Abstract
This study aims to demonstrate that the left and right anterior temporal lobes (ATLs) perform critical but unique roles in famous face identification, with damage to either leading to differing deficit patterns reflecting decreased access to lexical or semantic concepts but not their degradation. Famous face identification was studied in 22 presurgical and 14 postsurgical temporal lobe epilepsy (TLE) patients and 20 healthy comparison subjects using free recall and multiple choice (MC) paradigms. Right TLE patients exhibited presurgical deficits in famous face recognition, and postsurgical deficits in both famous face recognition and familiarity judgments. However, they did not exhibit any problems with naming before or after surgery. In contrast, left TLE patients demonstrated both pre-and postsurgical deficits in famous face naming but no significant deficits in recognition or familiarity. Double dissociations in performance between groups were alleviated by altering task demands. Postsurgical right TLE patients provided with MC options correctly identified greater than 70% of famous faces they initially rated as unfamiliar. Left TLE patients accurately chose the name for nearly all famous faces they recognized (based on their verbal description) but initially failed to name, although they tended to rapidly lose access to this name. We believe alterations in task demands activate alternative routes to semantic and lexical networks, demonstrating that unique pathways to such stored information exist, and suggesting a different role for each ATL in identifying visually presented famous faces. The right ATL appears to play a fundamental role in accessing semantic information from a visual route, with the left ATL serving to link semantic information to the language system to produce a specific name. These findings challenge several assumptions underlying amodal models of semantic memory, and provide support for the integrated multimodal theories of semantic memory and a distributed representation of concepts.
Keywords: Famous face naming and recognition, Epilepsy surgery, Models of semantic memory
1. Introduction
The anterior temporal lobes (ATLs) have been implicated as important regions for object naming and recognition in a wide range of patients [e.g., focal epilepsy, tumors, semantic dementia, Alzheimer’s disease (AD)] (Chan et al., 2004; Drane et al., 2008; Glosser et al., 2003; Warrington and Shallice, 1984), using a variety of neuroimaging paradigms in both patients and healthy controls (Grabowski et al., 2001; Martin and Chao, 2001; Noppeney et al., 2004), and through direct stimulation brain mapping to protect eloquent cortex during neurosurgery (Giussani et al., 2009). These findings have contributed significantly to models of semantic memory, with nearly all of these models placing importance on the ATL regions, and most assuming these regions play a role in activating stored object representations. Nevertheless, controversy exists regarding the specific role of these regions in semantic memory (Kiefer and Pulvermuller, 2012). Some models postulate that the left and right ATL regions form a unitary semantic storage system, which is amodal yet accessible from each input modality (e.g., Semantic Hub account: Lambon Ralph and Patterson, 2008; Patterson et al., 2007). Other models view the ATLs as including multiple processing regions that reactivate stored conceptual information residing in distributed neural networks (i.e., frequently presumed to be the primary sensory and motor areas where object perception occurs), and linking this information to the classic language network. This includes the modality-specific models of semantic memory, such as the convergence zone (CZ) model of Damasio, and Barsalou’s “embodied cognition” adaptations of the CZ model (Barsalou et al., 2008, 2003; Damasio, 1989; Damasio et al., 2004). In the current study, we investigate the error patterns of pre- and postsurgical temporal lobe epilepsy (TLE) patients using a famous face identification task along with a novel multiple choice (MC) recognition paradigm to explore the veracity of these models.
1.1. Support for the amodal models of semantic memory
Amodal models of semantic memory are typically based on research in semantic dementia demonstrating that degradation or loss of semantic knowledge is observed across all lexical and sensory processing modalities (e.g., no recognition of objects visually or from characteristic sounds, tastes, or smells, or the meaning of words representing these objects) (see Kiefer and Puvermuller for a thorough review, and representative articles such as Bozeat et al., 2000; Lambon Ralph et al., 2007; Luzzi et al., 2007). Semantic dementia is associated with damage to the temporal poles and lateral inferior temporal cortex of both cerebral hemispheres with sparing of the hippocampi. While this disease progresses into posterior TL regions and the frontal lobes, damage to the bilateral temporal poles appears to be sufficient to lead to the core semantic deficits observed (Patterson et al., 2007). The Hub account (Lambon Ralph et al., 2007; Patterson et al., 2007; Rogers et al., 2004) is probably the most well-known amodal model at present.
Hub model advocates suggest the extreme semantic knowledge deficits observed in semantic dementia could not result from a multimodal distributed model, as this would require a widespread disruption of brain regions in their view. This is because different types of knowledge would be widely represented throughout the brain. As semantic dementia primarily involves the bilateral ATL regions, they conclude these regions must act as a unitary, amodal representation or repository for conceptual information. They have noted that semantic memory is “largely subserved by a unitary and relatively homogeneous neural system in the anterior and lateral aspects of the temporal cortices bilaterally [p. 206, (Rogers et al., 2004)].” They also indicate that the amodal nature of the ATL system is underscored by very high correlations observed in semantic dementia patients between scores on different semantic tasks and strong item-specific consistency across modalities (Pobric et al., 2007). Thus, conceptual information would no longer be tied to the original sensory/motor processing regions where the experience of all objects initially occurred. They note that “the system acquires abstract representations whose similarity relations are not tied to any individual modality” (Rogers et al., 2004) (p. 206).
Hub proponents state that processing functions of the bilateral ATLs are partially redundant and non-unique (Lambon Ralph et al., 2012), with damage to either leading to graded deficits in all functions that this amodal region supports. This is based on their research showing that both the processing of words and pictures can be slowed by applying repetitive transcranial magnetic stimulation (rTMS) to either the left or right ATL of healthy adults (Lambon Ralph et al., 2009; Pobric et al., 2007, 2010). Finally, the Hub advocates (Lambon Ralph et al., 2012) point to functional neuroimaging studies that have found bilateral ATL activation during multimodal semantic processing as evidence of their model (Vandenberghe et al., 1996; Visser et al., 2010). They also indicate that the reason why numerous studies failed to find such activations is due to the methodological difficulties associated with obtaining adequate signal activations in this brain region.
1.1.1. More recent emphasis on Spokes of the hub
Hub advocates have more recently emphasized a Hub and Spoke account of semantic memory (Lambon Ralph et al., 2012; Pobric et al., 2007, 2010), suggesting there are both modality-specific regions and a unitary, amodal hub region. They put forward evidence that modality-specific disruptions can be elicited using rTMS in certain regions (e.g., inferior parietal lobule stimulation slowed the naming of highly manipulable man-made objects only), while more general semantic disruptions result from focal application of rTMS to either ATL region (ATL stimulation slowed naming of all living and non-living objects) (Pobric et al., 2010). They state that “concepts are formed from the interaction of various modality-specific sources of information with an ATL trans-modal representational hub” [p. 255, (Lambon Ralph et al., 2012)]. Damage to the bilateral ATL regions is said to lead to the multimodal degradation of knowledge seen in semantic dementia. They believe a “graceful degradation” results from redundancy built into the semantic hub, meaning that greater dysfunction will occur as larger portions of this bilateral region are damaged (Lambon Ralph et al., 2010). Deficits will first occur with items that are highly specific and less frequent in occurrence. While semantic deficits will occur with unilateral lesions, they will be less striking due to redundancy in the system, and have heretofore been missed by standard clinical assessments.
1.2. Challenges to the amodal models of semantic memory
Several recent discoveries challenge the Hub model. Animal and human studies have demonstrated sensory subdivisions within the ATLs, with auditory inputs tending to be more superior and visual inputs more inferior, suggesting greater anatomic separation of sensory function (Ding et al., 2009; Kondo et al., 2003; Poremba et al., 2003). Functional ATL subdivisions are also seen in functional neuroimaging studies based upon the sensory modality of the information being processed (Olson et al., 2007; Skipper et al., 2011). Contemporary reviews of semantic memory point out that the majority of functional neuroimaging studies do not find ATL activations with semantic tasks [see (Gainotti, 2011; Kiefer and Pulvermuller, 2012; Martin, 2007; Simmons and Martin, 2009)]. Several researchers have also suggested that the presence of ATL activations appear to reflect other processes (e.g., social processing) and note that activations during a given task may not reflect an essential role in that process (Olson et al., 2007; Simmons et al., 2010; Thompson-Schill, 2003).
While slowed word and picture naming with left and right rTMS occurs, this may simply be related to stimulation beyond the target zone resulting in bilateral dysfunction (Simmons and Martin, 2009). Even if one accepts that rTMS is only impacting the focal region where it is applied, slowing of performance does not establish the nature of the underlying processes involved. For example, naming a picture might be slower after left ATL stimulation due to disruption of regions dedicated to lexical processing while the same task might be slower after right ATL stimulation due to disruption of networks carrying out recognition processes. This same criticism may be applied to the recent unilateral ATL resection data of the Hub proponents showing slowed processing of semantic processing tasks resulting from resection of either TL region (Lambon Ralph et al., 2012). Recent research involving TLE, demonstrating category-related declines in object recognition following right TL resection or object naming following left TL resection (Drane et al., 2008, 2004; Glosser et al., 2003; Trautner et al., 2004; Yucus and Tranel, 2007), has often been interpreted to support modality-specific models of semantic memory (see reviews by Gainotti, 2011; Simmons and Martin, 2009). We have emphasized that deficit patterns are unique and exclusive following left or right ATL resections rather than graded in nature as predicted by the Hub model. More recent papers from the Hub position acknowledge that deficits may be more pronounced for one hemisphere than the other (i.e., greater language deficits from left ATL resection) (Lambon Ralph et al., 2012), but they maintain that the deficit patterns are relative in nature rather than exclusive as we have asserted (e.g., we have yet to observe naming declines in right TLE patients following surgery).
Despite differing task paradigms, strikingly similar double dissociations in object naming and recognition performances have also been demonstrated in early stage semantic dementia patients with asymmetric ATL atrophy (Snowden et al., 2004). Semantic dementia patients with greater left ATL atrophy were better at identifying famous faces than famous names (worse at the lexical-semantic task), while those with predominantly right ATL atrophy demonstrated the opposite pattern (worse with the visual-semantic task). These findings were considered “inconsistent with a unitary, amodal model of semantic memory (p. 869),” and were said to support semantic knowledge being represented by an integrated, multimodal network in which the left ATL preferentially processes verbal information and the right ATL processes visual information.
