Abstract
The large-conductance voltage- and Ca2+-activated K+ (BK) channel is a major regulator of detrusor smooth muscle (DSM) contractility thus facilitating urinary bladder function. Recent findings suggest that activation of β3-adrenoceptors causes DSM relaxation. However, it is unknown whether the β3-adrenoceptor-mediated DSM relaxation is BK channel-dependent during nerve-evoked contractions. To test this hypothesis, we induced nerve-evoked contractions in rat DSM isolated strips by using a tissue bath system equipped with platinum electrodes for electrical field stimulation (EFS). (±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy] acetic acid sodium hydrate (BRL37344), a β3-adrenoceptor agonist, significantly decreased the amplitude and muscle force of the 20 Hz EFS-induced DSM contractions in a concentration-dependent manner. The BRL37344 inhibitory effect was significantly antagonized by 1-(2-Ethylphenoxy)-3-[[(1S)-1,2,3,4-tetrahydro-1- naphthalenyl]amino]-(2S)-2-propanol hydrochloride (SR59230A), a β3-adrenoceptor antagonist. We further isolated the cholinergic from the purinergic component of the 0.5–50 Hz EFS-induced DSM contractions by using selective inhibitors, atropine as well as suramin and α,β- methylene-ATP, respectively. We found that BRL37344 inhibited both the purinergic and cholinergic components of the nerve-evoked contractions in rat DSM isolated strips. The pharmacological blockade of the BK channels with iberiotoxin, a selective BK channel inhibitor, increased the amplitude and muscle force of the 20 Hz EFS-induced contractions in rat DSM isolated strips. In the presence of iberiotoxin, there was a significant reduction in the BRL37344-induced inhibition of the 20 Hz EFS-induced contractions in rat DSM isolated strips. These latter findings suggest that BK channels play a critical role in the β3-adrenoceptor-mediated inhibition of rat DSM nerve-evoked contractions.
Keywords: BRL37344; SR59230A; iberiotoxin; atropine; suramin; α,β-methylene-ATP
1. INTRODUCTION
The ability of β3-adrenoceptor agonists such as (±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy] acetic acid sodium hydrate (BRL37344), mirabegron (YM178), solabegron (GW427353), FK-175, TAK677, TRK-380, CL316243, and CGP12177A to inhibit detrusor smooth muscle (DSM) myogenic contractions has been well documented (Biers et al., 2006; Fujimura et al., 1999; Hicks et al., 2007; Hristov et al., 2008; Kanie et al., 2012; Kullmann et al., 2009; Leon et al., 2008; Sato et al., 2007; Takasu et al., 2007; Takeda et al., 1999; Tyagi et al., 2009). These β3-adrenoceptor agonists have been shown to increase the intracellular cAMP levels in native DSM tissues as well as in heterologously expressed systems (Fujimura et al., 1999; Hicks et al., 2007; Kanie et al., 2012; Kullmann et al., 2009; Sato et al., 2007; Takasu et al., 2007). Specifically, in rat DSM isolated strips, FK-175 has been shown to increase the intracellular cAMP level by ~30% (Fujimura et al., 1999). The recent approval of mirabegron as the first β3-adrenoceptor agonist for the treatment of overactive bladder further outlines the importance of β3-adrenoceptors as pharmacological targets for overactive bladder. Since overactive bladder has myogenic and neurogenic origins with diverse mechanisms and etiology, it is still unknown whether this new therapeutic approach will be effective to treat all forms of overactive bladder. There has been a significant effort aiming to investigate the mechanism by which β3-adrenoceptor agonists induce DSM relaxation, especially during neurogenic contractions. However, the exact mechanism remains uncertain. Experiments conducted on DSM myogenic contractions suggest that the mechanism underlining the β3- adrenoceptor-mediated DSM relaxation involves the cAMP signaling pathway and the largeconductance Ca2+-activated K+ (BK) channels (Hristov et al., 2008; Michel and Vrydag, 2006; Petkov, 2012; Petkov and Nelson, 2005; Xin et al., 2012a; Xin et al., 2012b). Our group revealed a functional link between BK channels and β3-adrenoceptors during myogenic contractions, a mechanism which