). While WT allo-iTreg delayed disease onset, we observed better weight gain and lower clinical scores in animals transferred with C3ar1−/−C5ar1−/− allo-iTreg (Fig. 3A–B). Spleens and livers from recipients of C3ar1−/−C5ar1−/− allo-iTreg 4 weeks after transfer contained up to 6-fold more Foxp3GFP+ cells (Fig. 3C), indicating that absent C3aR/C5aR signaling augments long-term iTreg stability in vivo during GVHD.
FIGURE 3. Allo-iTreg deficient in C3aR/C5aR suppress acute GVHD and exhibit enhanced in vivo stability.
(A) Weight changes and (B) clinical scores in C.B.-17 scid mice that received CD45.1+ Tconv alone (black, n=4), or Tconv + WT (red n=7) or C3ar1−/−C5ar1−/− (blue, n=8) allo-iTreg. Normal growth curve in PBS controls (green, n=5). (C) Quantified percentages of Foxp3GFP+ iTreg among CD45.2+ T cells in the spleen and liver of recipients at 4 wk. Results are cumulative data of 3 independent experiments. Error bars indicate mean ± s.e.m. *p<0.05
C3aR/C5aR signaling modulates generation and stability of human iTreg
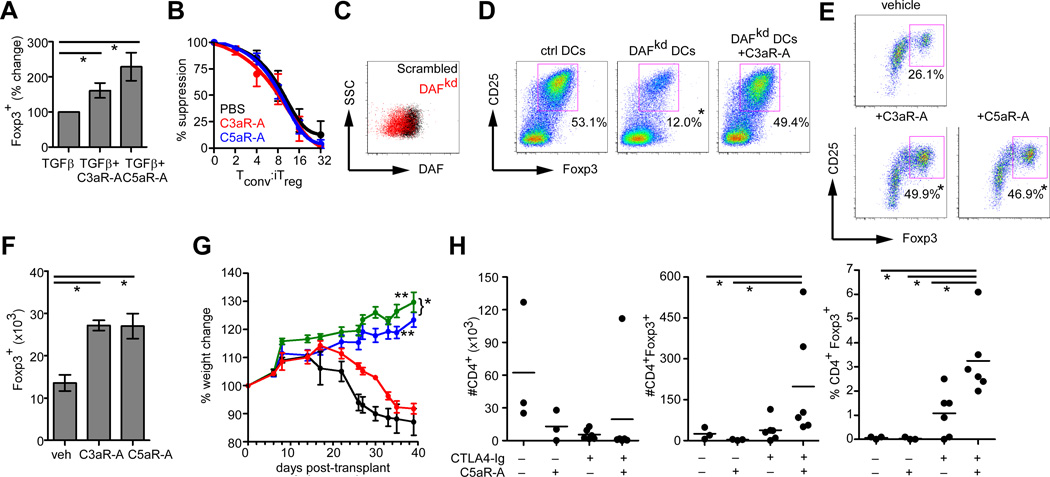
Verifying the murine findings with human cells, we observed that addition of recombinant C3a/C5a reduced Foxp3 expression during human iTreg generation (Supplemental Fig. 3) while antagonizing C3aR/C5aR signaling (small molecule inhibitors) augmented the frequency of Foxp3 expressers (Fig. 4A). The induced CD4+CD25+Foxp3+ cells generated ± C3aR/C5aR antagonists exhibited equal suppressive capacities (Fig. 4B). When we knocked down human DAF (DAFkd) on monocyte-derived DCs with siRNA (Fig. 4c) and used these APCs for in vitro iTreg generation, we observed 2–4 fold higher quantities of C3a/C5a from DAFkd cultures (p<0.05 by ELISA, not shown, verifying lifted restraint on local complement activation) which associated with generation of fewer Foxp3+ iTreg (Fig. 4D). The effects were reversed by adding a C3aR antagonist. Following restimulation in MLRs we observed higher percentages (Fig. 4E) and total numbers (Fig. 4F) of Foxp3+ cells in the presence of C3aR/C5aR antagonists, indicating that blocking C3aR/C5aR signaling augments phenotypic stability.
FIGURE 4. C3aR/C5aR signaling modulates generation and stability of human iTreg.