Snowden et al. (2004) highlighted an additional dissociation in performance between sense of knowing (familiarity judgment) and covert object recognition. They demonstrated that when semantic dementia patients with predominantly right ATL atrophy were presented with one famous and one non-famous face, they were significantly more likely to report that both faces were unfamiliar than healthy subjects, an AD comparison group, or semantic dementia patients with predominantly left ATL atrophy. Nevertheless, when forced to choose which of the faces was famous (despite having said that both were unfamiliar), semantic dementia patients with predominantly right ATL atrophy still performed significantly better than chance levels unlike these other groups. Likewise, studies with prosopagnosic patients (i.e., individuals with an inability to recognize familiar/famous faces) have demonstrated dissociations between familiarity judgments and covert recognition for visually presented objects (Rivolta et al., 2012, 2010; Schweinberger and Burton, 2003). Taken together, these findings suggest that simple binary decisions about whether one has seen a face or not can be made even without any overt awareness. However, it is not clear this would require successful semantic processing. Our findings with TLE patients add support to the performance dissociations recognized by Snowden and colleagues, demonstrating that deficits reflect problems accessing semantic concepts rather than primary perceptual limitations.
1.3. Current hypotheses
We advance evidence that the functions of the left and right ATL regions differ with respect to their roles in object processing, demonstrating that the left ATL is critical for lexical-semantic analysis while the right is more important for visual-semantic analysis. We restrict our focus in this paper to famous face recognition and naming, although we believe that similar results may be observed with some other semantic categories (particularly proper nouns, such as landmarks). Replicating our previous findings that left TLE patients exhibit famous face naming deficits and that right TLE patients exhibit famous face recognition deficits (Drane et al., 2008), we predict that adding a MC recognition format will reinforce the idea that each ATL region carries out a unique but integrated role in semantic processing, while demonstrating that these deficits do not reflect any degradation in semantic concepts.
We predict that left TLE patients will demonstrate no impairment in famous face recognition and can generate normal amounts of semantic information about famous individuals compared to a healthy comparison group. Their dysfunction in the area of famous face identification will be limited to a pure (spontaneous) naming problem, and they should be able to accurately select the name of most famous faces when provided with a four-choice MC paradigm using semantically related foils (comparable to the performance of healthy subjects and right TLE patients). However, they will rapidly lose access to this name once it is out of working memory, as we believe their deficit results from decreased coupling between the reactivated conceptual representation of each famous face and the classic language network consistent with the CZ (distributed) models of semantic memory. In contrast, we predict that right TLE patients will report a higher proportion of famous faces to be unfamiliar, and will spontaneously recognize significantly fewer famous faces than left TLE patients and healthy participants. This is based on our view that the right hemisphere plays a unique role in processes underlying familiarity and object recognition (Drane et al., 2008). However, we also predict right TLE patients will benefit from a MC recognition paradigm involving the presentation of four orally presented names, showing improvement even for those famous faces initially rated as unfamiliar. This is because we believe: (1) the research of Snowden et al. (2004) with semantic dementia patients with asymmetric atrophy of the right ATL suggests that visuo-perceptual processing and covert object recognition occurs normally in patients with right ATL damage despite impairments in sense of familiarity and spontaneous recognition, and (2) the lexical-semantic connection node of the semantic network is located in the left ATL region (remaining intact in right TLE patients) representing a separate, unique route to stored semantic knowledge that will facilitate recognition of visually presented famous faces. The left TLE patients and healthy subjects will not benefit as much from the MC recognition paradigm on famous faces rated as unfamiliar, as they do not experience familiarity or recognition deficits at baseline. We predict right TLE patients will produce an average amount of conceptual information about famous persons identified correctly with the MC paradigm who they initially reported to be unfamiliar. These findings will highlight that right TLE patients experience deficits involving familiarity and visual recognition of famous faces, but not degradation of conceptual information related to these persons.
2. Methods
2.1. Subjects
Famous face recognition and naming was assessed in 50 pre-or postsurgical TLE patients and 20 healthy comparison subjects. Most of these TLE patients will eventually complete both pre- and postsurgical assessments. We included both pre- and postsurgical individuals to compare the deficit patterns before and after surgery. All patients possessed a presurgical Wechsler Adult Intelligence Scale (WAIS)-III IQ of 70 or above, English as primary language, and left-hemisphere language dominance based on the intracarotid amobarbital (Wada) procedure (Wada and Rasmussen, 1960). We excluded 14 TLE patients, as 12 had diffuse brain injury resulting from head trauma (which could potentially obscure our current focus on unilateral ATL dysfunction) and two exhibited exclusively right hemisphere language on the Wada. This left 36 patients meeting inclusion criteria. We examined post-surgical data for those who had reached this stage of follow-up (n = 14) and presurgical data for those who had not (n = 22).
All patients completed a standard neuropsychological battery, the Wada assessment, an magnetic resonance imaging (MRI) scan of the brain, and video-encephalogram monitoring using standard scalp electrodes. Many also underwent Positron Emission Tomography (PET) or Single-Photon Emission Computed Tomography (SPECT) scanning. Additional invasive studies (i.e., grid and/or strip monitoring) were required to determine seizure localization in 14 of the 50 patients. Most patients with dominant hemisphere seizure onset also underwent intra- or extraoperative cortical stimulation language localization. These 36 TLE patients included 17 with seizures lateralized to the left and 19 with seizures lateralized to the right. The proportion of early to late seizure onset was evenly mixed (15 early to 21 late onset patients) although there were more patients with early onset in the right TLE group (Table 1). There were no significant differences between right and left unilateral TLE patients and healthy subjects in gender, education level, or age, either before or after surgery. Right and left TLE patients did not differ in number of antiepileptic drugs (AEDs) before or after surgery. Postsurgical changes in AED regimen are typically made 1 year after surgery at both epilepsy centers where patients were studied. There was a trend in the postsurgical sample for the left TLE patients to be younger, and to score lower on IQ testing, but these differences did not reach significance with the relatively small sample size resulting from this further subdivision of groups. Most postsurgical patients were assessed approximately 1 year after surgery (M = 14.7 months, SD = 6.8, range = 8–35 months).
Table 1.
Comparison of demographic and disease-related variables between the left and right temporal lobe seizure onset groups and a healthy comparison sample with regard to surgical status.
| Groups | Presurgical left TLE (n = 10)
|
Presurgical right TLE (n = 12)
|
Healthy comparison group (n = 20)
|
Statistical differences | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Age | 40.7 | 15.7 | 39.8 | 11.4 | 40.3 | 13.7 | ||
| Age of seizure onset | 26.1 | 20.0 | 12.3 | 8.5 | N/A | t = 4.76, p < .05 | ||
| Education | 15.2 | 2.4 | 15.9 | 2.9 | 15.4 | 2.5 | n.s. | |
| WAIS-III full-scale IQ | 101.1 | 8.7 | 109.0 | 9.6 | N/A | n.s. | ||
| Number of AEDs | 1.9 | .8 | 1.6 | .73 | N/A | n.s. | ||
| χ2 | p | |||||||
| Gender | 7 females/3 males | 8 females/4 males | 10 females/10 males | 1.46 | n.s | |||
| Postsurgical left TLE (n = 7)
|
Postsurgical right TLE (n = 7)
|
Healthy comparison group (n = 20)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Age | 32.7 | 12.1 | 39.4 | 9.5 | 40.3 | 13.7 | n.s. | |
| Age of seizure onset | 19.9 | 11.4 | 12.4 | 13.9 | N/A | t = 5.86, p < .05 | ||
| Education | 12.0 | 1.2 | 13.7 | 2.7 | 15.4 | 2.5 | n.s. | |
| WAIS-III full-scale IQ | 87.4 | 13.3 | 96.8 | 16.9 | N/A | n.s. | ||
| Number of AEDs | 2.0 | .6 | 1.9 | 1.0 | N/A | n.s. | ||
| χ2 | p | |||||||
| Gender | 2 females/5 males | 4 females/3 males | 10 females/10 males | 1.31 | n.s | |||
Note. n.s. = non-significant.
Twenty healthy subjects, included for comparison, were carefully screened for developmental (e.g., attention deficit hyperactivity disorder (ADHD), learning disabilities), psychiatric, substance abuse, and medical illnesses that might impact neurocognitive function. Inclusion criteria for the healthy controls included normal performances on a screening measure of depression (Beck Depression Inventory: Beck et al., 1996), an extended mental status exam (modified Cognistat: Drane et al., 2002; Drane et al., 2003), and a measure of symptom validity testing (oral form of Word Memory Test: Green and Astner, 1995). All healthy subjects also underwent an MRI of the brain at 3.0 T (Siemens Trio, Erlangen, Germany) with coronal rapid gradient-echo (MPRAGE) sampling (Repetition Time (TE) = 7.37, Echo Time (TE) = 3.42 msec, 1.0-mm isotropic resolution), reviewed by one of the co-authors (A.S.). An effort was made to match age, gender, and education of the healthy subjects to the two patient groups. Demographic information and seizure-related variables are presented in Table 1 for healthy subjects and TLE patients grouped by pre- and postsurgical status.
2.1.1. Surgical descriptions
Of the 36 patients whose error patterns are analyzed, 20 came from the University of Washington (UW) Regional Epilepsy Center (eight postsurgical cases: four left and four right) and 16 from Emory University Epilepsy Center (six postsurgical cases: three left and three right). The resections at the UW Regional Epilepsy Center typically consist of a tailored temporal lobectomy (Silbergeld and Ojemann, 1993), with electro-corticography and speech mapping (McKhann et al., 2000; Ojemann et al., 1989) to determine extent of lateral (superior, middle, inferior), basal temporal cortex, and hippocampal resection. Superior temporal gyrus resection is avoided other than the anterior 1 cm included in the temporal pole. When minimal lateral cortex is involved in the pathology, or in cases not using electrocorticography, only the anterior 3–4 cm of middle and inferior temporal cortex is resected to allow entry into the temporal horn of the lateral ventricle. The lateral amygdale is resected to the roof of the ventricle and the inferior uncus is resected in a subpial fashion. Basal temporal lobe is resected including parahippocampus and the hippocampal/parahippocampal resection is taken posteriorly to the tectal plate, or less aggressively if indicated by focal pathology or electrocorticography.
Of the six postsurgical patients from the Emory University Epilepsy Center, five underwent selective amygdalohippocampectomy and one underwent standard temporal lobectomy (right hemisphere). During selective amygdalohippocampectomy, exposure to the inferior horn of the lateral ventricle was through the inferior temporal sulcus to preserve as much of the temporal stem as possible, whereas anterior temporal lobectomy involved the resection of 3.5 cm from the temporal tip, superiorly limited by the superior pia of the middle temporal gyrus and inferiorly generally including the fusiform gyrus. The hippocampal and parahippocampal gyrus resection was carried posteriorly to the level of tectal plate in a standard fashion. MRI images from typical ATL and selective TL resections are in Fig. 1.