utilizes the ryanodine receptors of the sarcoplasmic reticulum to induce DSM relaxation (Hristov et al., 2008). Furthermore, the pharmacological blockade of BK channels with iberiotoxin, a selective BK channel inhibitor, caused a rightward shift of the concentration-response curve of the BRL37344 inhibitory effects on myogenic contractions of rat DSM isolated strips. The BRL37344-induced hyperpolarization of freshly isolated DSM cells was eliminated by iberiotoxin or ryanodine suggesting that activation of β3-adrenoceptors increases the BK channel activity and ultimately leads to DSM relaxation (Hristov et al., 2008). It has been demonstrated that in mouse DSM isolated strips selective inhibition of the BK channels and protein kinase-A with iberiotoxin and H-89, respectively, reduced the relaxant effect of isoproterenol, a non-selective β3-adrenoceptor agonist (Brown et al., 2008). The fundamental role of the BK channels in the β-adrenergic/protein kinase-A-mediated DSM relaxation is demonstrated by the observations that genetic ablation of the BK channel in a transgenic mouse model leads to development of a compensatory adaptive upregulation of the β-adrenergic/cAMP/protein kinase-A signal transduction pathway (Brown et al., 2008). However, these novel mechanisms have yet to be investigated in nerve-evoked DSM contractions. The present study utilizes tissue baths equipped with electrodes for electrical field stimulation (EFS), DSM strips isolated from rat urinary bladder, and various pharmacological modulators to assess β3-adrenoceptor and BK channel functional role in the nerve-evoked contractions.
2. MATERIALS AND METHODS
2.1 Isometric DSM tension recordings
The present study was performed according to the Animal Use Protocol #1747, reviewed and approved by the University of South Carolina Institutional Animal Care and Use Committee. In total, 41 male Sprague-Dawley rats (10–16 weeks old) weighing 361.7 ± 5.2 g on average were used. Isometric DSM tension recordings were conducted as previously described (Hristov et al., 2008; Soder and Petkov, 2011). Briefly, rats were euthanized with CO2, the urinary bladder was removed and stored in dissection solution (§2.2 Solutions and Drugs). The urinary bladder was then quickly sliced opened and the entire mucosa was removed before DSM strips (5–7 mm long and 2–3 mm wide) were dissected. Following dissection, DSM strips were mounted on a tissue holder equipped with a pair of platinum electrodes, then placed in thermostatically controlled (37°C) 10 ml tissue baths filled with Ca2+-containing physiological saline solution (PSS) (§2.2 Solutions and Drugs). EFS pulses had 20 V amplitude, 0.75 ms width, 3 s stimulus duration, and polarity was reversed for alternating pulses. One gram of tension was applied to each individual strip and the bath PSS was changed with fresh PSS every 15 min during an equilibration period of 45 to 60 min. Using a PHM-152I stimulator (Med Associates, Inc., Georgia, VT), stimulation of 20 Hz EFS frequency at 1 min intervals was applied or increasing EFS frequencies (0.5, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, 50 Hz) at 3 min intervals each were applied. The DSM response to EFS was recorded using MyoMed software (Med Associates).
2.2 Solutions and Drugs
The Ca2+-free dissection solution had the following composition (in mM): 80 monosodium glutamate; 55 NaCl; 6 KCl; 10 glucose; 10 N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid (HEPES); 2 MgCl2; and pH 7.3 adjusted with NaOH. The Ca2+- containing PSS was prepared daily and contained (in mM): 119 NaCl; 4.7 KCl; 24 NaHCO3; 1.2 KH2PO4; 2.5 CaCl2; 1.2 MgSO4; 11 glucose; and aerated with 95% O2/5% CO2 to obtain pH 7.4. Iberiotoxin, atropine, suramin, and α,β-methylene-ATP were purchased from Sigma-Aldrich (St. Louis, MO). BRL37344 and 1-(2-Ethylphenoxy)-3-[[(1S)-1,2,3,4-tetrahydro-1- naphthalenyl]amino]-(2S)-2-propanol hydrochloride (SR59230A) were purchased from Tocris Bioscience (Bristol, UK). BRL37344 was prepared daily in double-distillated water and heated at 60°C to be completely dissolved as suggested by the manufacturer.