(A) Percentage of Foxp3+ CD4+ cells on d5 of human iTreg induction cultures ± C3aR-A or C5aR-A (n=8). *p<0.05. (B) Suppressive capacities of CD25+ iTreg generated with C3aR-A (red), C5aR-A (blue) or PBS control (black). (C) Representative flow plot of DAF expression on humans DCs transfected with scrambled-targeted (black) or DAF-targeted (red) siRNA. (D) Plots depicting human Foxp3+ CD4+ cells generated with control or DAFkd DCs, ± C3aR-A (mean % Foxp3+ cells shown, n=4,*p<0.05 vs. other 2 groups). (E) Percentages and (F) total numbers of Foxp3 expressing, CD25-enriched human iTreg restimulated for 3d ± C3aR-A or C5aR-A. Numbers represent means and s.d. of 3 replicates, *p<0.05 vs. control. (G) Weights of NOD scid γcnull mice transferred with human PBMC treated with PBS (black), C5aR-A (red), CTLA4-Ig (blue), or CTLA-Ig + C5aR-A (green), n=4–6 per group *p<0.05 (CTLA4Ig vs. CTLA4Ig+C5aR-A), **p<0.05 vs. PBS and vs. C5aR-A alone. (H) Total numbers of human CD4+ cells (left) or CD4+Foxp3+ cells (middle) in the spleens, and percentages of Foxp3+ cells (right) within the human CD4+ gate, 6 wk after adoptive transfer (n=3–6/group), *p<0.05.
To assess effects of C5aR blockade in vivo we adoptively transferred human PBMC into NOD-scid IL2Rγnull recipients, an immunodeficient mouse that permits the expansion of human lymphocytes resulting in GVHD over 4–6 weeks (18), and notably lacks murine C5 (19). We tested the effects of administering a specific human C5aR antagonist (20) ± CTLA4Ig (Abetacept), previously shown to attenuate GVHD (21) and to facilitate iTreg generation (22, 23) on disease outcome. CTLA4Ig attenuated disease scores and weight loss compared to IgG-treated controls, and co-treatment with the C5aR-A further improved outcome (Fig 4G). Treatment with C5aR-A alone partially delayed disease onset.
At day 45 post-transfer, we quantified human CD4+Foxp3+ cells in the spleens of each animal. Treatment with C5aR-A+CTLA4Ig decreased the total number of human CD4+ cells yet increased the numbers of human CD4+Foxp3+ cells, resulting in an overall increase the percentage of CD4+Foxp3+ cells compared to untreated controls (or C5aR-A treated controls, Fig 4H). In contrast, CTLA4Ig alone reduced total CD4+ T cells without altering the number of CD4+Foxp3+ cells, consistent with previous reports (24, 25).
That C3aR and C5aR signaling on T cells modulates induction and stability of murine and human supports an expansive role for these receptors beyond their traditional contribution to innate immunity. Consistent with previous studies evaluating C3aR/C5aR modulation of Treg cells (9), our data indicate that the effects on iTreg are mediated through locally produced, spontaneously activated, immune cell derived complement. C3aR and C5aR signal in part through AKT-dependent Foxo1/3a phosphorylation, although others have demonstrated links to cAMP/CREB (26) which may contribute as well. Whether and how additional stimuli known to inhibit iTreg generation and stability (including IL-6) interact with C3aR/C5aR-initiated signals, including pFoxo1 will require additional studies. Our findings support the need to test C3aR/C5aR antagonism as a strategy to improve the success of adoptive iTreg immunotherapy aimed at preventing and/or treating GVHD, transplant rejection and potentially autoimmune diseases in humans.
Supplementary Material
Acknowledgements
The authors thank Parth Lakhani for technical assistance and the personnel of the Mount Sinai Flow Cytometry Shared Resource Facility for reliable cell sorting service.
Footnotes
The work was supported by an NIH R01AI043578 and an ARRA supplement to R01AI071185 awarded to PSH. W.H. is a recipient of a fellowship award from American Society of Transplantation. E.P.A. is supported by a FIS-BAE grant from the Spanish Ministry of Health, PC is a recipient of a fellowship grant from the American Heart Association and, W.v.d.T. was supported by an NIH T32 AI007605
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013 doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, Medof ME, Merad M, Heeger PS. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito R, Katano I, Kawai K, Hirata H, Ogura T, Kamisako T, Eto T, Ito M. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87:1654–1658. doi: 10.1097/TP.0b013e3181a5cb07. [DOI] [PubMed] [Google Scholar]
- 19.Lynch DM, Kay PH. Studies on the polymorphism of the fifth component of complement in laboratory mice. Exp Clin Immunogenet. 1995;12:253–260. [PubMed] [Google Scholar]
- 20.Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol. 2005;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- 21.Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, Gribben JG. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 22.Wallace PM, Johnson JS, MacMaster JF, Kennedy KA, Gladstone P, Linsley PS. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–610. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Lee RS, Rusche JR, Maloney ME, Sachs DH, Sayegh MH, Madsen JC. CTLA4Ig-induced linked regulation of allogeneic T cell responses. J Immunol. 2001;166:1572–1582. doi: 10.4049/jimmunol.166.3.1572. [DOI] [PubMed] [Google Scholar]
- 24.Charbonnier LM, Vokaer B, Lemaitre PH, Field KA, Leo O, Le Moine A. CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplant. 12:2313–2321. doi: 10.1111/j.1600-6143.2012.04184.x. [DOI] [PubMed] [Google Scholar]
- 25.Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, Batal I, Xiao X, Sayegh MH, Chandraker A. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. 12:846–855. doi: 10.1111/j.1600-6143.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 26.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2012;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.