Fig. 1.
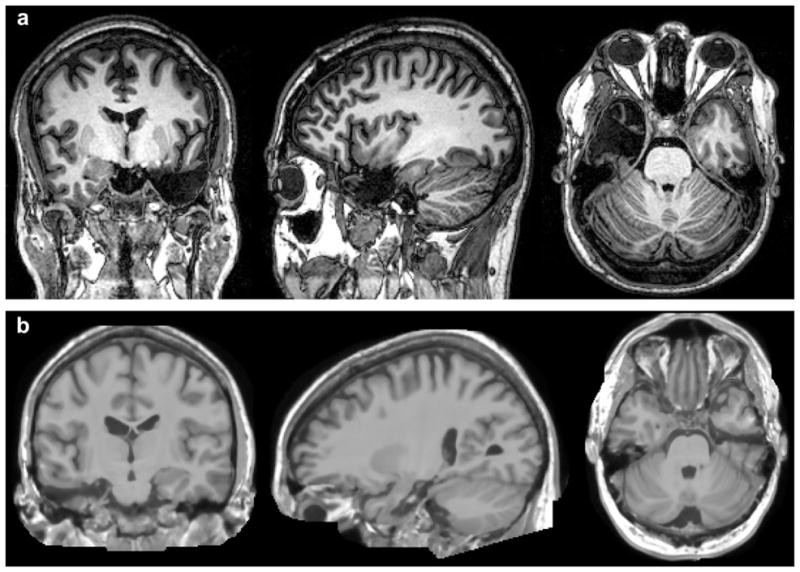
MRI scans demonstrating surgical resections: (a) tailored anterior temporal lobe (ATL) resection performed at the University of Washington – representative coronal, sagital, and axial slices (from left to right) in a patient undergoing left ATL resection; (b) selective amygdalohippocampectomy performed at Emory University – representative coronal, sagital, and axial slices (from left to right) in a patient undergoing a right temporal lobe resection.
2.2. Procedures
All patients were screened for aphasia using subtests from the Multilingual Aphasia Exam (Benton et al., 1983b) and measures of generative fluency (Benton et al., 1983b; Spreen and Strauss, 1998). They were screened for deficits in primary visuo-perception and visual-spatial processing using the Facial Recognition Test (Benton et al., 1983a), the Judgment of Line Orientation Test (Benton et al., 1983a), the Visual Object Space Perception Battery (Warrington and James, 1991), and an assessment of visual acuity (Snellen eye chart). No assessed patients showed any visuo-perceptual or visual-spatial abnormalities. No presurgical patients exhibited aphasia. One post-surgical left TLE patient experienced anomic aphasia, but was included in all study analyses. All subjects meeting inclusion criteria were assessed with a famous face recognition and naming test, based on the measure of Damasio and colleagues (Damasio et al., 1996; Tranel et al., 1997b). We have modified this measure by adding more recent images and a MC recognition format. All subjects were presented with face images on a laptop computer and asked to name the pictured individuals as quickly and accurately as possible. For those they could not name, they were trained to provide a description of the individual, including as much specific detail as possible, so an independent rater would be able to determine the person viewed. They were also asked to indicate whether each individual they could not name was familiar to them (i.e., have they seen this individual previously). Finally, after attempting to name the face and to provide a semantic description for those they could not accurately name, subjects were orally presented with four MC options and asked to select the correct choice. The four MC options included the target person and three semantically related foils. Whenever possible, we included one semantically related foil that also looked visually similar to the target image. For example, for the target stimuli, “Nelson Mandela,” the three foils included two other world leaders (i.e., Kofi Annan & Idi Amin) and an actor, Morgan Freeman, who played Nelson Mandela in a movie and had a somewhat similar appearance. We repeated the MC options as necessary and required subjects to guess on items for which they were uncertain. The MC paradigm was also administered for those famous faces said to be unfamiliar by each subject.
2.2.1. Iowa Famous Faces Test and MC paradigm
The Iowa Famous Faces Test (n = 155) included individuals known for involvement in sports, politics/world affairs, entertainment (actors, musicians), or with notoriety secondary to well-publicized events or crimes (Damasio et al., 1996). Persons are included who were famous during the first half of the 20th century (e.g., Charlie Chaplin, Winston Churchill, Adolph Hitler) through the current era (e.g., Oprah Winfrey, George W. Bush, Mel Gibson). No contextual information was included in any of these pictures that could provide clues to their background or reason for notoriety. Examples of these stimuli are in Fig. 2. Time limits were not imposed for these category-specific naming and recognition tasks. Responses were recorded to allow for review during scoring. Prior to the Famous Faces Test, each subject was given a practice item, with examples of how the person might be described had they not recalled their name.
Fig. 2.

Examples of stimuli from the famous face test used in the current study. Examples include Marilyn Monroe, Nelson Mandella, Adolph Hitler, and Michael Jackson.
Based on initial data collection, we knew that nearly all patients recognize some famous persons (accurate recognition based on their verbal description) and accurately identify them with the MC paradigm even when unable to name them. We incidentally noticed that some of the left TLE patients would “lose” the name over a matter of seconds after correctly choosing it during the MC paradigm. Given this observation, we began re-presenting the items on which this performance pattern occurred, for a second naming attempt. We examined naming performance on the first 20 re-presented items for the last 21 of the 36 patients and for 10 of the comparison subjects. This represented a consecutive series of patients recruited to the study, and included a mix of both pre- and postsurgical patients of both left and right TLE onset.
Scoring methodology was consistent with the procedures of Damasio and colleagues (Damasio et al., 1996; Tranel et al., 1997a). Item familiarity represents a subjective rating provided according to the procedures above. We calculated the percent of persons familiar to each patient out of the total number of test items. To receive credit for a correct naming response, subjects were required to provide complete object names and both the first and last names of individuals (except ones for whom one name is typical, e.g., “Madonna,” “Cher”). If a patient correctly named an item, they automatically received credit for recognition and familiarity, which were assumed to be required for accurate naming. For items not correctly named, transcribed responses were presented to raters who determined from the description alone the person in the picture without having in front of them either the face or the name. If this could be done, the item was scored as a correct recognition; if it could not, then the item was scored as a recognition failure.
As seen by reviewing examples of recognition and naming responses for the Famous Faces subtest in Table 2, correct recognition responses had to be applicable to a single stimulus. For example, “A former President of the US,” would not be specific enough to accurately establish the patient had recognized a picture of George W. Bush. However, adding the phrase, “His father was the Vice-President for Ronald Reagan and later President of the US himself. They both got us into wars in the Middle East,” would be considered an accurate recognition response. Similarly, it was not enough to simply provide a superordinate description of an object or person as some prior studies have done (e.g., “He is a male vocalist” would not be considered an accurate recognition for Michael Jackson, while “He is a rock star who was famous for dancing backwards and wearing a single glove” would be).
Table 2.
Examples of naming and recognition errors on famous face subtest.
| Famous faces – test stimuli | |
|---|---|
| Patient responses reflecting recognition errors | |
| Fidel Castro | “Middle East terrorist. He was killed already. I think that happened during Desert Storm.” |
| Brad Pitt | “Oh…he was great in the Titanic movie. He also acted in one where he pretended to be a doctor and other things…Catch Me if You Can.” |
| Bill Cosby | “OJ…He killed his wife and got away with it…He was a sports star a long time ago. I think he played football…” |
| Adolph Hitler | “I think he was a politician…very familiar…but I cannot place him…maybe in Congress a long time ago…” |
| Patient responses reflecting naming errors | |
| Angela Lansbury | “She is an actress. She had a T.V. show called Murder She Wrote.” |
| Martin Luther King, Jr. | “Famous black man from Atlanta…He was famous for his walk from Birmingham…helping get equality for blacks…He was shot in Memphis…A local road is named after him…” |
| Arnold Schwarzenegger | “The terminator...governor of California…his wife is one of the Kennedy’s …” |
We calculated a variety of primary and secondary scores for recognition and naming. The primary scores include: (a) Familiarity Score – representing the number of famous faces reported to be familiar divided by the total number of items. (b) Recognition Accuracy Score – based only on familiar faces, calculated by dividing the total number of faces correctly recognized by the total number of familiar faces. (c) Naming Accuracy (Recognized Faces) Score – based only on the recognized faces by adding the total number of faces correctly named and dividing this total by the number of recognized objects. (d) Percent of Items Identified with MC that were Recognized but not Named Score – calculated by dividing the number of items accurately identified during the MC paradigm by the number of MC items presented. (e) Naming Accuracy on Item Re-presentation Score – calculated the same as the Naming Accuracy score but is only based on the 20 items that were presented a second time. As described above, these items included the first 20 stimuli that the patient was able to recognize but not spontaneously name, that were identified by MC. (f) Percent of Unfamiliar Items Correct with MC Score – based only on items rated by each subject as unfamiliar, calculated by dividing the number of items correct with MC by the total number of unfamiliar items.
Consistent with our prior publications (Drane et al., 2008, 2009a; 2009b), we based the primary recognition accuracy score only on items that were familiar to each patient, as we did not want to penalize them for not having been previously exposed to a person. All patients and controls rated some of the famous faces as unfamiliar. This is not surprising, as many younger individuals may not have seen some of the famous persons from earlier decades (e.g., Winston Churchill, Johnny Carson), while older subjects have never seen some of the younger sports figures and entertainers (e.g., Miley Cyrus). As will be seen from our data, it is possible to ultimately recognize a face that one has rated as unfamiliar (this is actually a common occurrence among our right ATL patients and a primary finding that we are trying to covey in the paper). We based the primary Naming Accuracy score on the recognized faces only, as we believe you cannot adequately assess someone’s ability to name an object or face that has not been recognized.
The secondary scores were included as they reflect the practice of some researchers examining face processing, although we believe they tend to be misleading for a variety of reasons. These scores include: (a) Naming Accuracy (total faces) Score – based on the total number of correctly named famous faces divided by the total number of faces. (b) Recognition Accuracy (total faces) Score – based on the total number of correctly recognized face divided by the total number of faces. In short, we believe that the secondary naming score overestimates naming deficits in right TLE patients who are unable to recognize many faces and therefore cannot be expected to name them. Similarly, we believe that the secondary recognition measure overestimates deficits in all groups, as there are truly individuals who are not familiar to each person.