2.3 Data analysis and statistics
For the 20 Hz EFS-induced contractions, a 5 min period prior to the first BRL37344 application (10 nM) was taken as a control and the responses to subsequent BRL37344 application (10 nM–100 µM) were normalized to that control, which was taken to be 100%. During cumulative addition of BRL37344, the effect of each BRL37344 concentration (10 nM–100 µM) on 20 Hz EFS-induced contraction amplitude and muscle force (determined by integrating the area under the curve of the EFS-induced contractions) were evaluated by analyzing the 5 min period prior to the following BRL37344 concentration application. For the 0.5–50 Hz EFS-induced contractions, the contraction amplitude at EFS frequency of 50 Hz prior to BRL37344 application (control conditions) was taken to be 100% and the data were normalized. The EFS-induced DSM contractions were analyzed using MiniAnalysis software version 6.0.7 (Synaptosoft, Inc., Decatur, GA). GraphPad Prism 4.03 software (GraphPad Software Inc., La Jolla, CA, USA) was used for further statistical analysis. For data illustrations, CorelDraw Graphic Suite X3 software (Corel Co., Ottawa, Canada) was used. The results are summarized as means ± SEM; n represents the number of DSM isolated strips and N, the number of rats. Statistical significance was evaluated using paired or unpaired Student’s t-test. A P-value <0.05 was considered statistically significant.
3. RESULTS
3.1 BRL37344 inhibitory effect on EFS-induced DSM contractions is antagonized by SR59230A, a β3-adrenoceptor antagonist
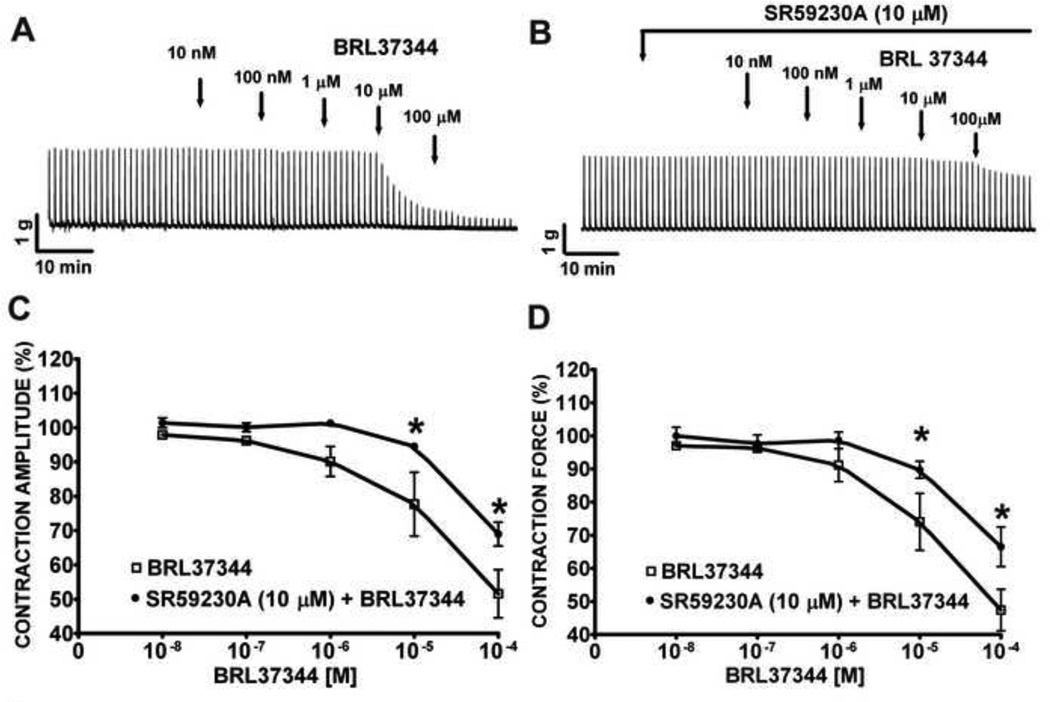
We investigated how the β3-adrenoceptor agonist, BRL37344, affects nerve-evoked contractions of rat DSM isolated strips. We found that BRL37344 decreased the amplitude and muscle force of the 20 Hz EFS-induced contractions of rat DSM isolated strips in a concentration-dependent manner. BRL37344 (10 nM–1 µM) had no significant inhibitory effect; however, BRL37344 at concentrations of 10 µM and 100 µM statistically significantly decreased the amplitude of the 20 Hz EFS-induced DSM contractions by 22.3±9.3% and 48.4±7.0%, respectively, and the muscle force by 26.0±8.6% and 