Both primary and secondary recognition scores tend to be biased against the left TLE patients, as these scores are based on a verbal response, and this group has a disproportionate problem with naming. Therefore, recognition deficits tend to be overestimated with these measures in the left TLE group. For example, one of our postsurgical TLE patients exhibited an anomic aphasia postoperatively, and appeared unable to access the correct words to allow for adequate description, artificially lowering her recognition score. Because of para-phasic substitutions in verbal responses (e.g., she referred to all political figures as “kings”), she could not receive credit for recognition on the standard criteria of a blind rater being able to identify the person from the description alone. For example, when presented with a picture of Sonny Bono, she stated: “That’s that guy that sang with the girl with the long dark hair that you showed me earlier (i.e., Cher). They had the show together. He later became a king and died while he was doing that thing on the snow.” Of course, for an aphasic patient, this appears to be a generally good description of this individual, as Sonny Bono hosted a variety show with Cher, eventually became a US Congressman (he was also mayor of Palm Springs before going to Congress), and later died in a skiing accident. However, if blind to the target identity, this verbal description would not be adequate.
2.3. Statistical methodology
Statistical analyses compared the TLE groups on a variety of variables across different assessment conditions. We used non-parametric tests for analyses involving the modified Iowa Famous Faces Test (e.g., Kruskal–Wallis Test, Mann–Whitney U-Test), as data from this measure were not normally distributed. In addition, as most performance scores were reported as percentages, we treated these as proportions and carried out an arcsine-root transformation. This transformation controls for possible violations of the assumptions underlying the calculation of p-values and confidence limits that can be introduced when using a count or proportion as a dependent variable (Zar, 1996). All patients were administered the same number of test items, eliminating the need to use weighted proportions for most analysis. However, we used weighted proportions for two of the error analyses that involved different numbers of items being examined for each subject, as this controls for potential error resulting from such differences. When results of the Kruskal–Wallis Test revealed significant effects, follow-up analysis was conducted to evaluate pairwise differences between the three groups for the relevant condition, controlling for Type I error across groups using a modified Bonferroni approach (Holland and Copenhaver, 1988). Analysis of variance (ANOVA) was used for data that was normally distributed. All statistical analyses were completed using version 19 of SPSS (SPSS Inc., 2009). Effect sizes are provided for all analyses.
3. Results
3.1. Famous face recognition and naming performance in left and right TLE patients and a healthy sample: face identification problems are restricted to naming only for patients with left TLE but reflect problems with recognition and familiarity for patients with right TLE
We first compared our left and right TLE seizure onset groups and a healthy sample on the familiarity, recognition, and naming measures from our modified Iowa Famous Faces Test using a series of non-parametric tests. Prior to completing these analyses, all percent accuracy scores were converted to transformed proportions as discussed in the methodology section.
A significant difference was found between the post-surgical left and right TLE patients and the healthy subjects on the Familiarity Score for the modified Iowa Famous Faces Test, but not for their presurgical counterparts (See Table 3). The proportion of variability in the ranked dependent variable accounted for by subject group was .40 for the post-surgical comparison (i.e., effect size), suggesting a moderate relationship between familiarity performance and group membership. Follow-up analysis with modified Bonferroni correction revealed that the postsurgical right TLE group performed significantly worse than the healthy sample (p = .001), but no other pairwise differences were significant (Fig. 3).
Table 3.
Comparison of percent accuracy scores for familiarity, recognition, and naming of famous faces for the left and right TLE patients and a healthy comparison sample grouped by surgical status.
| Groups | Presurgical left TLE (n = 10)
|
Presurgical right TLE (n = 12)
|
Healthy comparison group (n = 20)
|
Statistical differences | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Famous Faces | |||||||
| Familiarity Score | 88.3% | 9.6 | 90.9% | 9.0 | 94.8% | 5.8 | χ2 = 3.99, n.s. |
| Recognition Accuracy Score (out of familiar faces) | 81.6% | 7.8 | 73.8%€ | 17.6 | 87.6%€ | 10.8 | χ2 = 7.17, p < .03 |
| Recognition Accuracy Score (total faces) | 73.5% | 12.3 | 66.4%€ | 18.3 | 83.2%€ | 14.1 | χ2 = 8.35, p < .02 |
| Naming Accuracy (total faces) | 48.8%‡∞ | 22.7 | 58.5%‡€ | 19.2 | 77.2%∞€ | 15.6 | χ2 = 11.08, p < .003 |
| Naming Accuracy (recognized faces) | 65.1%∞€ | 23.5 | 86.9%€ | 9.9 | 92.2%∞ | 5.3 | χ2 = 15.16, p < .001 |
| Mean Response Time (sec) | 6.33∞ | 2.55 | 5.88€ | 2.20 | 3.92∞€ | .99 | F = 7.28, p < .01 |
| Postsurgical left TLE (n = 7)
|
Postsurgical right TLE (n = 7)
|
Healthy comparison group (n = 20)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Famous Faces | |||||||
| Familiarity Score | 86.9% | 8.5 | 80.4%€ | 10.2 | 94.8%€ | 5.8 | χ2 = 13.66, p < .002 |
| Recognition Accuracy Score (out of familiar faces) | 81.0%€ | 8.7 | 64.0%€∞ | 12.5 | 87.6%∞ | 10.8 | χ2 = 14.13, p < .001 |
| Recognition Accuracy Score (total faces) | 72.1% | 8.1 | 54.5%€ | 14.7 | 83.2%€ | 14.1 | χ2 = 17.41, p < .001 |
| Naming Accuracy (total faces) | 20.7%‡€ | 11.4 | 50.1%‡∞ | 12.8 | 77.2%€∞ | 15.6 | χ2 = 22.33, p < .001 |
| Naming Accuracy (recognized faces) | 30.6%‡€ | 18.1 | 90.3%‡ | 5.9 | 92.2%€ | 5.3 | χ2 = 16.61, p < .001 |
| Mean Response Time (sec) | 7.49∞ | 2.02 | 5.73 | 2.24 | 3.92∞ | .99 | F = 13.69, p < .001 |
Note. Statistical comparisons were made using the Kruskal–Wallis Test with transformed proportions derived for each variable. Follow-up tests (i.e., Mann–Whitney U-Test) were completed to evaluate pairwise differences between the three groups, controlling for Type I error across tests using the Bonferroni approach. Groups with the following matching superscripts differ significantly. However, percent accuracy scores are presented for clarity. n.s. = non-significant.
Fig. 3.
Percentage of famous faces rated as familiar by each diagnostic group.
A significant difference was found between the presurgical TLE patients and the healthy group on the Recognition Accuracy Score of the Iowa Famous Faces Test. The effect size was small for this comparison (r2 = .18), suggesting a weak to moderate relationship between famous face recognition performance and group membership. Follow-up pairwise comparisons indicated that recognition accuracy was significantly worse for the presurgical right TLE group compared to the healthy sample (p < .02), but this comparison did not remain significant after application of the correction factor. When considering the postsurgical TLE samples, a significant difference was again found across groups for the Recognition Accuracy Score, with a moderate effect size (r2 = .47) suggesting a fairly strong relationship between famous face recognition performance and group membership. The right TLE group again performed significantly worse than the healthy sample (p < .001), and also performed significantly worse than the left TLE group (p < .01). Of note, using the secondary recognition measure, the Recognition Accuracy (Total Faces) Score, one can see that the same essential pattern is maintained, although all groups perform worse when recognition is based out of all objects rather than those rated as familiar Figs. 4 and 5.
Fig. 4.
Percentage of famous faces recognized by each diagnostic group based on: (a) all familiar items, and (b) total items.
Fig. 5.
Percentage of famous faces named by each diagnostic group based on: (a) all recognized items, and (b) total items.
A significant difference before surgery was observed between the TLE patients and healthy subjects on the Naming Accuracy (Recognized Faces) Score, again with a moderate effect size (r2 = .37). Follow-up pairwise comparisons with modified Bonferroni correction indicated this score was significantly worse for the presurgical left TLE group compared to the other two groups (p < .001 for both comparisons). The same pattern for naming accuracy was again observed comparing the postsurgical TLE patients and healthy sample (r2 = .46). Follow-up pairwise comparisons with modified Bonferroni correction indicated that the Naming Accuracy (Recognized Faces) Score was significantly worse for the postsurgical left TLE group compared to either other group (p < .001 for both comparisons).
We created a Naming Accuracy (Total Faces) Score in order to demonstrate that right TLE patients will appear worse than healthy subjects if their recognition performance is not considered on naming tasks. This was not one of our primary analyses but this serves as reminder that total naming scores will appear decreased for right TLE patients if recognition performance is not considered. Naming cannot be validly assessed in such individuals if the famous face cannot be recognized Figs. 6 and 7.
Fig. 6.
Mean response time for famous faces named by each diagnostic group based.
Fig. 7.
Percentage of famous faces named on representation by diagnostic group out of items that were correct with MC but not spontaneously named initially.
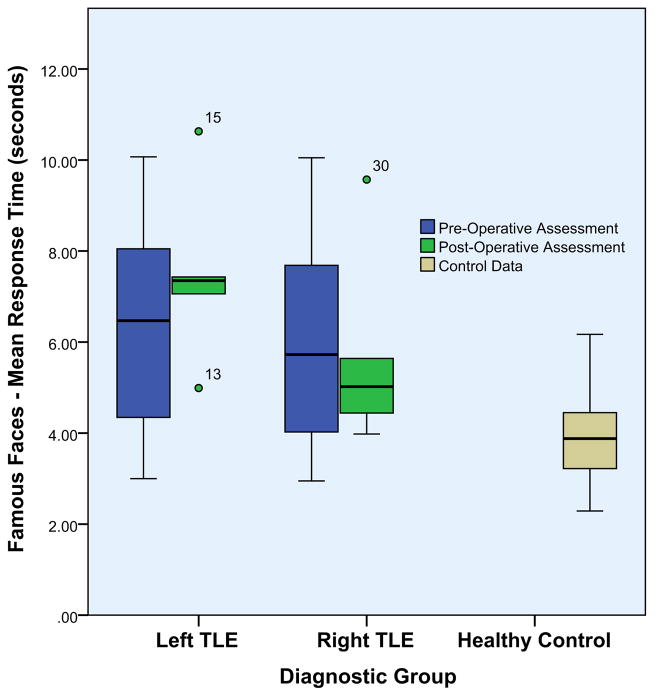
We also examined the mean response time for famous face naming for each group, as some of the recent work of the Hub proponents has suggested subtle declines in response speed for naming of pictures or words with rTMS applied to either unilateral ATL region in healthy controls (Pobric et al., 2010) and similar declines observed in postsurgical TLE patients regardless of side of surgery (Lambon Ralph et al., 2012). Left TLE patients performed more slowly than healthy comparison subjects and right TLE patients both pre- and postsurgically, although the postsurgical difference between TLE groups did not reach significance. Postsurgical left TLE patients were significantly slower than their presurgical counterparts. Right TLE patients performed significantly slower than healthy comparison subjects pre- and postsurgically, although the latter comparison did not reach statistical significance. Interestingly, the mean response speed of the postsurgical right TLE group was no slower than the presurgical right TLE group Fig. 8.
Fig. 8.
Percentage of famous faces correct with the MC paradigm that were initially rated as unfamiliar.
3.2. Demonstration that both left and right TLE patients as well as healthy subjects will perform comparably with a MC paradigm for famous faces that they are able to recognize (verbally describe but not name)
For this analysis, we compared the left and right TLE groups and the healthy sample on their Percent of Items Identified with MC that were Recognized but not Named scores using a Kruskal–Wallis Test, after converting the percentage scores into a transformed proportion and applying a weighting factor. A weighting factor was used because the number of items examined differed for each subject. The left TLE group was able to recognize but not name a significantly greater number of famous faces compared to the other two groups, yet performed comparably to them in terms of MC selection on these specific items. For the entire sample without regard to surgical status, the left TLE, right TLE, and healthy groups all had a better than 95% accuracy on the MC paradigm, with no significant difference in these scores across groups (Table 4). This demonstrates that all TLE patients and healthy subjects can select the correct name using a MC format for nearly all famous faces they are able to recognize (based on their verbal description of such individuals).
Table 4.
Percent of famous faces that were correctly identified with the MC paradigm that had been recognized but not named.
| Groups | Presurgical left TLE (n = 17)
|
Presurgical right TLE (n = 19)
|
Healthy comparison group (n = 20)
|
Statistical differences | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Number of famous faces that were recognized but not named | 47.3 | 29.7 | 13.4 | 10.3 | 9.4 | 1.4 | F = 24.3, p < .001 |
| % of famous faces correct with MC paradigm that had been recognized but not named | 96.1% | 4.5 | 97.2% | 5.2 | 98.0% | 2.9 | χ2 = 8.5, n.s. |
Note. Statistical comparisons were made using the Kruskal–Wallis Test or an ANOVA as appropriate. Transformed proportions derived from the percent accuracy scores were used when appropriate, along with a weighting factor to correct for differences between subjects regarding the number of famous faces that were recognized but not named.
3.3. Demonstration that left TLE patients rapidly lose access to famous face names while right TLE patients and comparison subjects do not
Next, for a consecutive subset of TLE patients from each group (left TLE = 10, right TLE = 12) and a like number of healthy subjects (n = 10), we re-presented the first 20 famous faces that they accurately recognized based on their verbal description but were only able to specifically identify by name when presented with the MC paradigm. These subjects were asked to spontaneously name these famous faces again. Some of the healthy subjects had fewer than 20 famous faces that they recognized (by verbal description) but could not name. Therefore, for the healthy sample at the end of the test, we represented all of the famous faces that met this criterion, up to a total of 20 (mean = 10.8, range = 4–20), and used a weighting factor when carrying out the appropriate non-parametric analyses. We present this data for the total sample and by surgical status subgroups (see Table 5 and Fig. 7). The right TLE patients were able to name over 90% of these faces regardless of surgical status, and the healthy subjects were accurate nearly 100% of the time. However, the left TLE group exhibited a significant naming deficit on re-presentation that was present both pre- and postsurgically, but appeared worse for the postsurgical patients. These comparisons reached statistical significance using Kruskal–Wallis Tests with the transformed proportions for the total sample, and for the presurgical and postsurgical subgroups. The proportion of variability in the ranked-dependent variable accounted by subject group for the total sample was .81, suggesting an extremely strong relationship between this variable and group membership. Although both groups correctly identified the visually presented faces minutes earlier with the MC paradigm, the left TLE group frequently could no longer report the name. In general, it appears that this pattern correlates with the famous face naming ability of the left TLE patients (i.e., left TLE patients with worse famous face naming have the most severe deficits in naming on re-presentation). The best performances on this measure from the left TLE sample included three presurgical patients performing in the 40–65% range. For three of the left postsurgical TLE patients for whom we “tested the limits” on some additional items after completing the procedure as designed, the time lag before they “lost” the name was no more than 30–60 sec for most items.
Table 5.
Percent of famous faces that were correctly named on re-presentation following successful identification with the MC paradigm, and comparison of the same groups on working memory tasks.
| Groups | Left TLE(n = 10)
|
Right TLE (n = 12)
|
Healthy comparison group (n = 10)
|
Statistical differences | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| % of famous faces named on re-presentation | 33.3% | 22.4 | 92.2% | 10.3 | 98.9% | 3.3 | χ2 = 357.6, p < .001 |
| WAIS-III digit span | SS = 8.2 | 3.3 | SS = 8.7 | 3.1 | N/A | t = .07, n.s. | |
| WAIS-III letter number sequencing | SS = 9.4 | 3.4 | SS = 10.2 | 2.4 | N/A | t = .21, n.s. | |
| Presurgical left TLE (n = 5)
|
Presurgical right TLE (n = 7)
|
Healthy comparison group (n = 10)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| % of famous faces named on re-presentation | 49.0% | 29.0 | 93.0% | 6.9 | 98.9% | 3.3 | χ2 = 227.8, p < .001 |
| Postsurgical left TLE (n = 5)
|
Postsurgical right TLE (n = 5)
|
Healthy comparison group (n = 10)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| % of famous faces named on re-presentation | 26.0% | 14.7 | 91.0% | 8.4 | 98.9% | 3.3 | χ2 = 218.8, p < .001 |
Note. Statistical comparisons were made using the Kruskal–Wallis Test or an ANOVA as appropriate. Transformed proportions derived from the percent accuracy scores were used for all percentages. A weighting factor was required for this analysis as some healthy control subjects did not have 20 re-presented famous faces. Therefore, the N size for this variable is based on the number of observations rather than number of subjects. n.s. = non-significant.
3.3.1. Ruling-out the impact of attention/working memory or episodic memory dysfunction as the cause of rapid loss of famous face names
Having demonstrated a difference in performance distinguishing the left TLE sample from the right TLE sample and healthy controls, we compared the patient groups on the Digit Span and Letter-Number Sequencing subtest of the WAIS-III to examine the possible impact of working memory on this discrepancy. We sought to determine if the rapid loss of the famous face name exhibited by the left TLE group related to their working memory performance rather than a primary problem with naming. Some data suggest auditory working memory can be negatively impacted by lesions of the dominant temporal lobe (particularly the lateral temporal region) (Helmstaedter et al., 1997; Ojemann et al., 1988). There were no significant differences between the patient groups on these measures (Table 5), suggesting that rapid loss of the correct name by the left TLE patients reflects a naming deficit rather than a working memory/attention issue.
3.4. Demonstrating that famous faces rated as unfamiliar can be recognized by the right TLE group when provided with a lexical recognition format (i.e., choice of four names), while this pattern of improvement is not observed in the left TLE group or healthy subjects
Finally, we compared the ability of the left and right TLE patients both pre- and postsurgically and the healthy subjects to correctly identify famous faces with the MC paradigm that they had initially rated as unfamiliar (Percent of Unfamiliar Items Correct with MC Score). For nearly all patients and comparison subjects, some famous faces have not been encountered previously and are unfamiliar. When comparing the transformed proportions using a Kruskal–Wallis Test, we weighted each proportion to control for different numbers of famous faces rated as unfamiliar and found a significant difference between groups both pre- and postsurgically. The effect size was weak presurgically (r2 = .04), but was very strong postsurgically (r2 = .72). Follow-up pairwise comparisons with modified Bonferroni correction indicated that the Percent of Unfamiliar Items Correct with MC Score was significantly better for the presurgical right TLE group compared to the presurgical left TLE group only (p < .001). However, neither presurgical group differed significantly from the healthy sample. In contrast, the postsurgical right TLE group performed significantly better than either of the other groups (p < .001 for both comparisons). The postsurgical left TLE group and healthy sample performed in the range of 35% accuracy, barely better than chance, while the right TLE group achieved 70% accuracy as a group. After noticing that the right TLE patients were clearly vocalizing their shift in performance with the MC paradigm (i.e., noting that they could indeed recognize the pictured individual with the additional cueing), we began asking them to subsequently provide semantic information about the person now that they knew who they were. Universally, the right TLE patients could now provide very thorough verbal descriptions of each famous face identified with the MC paradigm, demonstrating that their semantic knowledge appears grossly intact, and that their primary deficit involves accessing it solely through a visual route. Of note, however, the right TLE subjects frequently reported that the famous faces did not look quite right despite being able to correctly identify the person when provided with MC options (Table 6).
Table 6.
Percent of items initially unrecognized and rated unfamiliar that were correctly recognized with the MC paradigm.
| Groups | Presurgical left TLE (n = 9)
|
Presurgical right TLE (n = 12)
|
Healthy comparison group (n = 15)
|
Statistical differences | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Famous faces | |||||||
| % of items correct with MC paradigm that had been rated as unfamiliar | 35.4% | 20.3 | 50.7% | 22.3 | 38.8% | 16.0 | χ2 = 20.85, p < .001 |
| Postsurgical left TLE (n = 7)
|
Postsurgical right TLE (n = 7)
|
Healthy comparison group (n = 15)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Famous faces | |||||||
| % of items correct with MC paradigm that had been rated as unfamiliar | 32.8% | 13.4 | 69.7% | 10.0 | 38.8% | 16.0 | χ2 = 378.97, p < .001 |
Note. Statistical comparisons were made using the Kruskal–Wallis Test with transformed proportions derived from the percent accuracy scores. In addition, a weighting factor was applied to correct for differences between subjects regarding the number of items they rated as unfamiliar. One left TLE patient and five healthy comparison subjects did not rate any faces as unfamiliar.