52.6±6.2%, respectively (n=8, N=6, P<0.05; Fig. 1A, C, and D). Pre-incubation of rat DSM strips with SR59230A (10 µM), a β3-adrenoceptor antagonist, significantly reduced the BRL37344 inhibitory effect on the 20 Hz EFS-induced contractions (Fig. 1B-D). SR59230A attenuated the relaxant effect of BRL37344 at 10 µM and 100 µM on the contraction amplitude from 22.3±9.3% to 5.5±0.7%, and from 48.4±7.0% to 31.0±3.5%, respectively; and also on the contraction force from 26.0±8.6% to 10.0±2.6%, and from 52.6.0±6.2% to 33.5±6.0%, respectively (n=11, N=6, P<0.05; Fig. 1C, D). Time control experiments performed in the absence of BRL37344 and SR59230A showed no statistically significant difference in the 20 Hz EFS-induced contraction amplitude and force (n=6, N=6, P>0.05; data not illustrated). These findings suggest that the inhibitory effects on EFS-induced contractions induced by the β3- adrenoceptor receptor agonist, BRL37344, can be antagonized by SR59230A.
Figure 1. BRL37344 decreases the amplitude and muscle force of the 20 Hz EFS-induced contractions in rat DSM isolated strips.
A) This original DSM tension recording illustrates BRL37344 (10 nM–100 µM) inhibitory effects on 20 Hz EFS-induced contractions. This particular original recording shows a bit greater degree of inhibition than the average observed. B) This original DSM tension recording illustrates the attenuation of BRL37344 (10 nM–100 µM) inhibitory effects on 20 Hz EFS-induced DSM contractions in the presence of SR59230A (10 µM). C-D) These cumulative concentration-response curves illustrates SR59230A antagonistic effects against BRL37344 inhibitory effects on EFS-induced contraction amplitude and force, respectively (n=11, N=6; *P<0.05).
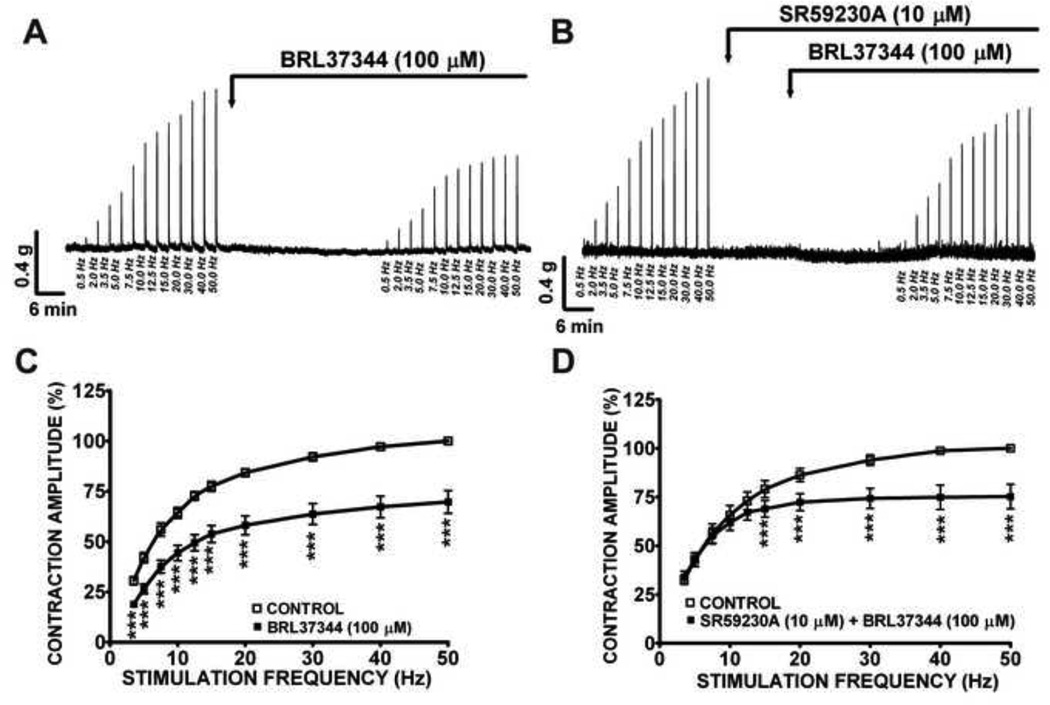
We also investigated how BRL37344 modulates the two main components of the nerve-evoked contractions, namely the cholinergic and the purinergic components, under a wide range of stimulation frequencies (0.5–50 Hz). First, we evaluated the changes in the amplitude of rat DSM EFS-induced contractions following application of BRL37344 (100 µM). We found that BRL37344 significantly inhibited EFS-induced contractions at frequencies ranging from 3.5 to 50 Hz (Fig. 2A, C). At the maximal stimulation frequency of 50 Hz, BRL37344 caused a 30.3±6.0% decrease in the amplitude of the EFS-induced DSM contractions (n=12, N=6, P<0.005; Fig. 2C). This BRL37344 inhibitory effect was significantly antagonized by SR59230A (10 µM) at low stimulation frequencies (3.5–12.5 Hz), but not at higher frequencies (15–50 Hz) (n=8, N=3, P<0.005; Fig. 2B, D). SR59230A (10 µM) per se did not have any effect on the nerve-evoked DSM contractions (n=8, N=3, P>0.05). These data show that the β3- adrenoceptor agonist, BRL37344, decreases rat DSM nerve-evoked contractions.
Figure 2. BRL37344 reduces rat DSM EFS-induced contractions generated by a wide range of stimulation frequencies (0.5–50 Hz).
This original DSM tension recording illustrates BRL37344 (100 µM) inhibitory effects on DSM EFS-induced contractions in the absence (A) or presence (B) of SR59230A (10 µM). C) The EFS frequency-response curves for the 0.5–50 Hz EFS-induced DSM contractions in response to 100 µM BRL37344 (n=12, N=6). D) The EFS frequency-response curves for BRL37344 (100 µM) inhibitory effects on 0.5–50 Hz EFS-induced DSM contractions in the presence of SR59230A (10 µM) (n=8, N=3; ***P<0.005).
3.2 BRL37344 inhibitory effect on nerve-evoked contractions of rat DSM isolated strips: Role of cholinergic and purinergic components
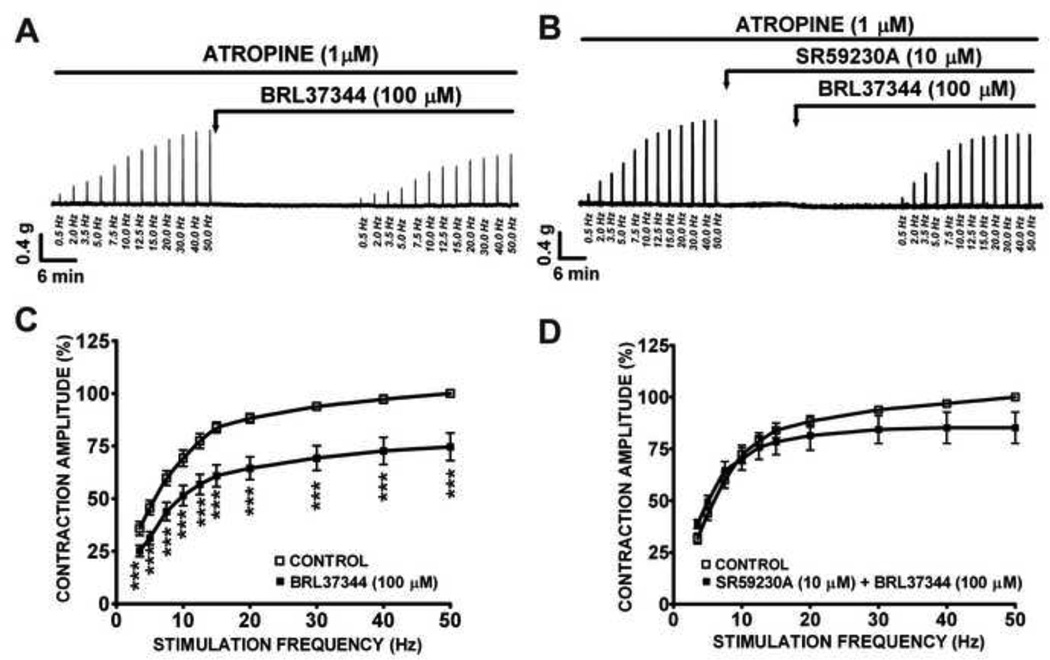
We further separated the cholinergic component from the purinergic component of the nerve-evoked contractions by using inhibitors of these two components. In the presence of atropine (1 µM), which was used to block the cholinergic component of the nerve-evoked contraction, BRL37344 (100 µM) significantly decreased the amplitude of the EFS-induced DSM contractions at EFS stimulation frequencies ranging from 3.5 Hz to 50 Hz (Fig. 3A). In the presence of atropine, at the maximal stimulation frequency of 50 Hz, BRL37344 (100 µM) caused a 25.4±6.6% decrease in the amplitude of the EFS-induced contractions (n=15, N=8, P<0.005; Fig. 3C). This BRL37344 inhibitory effect was antagonized by SR59230A (10 µM) at all EFS stimulation frequencies (3.5–50 Hz) (n=13, N=5; P>0.05; Fig. 3B, D). These data suggest that BRL37344 relaxes the EFS-induced contractions of rat DSM isolated strips via inhibition of the purinergic component of the EFS-induced DSM contractions.