4. Discussion
Our data demonstrate that unilateral ATL dysfunction observed both before and after surgery in TLE leads to unique deficits in famous face identification that differ between the left and right temporal lobes. These findings suggest that each ATL plays a different role in identifying visually presented famous faces, with the right ATL playing a fundamental role in accessing semantic information from a visual route, and the left ATL primarily serving to link the pertinent semantic/conceptual information to the language system to produce a specific name (i.e., lexical information). Moreover, several double dissociations in face identification performance emerge when comparing TLE patients with primarily left or right ATL dysfunction using our MC paradigm. These findings support a model of semantic processing that integrates multiple independent processing routes to semantic concepts with several unique deficit patterns emerging from damage to different sensory or lexical pathways. We also demonstrate that deficits from disruption of a single route to semantic concepts can be alleviated by altering task demands to provide information allowing access to semantic concepts by another route. For example, right ATL patients exhibiting recognition and familiarity deficits in face identification (i.e., dysfunction in the visual-semantic route of processing) performed normally when provided with information facilitating the lexical-semantic route of processing (i.e., choice of names). The alleviation of face identification deficits by the provision of cueing, regardless of the underlying mechanism, also highlights that unilateral ATL injury results in problems with access to stored semantic concepts rather than their degradation. These findings challenge many assumptions underlying the amodal models of semantic memory, and are more consistent with the integrated multimodal theories of semantic memory and a distributed representation of concepts.
4.1. Unique deficits in face identification resulting from left or right ATL dysfunction
Patients with right TLE exhibited deficits in famous face identification related to familiarity and visual recognition without any visuo-perceptual or visual-spatial processing limitations. Recognition of famous faces was decreased for the right TLE group before and after surgery compared to the other two groups, although it was worse for the postsurgical patients. Familiarity deficits were only observed at the group level for the postsurgical right TLE patients. These findings highlight differences in familiarity and recognition processes, as differential patterns of decline demonstrate these processes are dissociable, and that both can be disrupted by right ATL dysfunction. In contrast, right TLE patients could successfully name famous faces they could recognize, at a rate comparable to the healthy subjects, and exhibited a normal ability to benefit from lexical cueing (i.e., provision of four possible names) for faces they recognized but could not name. Post-surgical right TLE patients gained significant benefit from lexical cueing even on faces they reported as unfamiliar (approximately 70% accuracy on such items at the group level), while left TLE patients and healthy subjects performed at or only slightly above chance levels with such faces.
Overall, it appears right TLE patients experience a disruption between the completion of visuo-perceptual processing and access to stored semantic memory. Provision of names facilitates access to semantic concepts, presumably by allowing activation of other neural pathways (e.g., lexical-semantic). For example, it is possible that the four names provided by the MC paradigm allow individuals to generate semantic information (including visual/physical attributes) associated with each name that can then be used to reappraise faces initially rated as “unfamiliar,” and may often be enough to match the correct name with the face. The majority of these faces were obviously not truly unfamiliar to the right TLE patients, as they recognized them when provided with cueing. This is a pattern of performance observed in patients with prosopagnosia (Rivolta et al., 2010). While healthy subjects and left TLE patients would also theoretically benefit in the same manner, this is not reflected in their performance, as they are already able to recognize most individuals truly familiar to them due to their presumably intact right hemispheres. These two groups are likely to be more accurate than the right TLE patients when reporting whether or not a famous face is familiar to them, and less likely to show improvement with lexical cueing on such items. As several of the left TLE and healthy subjects performed slightly above chance levels, the same process may be benefitting them on famous faces that are less well known to them (e.g., they may have rated an occasional person as unfamiliar although they may have had some previous albeit limited exposure to them). Barsalou et al. (2003) have written theoretically about the possibility of benefitting in this manner based on a multimodal framework of semantic processing.
Left TLE patients exhibited a problem with famous face identification restricted to accessing names when provided with the visual stimulus, but had no difficulty accessing semantic information from the visual route. They had little or no difficulty recognizing famous faces (based on their verbal descriptions of familiar faces), both pre- and postsurgically, and showed intact familiarity scores. One could argue that they also have difficulty moving from semantic concepts to a specific target name. Although they generated a great deal of specific information about most famous faces described as familiar, they typically could not move beyond this level of processing without cueing. Once provided with MC options, however, they were as good as healthy subjects or right TLE patients at selecting the correct name for those persons they had accurately recognized based on their verbal descriptions. In contrast, while the other groups performed at better than 90% accuracy when retested on the same famous faces after a short delay, left TLE patients failed to recall the name on representation of the face which was not associated with a deficit in primary attention/working memory. More work is required to clarify the possible contribution of auditory/verbal memory deficits upon this pattern of naming dysfunction observed in left TLE patients. As patients with aphasia sometimes exhibit better performance when provided with object naming tasks allowing them to match words with pictures, we may simply be seeing an extension of this finding to the area of famous faces. Overall, the central point is that left TLE patients exhibit a primary problem with access to names suggesting a semantic-lexical disruption in processing.
4.2. Theoretical implications of our results for semantic processing models
4.2.1. Multimodal versus amodal semantic processing
Our data provide support for the integrated multimodal models of semantic processing in which distinct, dissociable functions are performed by the left and right ATLs (Barsalou et al., 2003; Damasio, 1989). In contrast, amodal models of semantic memory, such as the Hub account, suggest that both ATL regions provide redundant functions, and therefore injury to either ATL should lead to a similar type of deficit that cuts across all modalities (including lexical as well as all sensory perceptual). The distinct deficit patterns exhibited by left and right TLE patients on our famous face identification task demonstrate that each temporal lobe engages in a different aspect of processing related to the identification of a famous face from a perceptual image, and that their integrated function is required for successful task completion.
Snowden et al. (2004), as noted in the introduction, raised similar objections to the amodal models of semantic memory using a different assessment paradigm and patient population. However, others have argued that these findings might be explained on the basis of “pre-semantic processing” factors rather than processing differences between the left and right ATL regions (Haslam et al., 2004). For example, they felt that the successful performance of the semantic dementia patients with right greater than left ATL damage on the forced choice selection tasks might be based solely on a sense of familiarity for one object without any activation of semantic/lexical concepts (similar to the covert recognition of prosopagnosic patients). In the current study, however, postsurgical right TLE patients demonstrated an ability to select the appropriate name for famous faces rated as unfamiliar from a four-item array of orally presented options. Given that all three of the lexical foils for each item were famous names (rather than made-up names) and were usually semantically related to the target famous face in some manner, right TLE patients in our sample could not have successfully performed this task only on the basis of covert recognition. As most of the famous face names and foils would have been recognized by all subjects, correct selection of the target name (above a chance level of performance) would require being able to generate some basic semantic information that could be used to reappraise the target face. Thus, our findings cannot be explained on the basis of pre-semantic perceptual processing, and again point to differences in access to semantic concepts based on the modality of presentation of the stimuli eliciting this information.
4.2.2. Semantic-lexical route versus lexical-semantic route
There appears to be one glaring difference between our data and that arising from research with semantic dementia patients relevant to a discussion of semantic memory models. Research with semantic dementia patients has routinely found deficits with both face stimuli (visual) and name (lexical) stimuli, while we have yet to find any non-aphasic TLE patients that have lexical comprehension difficulties. While our left TLE patients have trouble accessing specific names, they do not exhibit difficulty retrieving semantic information when provided with a name. Both left and right TLE patients were capable of accurately choosing the correct name from a four-item array when they recognized the visual face (based on their verbal description) and were also capable of providing semantic information when provided with only a famous name. This suggests that semantic dementia patients likely experience brain dysfunction extending beyond the region of that experienced by TLE patients or resulting from an ATL resection. This likely represents greater involvement of the superior temporal gyrus and/or more posterior portions of the inferior and middle temporal gyri. The superior temporal gyrus is known to be involved in auditory processing, and is generally spared in resective epilepsy surgery apart from the most anterior tip that is included in temporal pole resection. In addition, a couple of recent studies have pointed to a central role of the posterior middle temporal gyrus in language comprehension (Binder et al., 2009; Turken and Dronkers, 2011), which is also rarely included in an epilepsy surgery resection. It is possible that semantic dementia patients have damage to an area more important for lexical processing, which decreases their ability to access semantic concept stores through this route. It also suggests that the lexical-semantic route is not included in the more anterior portions of the left inferior or middle temporal gyrus. The route that is impaired seems to be an intermediate processing step that relays the retrieved semantic information on to the language system. This again points to a separation of routes to conceptual stores rather than an amodal processing system, and suggests that left TLE patients experience a disruption in a semantic to lexical route but not in a presumed lexical to semantic route (which more likely arises from the more posterior regions of the temporal lobe).
4.2.3. Bilateral ATLs as a redundant hub or integrated, unique processors?
The Hub proponent’s position that the ATLs form a bilateral, amodal, unitary hub has led most to assume that damage to either the left or right ATL should result in dysfunction that cuts across all concepts and modalities equally [see reviews by Gainotti (2011); Kiefer and Pulvermuller (2012); Martin (2007)] and to raise objections to the model when research with patients with such unilateral dysfunction did not do so. More recently, the Hub proponents have conducted their own studies with unilateral TL dysfunction (e.g., rTMS studies with healthy adults, postsurgical assessment of TL epilepsy patients), and concluded that semantic deficits resulting from unilateral damage are very mild due to the redundancy built into the system (Lambon Ralph et al., 2010; Lambon Ralph et al., 2012; Lambon Ralph et al., 2009; Pobric et al., 2010). They have also suggested that there may seem to be greater deficits in some types of processing (e.g., naming deficits with left unilateral damage) because of closer association to processing regions that may be represented more unilaterally (e.g., language, visual processing of objects).
Instead, we believe that the absence of semantic dementia occurs because the stored semantic concepts are not located in this region, and the two ATL regions play different roles in accessing this information. While Hub proponents state that semantic dementia results from wiping out the redundant repository of semantic information ascribed to the ATL regions, we hold, instead, that bilateral ATL damage destroys the primary access points (or CZs) allowing access to the stored information. Damage to either ATL results in predictable patterns of decreased access to either lexical or semantic information, but damage to both ATLs is required to eliminate enough access points to lead to semantic dementia (and likely requires inclusion of more posterior TL regions to affect a lexical-semantic route). Of note, Gainotti recently proffered a similar conclusion from extensive reviews of the literature examining category-related naming and recognition deficits and famous face processing, also attributing semantic dementia to damage occurring to two independent but integrated processing regions located in the bilateral ATLs (Gainotti, 2011). One potential difference in our view of semantic processing from that of Gainotti is that we draw a greater distinction between lexical-semantic and semantic-lexical routes, and posit that the former is more posteriorly located in accordance with more recent neuroimaging data (Binder et al., 2009; Turken and Dronkers, 2011).
The Hub proponents would likely have a different interpretation for our data. While we see it strongly supporting different processing functions for the left and right ATL regions, they would perhaps suggest that damage to each ATL region is resulting in both recognition and naming deficits in each patient that are less dramatic. While our primary naming score supports the notion of segregated processing, one could suggest that the secondary score (i.e., naming based on all objects rather than those recognized) could reflect “graded degradation.” Similarly, both recognition scores could be interpreted as showing some degree of impairment for both left and right TLE patients. Instead, we believe these scores are misleading, as described earlier in the paper. For example, we consider it an error to base naming scores on faces that individuals have not recognized. This error has been perpetuated by a number of studies that have failed to distinguish between naming and recognition processes. We feel that the more reasonable interpretation of our naming data, as stated, is that it is impaired for left TLE patients only. Even if right TLE patients have some baseline naming deficits (which could be related to baseline restrictions in learning associated with their epilepsy), we have never observed a decline in naming following right TLE resection. A similar pattern has been clearly demonstrated for naming man-made objects in TLE patients. While both left and right TLE patients have baseline naming deficits for these objects relative to controls, only left TLE patients experience naming declines after unilateral ATL resection (Saykin et al., 1995).
Our recognition scores can be even more misleading in some respects. While the right TLE patients clearly exhibit the worse recognition scores, the left TLE patients exhibit scores on these measures that fall between the right TLE patients and the healthy subjects. However, we feel that this results from the verbal nature of our recognition task, which biases the task toward underestimating the performance on left TLE patients who are more likely to have naming deficits. As noted in our methodology section, several of the left TLE patients with severely defective naming ability lost credit for faces that they actually appeared to recognize. For example, the one left TLE patient with postsurgical anomia received a recognition score of around 40% although a less stringent interpretation of her responses suggests she actually recognized more than 80% of the famous faces, on par with her presurgical performance. We are developing an alternative method for scoring recognition for these naming-impaired individuals to be used in future work. Overall, we believe that these issues will be better resolved once studies are completed in epilepsy with thorough pre/post surgical designs, as we predict that naming declines will only occur following left ATL resections and recognition deficits will only result from right ATL resections. To date, group level studies of famous face recognition and naming in epilepsy (and semantic processing) have all examined pre- or postsurgical data cross-sectionally (e.g., Drane et al., 2008; Glosser et al., 2003; Seidenberg et al., 2002) or examined postsurgical data only (Lambon Ralph et al., 2012).
Based on our current data, we cannot definitively conclude that semantic information is ultimately stored in a distributed fashion involving multiple interconnected and overlapping neural networks or a unitary amodal repository. However, our data suggest that processing in the bilateral ATLs is unique and non-redundant, demonstrating that there are separable routes back to semantic/conceptual stores whether or not these ultimately reside in a unitary amodal location or a distributed network. In addition, these routes still function in a modality-specific manner in the ATL region. If an amodal hub exists, our data suggest that it would have to be located more posteriorly. We continue to favor a model of concept representation that is multimodal and distributed in nature, based on a variety of research findings dealing with cerebral activation patterns during the presentation of stimuli and during paradigms involving their imagined recall (Borst and Kosslyn, 2008), evidence from electrophysiological and neuroimaging studies of conceptual information that show clear activation of sensory and motor areas (Kiefer, 2005; Martin and Chao, 2001; Simmons and Martin, 2009), physiological evidence of ATL regions that are modality specific (Ding et al., 2009; Kondo et al., 2003), and general theoretical considerations (Barsalou et al., 2008, 2003; Kiefer and Pulvermuller, 2012). We believe there are likely multiple, hierarchically arranged CZs (including several trans-modal regions) that allow individuals to recreate semantic content by running simultaneous simulations based on original sensory, motor, and lexical input.
4.2.4. Access problems versus degradation of semantic concepts
A theoretical issue long debated in semantic memory research has been whether observed deficits reflect a problem with access to semantic concepts or its complete degradation (Hodges et al., 1992; Rogers and Friedman, 2008). This debate has most often arisen between researchers studying patients with semantic dementia or AD, conditions typically reflecting extensive bilateral cerebral damage although asymmetric cases do occur in the early disease stages. These studies typically show that a given patient group either: (1) benefits from some form of cueing (like our own data) or implicit priming, suggesting that semantic concepts are present but less accessible (Nebes, 1989), or (2) does not benefit from cueing or priming, suggesting that the semantic concepts are degraded (Hodges and Patterson, 1995; Hodges et al., 1992). Instead, we suggest that the two behavioral patterns reflect differences in the amount and location of cerebral damage, with the former suggesting less extensive or perhaps only unilateral dysfunction. Those with bilateral ATL damage would have no access to stored semantic information, whether or not such information remains elsewhere in the brain. The concerns voiced by Hub proponents that semantic dementia patients do not have damage extensive enough to wipe out widely distributed networks of semantic concepts would be overcome, as the central problem with semantic retrieval would always be one of access (rather than degradation) in the absence of extensive damage to the entire cortex.
4.3. Limitations of the current study
Epilepsy surgery candidates are not always easy to match on variables that can affect the presence of dysfunction and the potential for postsurgical decline (e.g., age of seizure onset, extent of presurgical MRI abnormalities, differences in AED regimens). In the current study, for example, while early and late seizure onset patients were equally included at the level of the total sample, the right TLE patients tended to have an earlier onset of seizures than the left TLE group. As early seizure onset patients are more likely to have undergone reorganization of language and other functions, and are therefore less likely to show deficits in these areas (particularly following surgery), this could have biased our results against finding dysfunction in this group.
For example, although the presurgical right TLE patients had worse recognition than the other two groups, our findings were not as strong as previously reported in other samples from our epilepsy programs (Drane et al., 2008), which could have been due to a higher portion of patients without deficits due to early brain reorganization. In the current sample, five of the seven postsurgical right TLE patients had seizure onsets before age 5, and four of these subjects showed minimal or no deficits on the recognition tests and no naming deficits. However, they exhibited the same error patterns that we have attributed to unilateral right ATL damage on the items missed. In contrast, only one of the eight left postsurgical TLE patients had seizure onset before 10 years of age. With the inclusion of additional late onset right TLE patients, it is possible that problems with familiarity would have been observed in pre-surgical patients and likely that right TLE recognition scores would have beenlower, consistent with our prior work.Overall, while this problem may have obscured potential findings, we reported several group differences across tasks, with several of these having very large effect sizes, and clearly demonstrated different error patterns between left and right ATL groups.
Hub accounts have sometimes ascribed findings from epilepsy patients inconsistent with this model to a possible reorganization of function (Lambon Ralph et al., 2012). While this may be true for some of the early onset patients as described above, we do not believe this holds for subjects with late seizure onset. Such patients are much more likely to have typical functional brain organization, and are much more likely to decline with surgery than their early onset counterparts. For example, multiple papers have demonstrated significant decline in late onset TLE patients in the areas of object naming and episodic memory (Griffin and Tranel, 2007; Hermann et al., 1995; Stroup et al., 2003; Yucus and Tranel, 2007). In addition, our late onset TLE patients do show decline on our famous face identification paradigm, and look remarkably similar to other patient groups that would have been unlikely to have experienced reorganization of function prior to their neurological injury or disease (e.g., stroke) onset, that have been previously tested on these tasks (Damasio et al., 1996; Drane et al., 2009b; Tranel et al., 1997a). While functional postsurgical reorganization is also reported to occur in some patients (Goradia et al., 2011; Yogarajah et al., 2010), we have yet to identify any improvement on our primary naming and recognition measures in adults beyond that occurring over the first few weeks following surgery. We have found that surgery-related deficits on these tasks are often profound, particularly in patients with adult seizure onset, and remain unchanged over time (Drane et al., 2009a).
Although not a limitation of the study per se, we have chosen not to attempt to sort out the effect of specific neuro-anatomical alterations resulting from surgery on functional performance, as the focus of this paper has been on examining the broader extent of these deficits and their implications for theories of semantic processing. There are several potential regions and interconnections that may be contributing to the various deficit patterns described. The relative impact of damage to various regions is the focus of ongoing studies from our group detailing pre- to postsurgical performance changes within each patient in relation to structural and functional changes in brain anatomy and connectivity.
4.4. Clinical relevance
Famous face processing deficits clearly occur presurgically in patients with epilepsy and worsen in many undergoing resective surgery even when they undergo standard language mapping techniques. Deficits can be pronounced despite being under-appreciated in the epilepsy surgery community. For example, at least two left TLE patients from the current postsurgical sample (n = 7) could not reliably name their own family members. By gaining a better understanding of these functions and how they are impaired, it may be possible to better predict who is at risk for decline in these areas and to plan and alter surgery to avoid such deficits. This could involve stimulation language mapping with a wider range of objects (standard mapping typically only involves man-made objects in most epilepsy centers) or using neuroimaging techniques to examine regional connectivity to avoid target regions or pathways. Finally, it may be possible to tailor surgery to avoid critical regions or pathways, or to use alternative less invasive techniques to complete the resection altogether (e.g., Gamma Knife surgery, laser technology) (Quigg et al., 2011; Sherman et al., 2011).
4.5. Conclusions and future directions
These findings strengthen our belief that famous face identification reflects a multistage process that involves several integrated sensory and lexical processing components that can be dissociated from one another with damage to different facets of the presumed network. These processing routes appear to remain modality specific in the bilateral ATL regions, and both ATLs make unique contributions to famous face identification. Our data suggest that the right ATL plays a critical role in visual-semantic processing while the left ATL is important for mediating between reactivated semantic concepts and the lexical system. While the left ATL is likely specialized for lexical-semantic processing as well, this route to semantic concepts is not typically disturbed in TLE patients prior to, or after surgery, likely because of lack of involvement of more posterior TL regions. Moreover, defects in the component processes of famous face identification reflect problems with access to semantic concepts rather than their degradation. These findings provide further evidence that the ATLs play a role in semantic processing, but do not support the theory that they serve as an amodal repository for semantic concepts.
Future research should continue to use available neuro-imaging and electrophysiological technologies to elucidate the actual systems (and specific brain regions) underlying these processes in healthy subjects and relevant patient groups, with emphasis being shifted to the interconnectivity necessary to support these functions. Extension of these behavioral and neuroimaging paradigms to other object types will also be useful, as similar processing may occur for some other objects. Finally, research needs to continue exploring the possible component processes of semantic processing (e.g., visual-semantic, lexical-semantic, semantic-lexical, auditory-semantic routes) and the more global integration of these processing components, incorporating designs that allow for pre/post-analysis whenever possible.