Figure 3. In the presence of atropine, BRL37344 significantly inhibited the amplitude of the 0.5–50 Hz EFS-induced contractions of rat DSM isolated strips.
This original DSM tension recording illustrates BRL37344 (100 µM) inhibitory effects on EFS-induced DSM purinergic contractions in the absence (A) or presence (B) of SR59230A (10 µM). C) These EFS frequency-response curves show the BRL37344 inhibitory effects on the nerve-evoked DSM contractions in the presence of 1 µM atropine (n=15, N=8; ***P<0.005). D) These EFS frequency-response curves show that SR59230A blocks BRL37344 inhibitory effects on the EFS-induced DSM contractions (n=13, N=5, P>0.05).
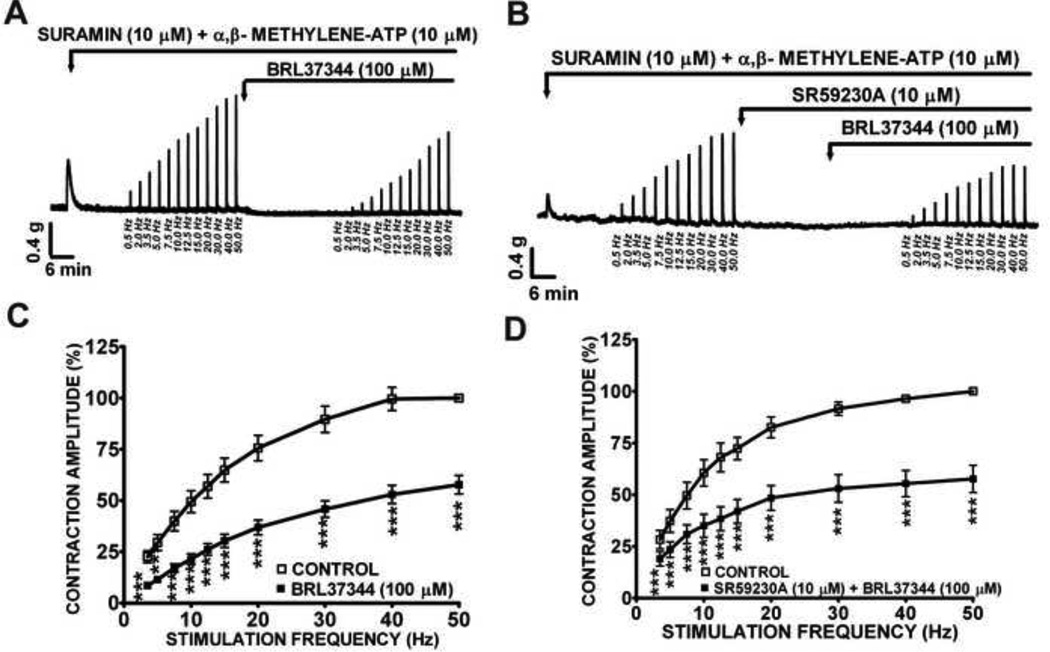
In order to investigate the cholinergic component of the EFS-induced contractions, we blocked the purinergic component of the EFS-induced contractions with suramin (10 µM) and α,β-methylene-ATP (10 µM) (Heppner et al., 2005; Heppner et al., 2009; Soder and Petkov, 2011;Thorneloe et al., 2005; Werner et al., 2007). These two inhibitors have different mechanism of action. While suramin inhibits the purinergic receptor directly, α,β-methylene-ATP first activates the receptors but then quickly desensitizes the receptors. Thus, the combined use of both compounds secures higher degree of purinergic receptor inhibition. It has been shown that the combination of these two purinergic inhibitors decreases the number of spontaneous global Ca2+ flashes and also nearly abolished the local Ca2+ transients evoked by EFS suggesting that these two compounds combined completely block the purinergic component of the nerve-evoked contractions in DSM (Heppner et al., 2005). In the presence of suramin (10 µM) and α,β- methylene-ATP (10 µM), BRL37344 significantly decreased the amplitude of the EFS-induced contractions in rat DSM isolated strips at a wide range of EFS stimulation frequencies (3.5–50 Hz) suggesting that BRL37344 inhibited the cholinergic component of the EFS-induced contractions (Fig. 4A, C). BRL37344 (100 µM) inhibited EFS-induced contraction amplitude by 42.3±4.5% at the maximal stimulation frequency of 50 Hz (n=12, N=5, P<0.005; Fig. 4C). This BRL37344 inhibitory effect on the cholinergic component was slightly reduced at low stimulation frequency (3.5–20 Hz) by SR59230A (10 µM) (n=9, N=5; P<0.05; Fig. 4B, D).
Figure 4. BRL37344 effects on the amplitude of the 0.5–50 Hz EFS-induced contractions of rat DSM isolated strips in the presence of suramin and α,β-methylene-ATP.
This original DSM tension recording of EFS-induced DSM cholinergic contractions illustrates BRL37344 (100 µM) inhibitory effects in the absence (A) or presence (B) of SR59230A (10 µM). Application of α,β-methylene-ATP, a purinergic receptor agonist caused a transient contraction (A and B) of the DSM followed by desensitization and inhibition of the receptor. C-D) These EFS frequency-response curves show BRL37344 inhibitory effect on EFS-induced DSM contraction amplitude in the absence (n=12, N=5) (C) or presence (D) of 10 µM SR59230A (n=9, N=5; ***P<0.005).
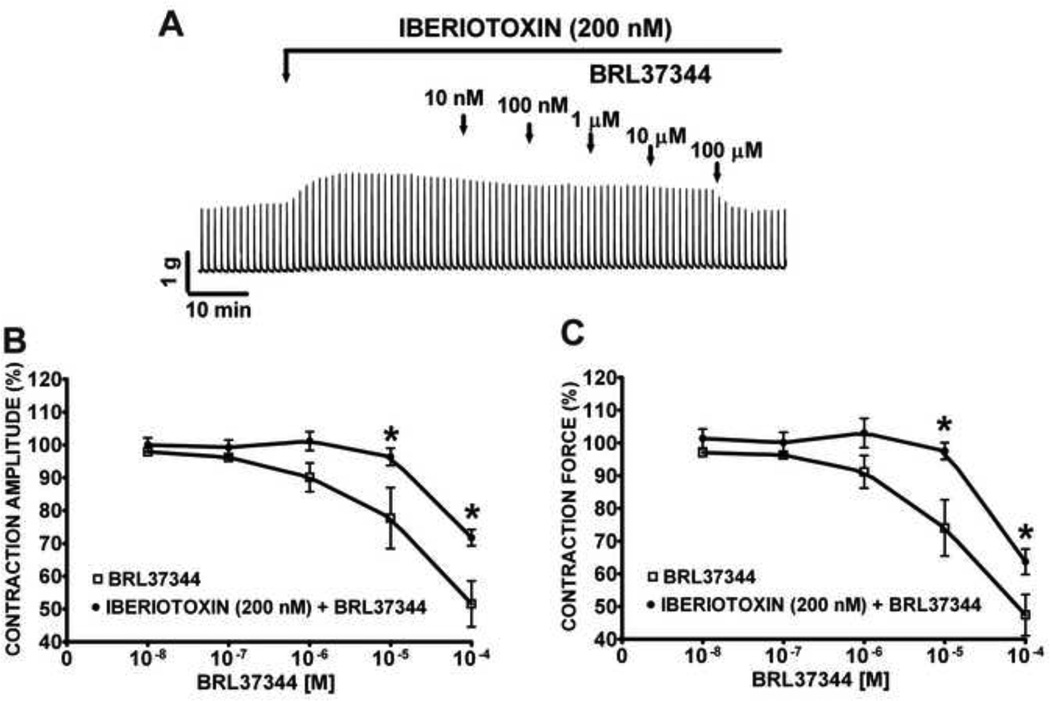
3.3 BRL37344 inhibitory effect on EFS-induced contractions is antagonized by iberiotoxin, a selective BK channel blocker
In this experimental series, we investigated whether the BK channel plays a role during the β3-adrenoceptor-mediated inhibition of nerve-evoked contractions in rat DSM. We pretreated rat DSM isolated strips with iberiotoxin (200 nM) prior to BRL37344 (10 nM–100 µM) application (Fig. 5A). We found that blocking the BK channels with iberiotoxin significantly antagonized BRL37344 inhibitory effects on the amplitude and muscle force of the 20 Hz EFS-induced DSM contractions (Fig. 5B-C). The BRL37344 inhibitory effects on 20 Hz EFS-induced DSM contractions were significantly reduced as illustrated by reduction in the potency of BRL37344 inhibitory effect on the EFS-induced contraction amplitude and force (n=7, N=5, P<0.05; Fig. 5B-C). These findings suggest that BK channels and β3-adrenoceptors work in synergy to oppose EFS-induced contractions in rat DSM isolated strips.
Figure 5. Blockade of BK channels with iberiotoxin reduces the BRL37344 inhibitory effects on 20 Hz EFS-induced contractions of rat DSM isolated strips.