Acknowledgments
This research was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institute of Health (NIH) (Grant Numbers: K23 NSO49100, K02 NS070960, and NS19632). Dr Tranel is also supported by the National Institute of Drug Abuse (Grant Number: DA022549). This study was approved by the Institutional Review Boards of the University of Washington Medical School (application #05-5738-G 01) and Emory University School of Medicine (application #IRB00010651), where all data were collected. Portions of this paper were presented at the 2009 meeting of the American Epilepsy Society. We would like to thank Gail Rosenbaum, Nathan Hantke, Jaclyn Tucker, Brie Sullivan, Eric Samuelson, Michele Price, and Paul Woody for their assistance in collection and management of the neurocognitive data included in this study.
References
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Santos A, Simmons WK, Wilson CD. Language and simulation in conceptual processing. In: Vega MD, Glenberg AM, Graesser AD, editors. Symbols, Embodiment, and Meaning. Oxford: Oxford University; 2008. pp. 245–283. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory – II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Benton AL, Hamsher K, Nils RV, Spreen O. Contributions to Neuropsychological Assessment. NY: Oxford University Press; 1983a. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1983b. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst GK, Kosslyn S. Visual mental imagery and visual perception: Structural equivalence revealed by scanning processes. Memory and Cognition. 2008;36:849–862. doi: 10.3758/mc.36.4.849. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38(9):1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Chan AS, Sze SLM, Cheung M. Neuroanatomical basis in the temporal lobes for processing living things. Neuropsychology. 2004;18(4):700–709. doi: 10.1037/0894-4105.18.4.700. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio A. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: A combined analysis of multiple cytoarchitectonic, chemoarcthitectonic, and pathological markers. Journal of Computational Neurology. 2009;514:595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Yuspeh RL, Klingler LK, Huthwaite JS, Mrazik M. Introduction of a total composite score for the Cognistat (NCSE) Journal of the International Neuropsychological Society. 2002;8(2):154–155. [Google Scholar]
- Drane DL, Yuspeh RL, Huthwaite JS, Klinger LK, Foster LM, Mrazik M, et al. Healthy older adult performance on a modified version of the Cognistat (NCSE): Demographic issues and preliminary normative data. Journal of Clinical and Experimental Neuropsychology. 2003;25(1):133–144. doi: 10.1076/jcen.25.1.133.13628. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Tranel D, Ojemann JG, Miller JW. Category-specific naming and recognition deficits in patients with temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2004;10(Suppl 1):202. [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy. Neuropsychologia. 2008;46(5):1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Aylward E, Phatak V, Hebb A, Tranel D, et al. Category-related naming and recognition deficits in epilepsy surgery patients reflect disconnection syndromes. Epilepsia. 2009a;50(Suppl 11):281. [Google Scholar]
- Drane DL, Ojemann GA, Ojemann JG, Aylward EA, Silbergeld DL, Miller JW, et al. Category-specific recognition and naming deficits following resection of a right anterior temporal lobe tumor in a patient with atypical language lateralization. Cortex. 2009b;45(5):630–640. doi: 10.1016/j.cortex.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. The organization and dissolution of semantic-conceptual knowledge: Is the ‘amodal hub’ the only plausible model? Brain and Cognition. 2011;75:299–309. doi: 10.1016/j.bandc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Giussani C, Roux FE, Bello L, Lauwers-Cances V, Papagno C, Gaini SM, et al. Who is who: Areas of the brain associated with recognizing and naming famous faces. Journal of Neurosurgery. 2009;110:289–299. doi: 10.3171/2007.8.17566. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Goradia D, Chugani HT, Govindan RM, Behen M, Juhasz C, Sood S. Reorganization of the right arcuate fasciculus following left arcuate fasciculus resection in children with intractable epilepsy. Journal of Child Neurology. 2011;26:1246–1251. doi: 10.1177/0883073811402689. [DOI] [PubMed] [Google Scholar]
- Grabowski T, Damasio H, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entries. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Astner K. Manual for the Oral Word Memory Test. North Carolina: CogniSyst; 1995. [Google Scholar]
- Griffin S, Tranel D. Age of seizure onset, functional reorganization, and neuropsychological outcome in temporal lobectomy. Journal of Clinical and Experimental Neuropsychology. 2007;29(1):13–24. doi: 10.1080/13803390500263568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam C, Kay J, Hanley JR, Lyons F. Biographical knowledge: Modality-specific or modality neutral? Cortex. 2004;40:451–466. doi: 10.1016/s0010-9452(08)70139-4. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Grunwald T, Lehnertz K, Gleissner U, Elger CE. Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: Evidence from temporal lobe epilepsy. Brain and Cognition. 1997;35(1):110–131. doi: 10.1006/brcg.1997.0930. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Haltiner AM, Wyler AR. Relationship of age at onset, chronological age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–145. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33(4):441–459. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Holland BS, Copenhaver MD. Improved Bonferonni-type multiple testing procedures. Psychological Bulletin. 1988;104:145–149. [Google Scholar]
- Kiefer M. Repetition priming modulates category-related effects on event-related potentials: Further evidence of multiple semantic systems. Journal of Cognitive Neuroscience. 2005;17(2):199–211. doi: 10.1162/0898929053124938. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Pulvermuller F. Conceptual representations in mind and brain: Theoretical developments, current evidence, and future directions. Cortex. 2012;48(7):805–825. doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kondo HS, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K. Generalization and differentiation in semantic memory: Insights from semantic dementia. Annals of the New York Academy of Science. 2008;1124:61–76. doi: 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: Evidence from semantic dementia, HSVE, and a neural network model. Brain. 2007;130:1127–1137. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex. 2009;19:832–838. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolloti L, Manes F, Patterson K. Taking both sides: Do unilateral temporal lobe lesions disrupt semantic memory? Brain. 2010;133:3243–3255. doi: 10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Ehsan S, Baker GA, Rogers TT. Semantic memory is impaired in patients with unilateral temporal lobe resection for temporal lobe epilepsy. Brain. 2012;135:242–258. doi: 10.1093/brain/awr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45(8):1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- McKhann GM, 2nd, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann GA. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. Journal of Neurosurgery. 2000;93(1):44–52. doi: 10.3171/jns.2000.93.1.0044. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Semantic memory in Alzheimer’s disease. Psychological Bulletin. 1989;106(3):377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42(9):1269–1280. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Creutzfeldt O, Lettich E, Haglund MM. Neuronal activity in human lateral temporal cortex related to short-term verbal memory, naming and reading. Brain. 1988;111(Pt. 6):1383–1403. doi: 10.1093/brain/111.6.1383. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery. 1989;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Natures Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: Mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Current Biology. 2010;20(10):964–968. doi: 10.1016/j.cub.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Saunders RC, Crane AM, Cook M, Sokoloff L, Mishkin M. Functional mapping of the primate auditory system. Science. 2003;299:568–572. doi: 10.1126/science.1078900. [DOI] [PubMed] [Google Scholar]
- Quigg M, Broshek D, Barbaro NM, Ward MM, Laxer KD, Yan G, et al. Neuropsychological outcomes after Gamma Knife radiosurgery for mesial temporal lobe epilepsy: A prospective multicenter study. Epilepsia. 2011;52(5):909–916. doi: 10.1111/j.1528-1167.2011.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta D, Schmalzl L, Coltheart M, Palermo R. Semantic information can facilitate covert face recognition in congenital prosopagnosia. Journal of Clinical and Experimental Neuropsychology. 2010;32(9):1002–1016. doi: 10.1080/13803391003662710. [DOI] [PubMed] [Google Scholar]
- Rivolta D, Palermo R, Schmalzl L, Coltheart M. Covert face recognition in congenital prosopagnosia: A group study. Cortex. 2012;48(3):344–352. doi: 10.1016/j.cortex.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Friedman RB. The underlying mechansims of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia. 2008;46(1):12–21. doi: 10.1016/j.neuropsychologia.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Stafiniak P, Robinson LJ, Flannery KA, Gur RC, O’Connor MJ, et al. Language before and after temporal lobectomy: Specificity of acute changes and relation to early risk factors. Epilepsia. 1995;33:1071–1077. doi: 10.1111/j.1528-1157.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Burton AM. Covert recognition and the neural system for face processing. Cortex. 2003;39(1):9–30. doi: 10.1016/s0010-9452(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Griffith R, Sabsevitz D, Moran M, Haltiner A, Bell B, et al. Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2002;40:446–456. doi: 10.1016/s0028-3932(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Sherman JH, Hoes K, Marcus J, Komotar RJ, Brennan CW, Gutin PH. Neurosurgery for brain tumors: Update on recent technical advances. Current Neurology and Neuroscience Reports. 2011;11(3):313–319. doi: 10.1007/s11910-011-0188-9. [DOI] [PubMed] [Google Scholar]
- Silbergeld DL, Ojemann JG. The tailored temporal lobectomy. Neurosurgical Clinics of North America. 1993;4(2):273–281. [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. Journal of the International Neuropsychological Society. 2009;15:645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PSF, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cerebral Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper LM, Ross LA, Olson IR. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49(12):3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127:860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss EH. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1998. [Google Scholar]
- SPSS Inc. SPSS 19.0. Upper Saddle River, New Jersey: Prentice-Hall, Inc; 2009. [Google Scholar]
- Stroup ES, Langfit JT, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy. Neurology. 2003;60:1266–1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997a;35(10):1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Logan CG, Frank RJ, Damasio AR. Explaining category-related effects in the retrieval of conceptual and lexical knowledge for concrete entities: Operationalization and analysis of factors. Neuropsychologia. 1997b;35(10):1329–1339. doi: 10.1016/s0028-3932(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Trautner P, Dietl T, Staedtgen M, Mecklinger A, Grunwald T, Elger CE, et al. Recognition of famous faces in the medial temporal lobe: An invasive ERP study. Neurology. 2004;63(7):1203–1208. doi: 10.1212/01.wnl.0000140487.55973.d7. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383 doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MA. The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48:1689–1696. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: Experimental and clinical observations. Journal of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. Visual Object and Space Perception Battery. Edmunds, UK: Thames Valley Company; 1991. [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;197:829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133(Pt. 8):2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucus C, Tranel D. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia. 2007;48(12):2241–2252. doi: 10.1111/j.1528-1167.2007.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostastistical Analysis. New Jersey: Prentice Hall; 1996. [Google Scholar]