A) This original DSM tension recording illustrates the reduction of BRL37344 (10 nM–100 µM) inhibitory effects on 20 Hz EFS-induced DSM contractions by BK channels blockade with 200 nM iberiotoxin. These cumulative concentration-response curves show that pretreatment of DSM isolated strips with 200 nM iberiotoxin opposes BRL37344 inhibitory effects on EFS-induced DSM contraction amplitude (B) and force (C) (n=7, N=5; *P<0.05).
4. DISCUSSION
The present study provides evidence that the β3-adrenoceptor agonist BRL37344-mediated relaxation of rat DSM nerve-evoked contraction is regulated by the BK channels. This BRL37344-induced DSM relaxation involved inhibition of both cholinergic and purinergic components of the nerve-evoked contractions.
4.1 BRL37344 reduces nerve-evoked contractions in rat DSM isolated strips: The role of purinergic and cholinergic components
During the voiding phase of the micturition process, the parasympathetic nerves become activated and acetylcholine and ATP, the two main excitatory neurotransmitters in the bladder are released (Andersson and Arner, 2004). ATP activates purinergic P2X receptors while acetylcholine activates M2/M3 muscarinic cholinergic receptors to induce DSM contractions and facilitate voiding (Andersson and Arner, 2004). In our study, bladder nerves were stimulated with a wide range of EFS frequencies in order to activate these two neurogenic pathways. It has been demonstrated that the activation of both purinergic and cholinergic pathways is EFS frequency-dependent (Heppner et al., 2009). At low stimulation frequencies (≤ 20 Hz), the purinergic pathway is predominant, contrary to the cholinergic component which predominates at high frequencies (≥ 20 Hz) (Brading and Williams, 1990; Heppner et al., 2009; Werner et al., 2007).
In a previous report, we showed in guinea pig DSM isolated strips that the BRL37344 inhibitory effect on EFS-induced contractions was not mediated by β3-adrenoceptors suggesting important species differences (Afeli et al., 2012). We further showed that L-755,507, a selective β3- adrenoceptor agonist, failed to inhibit the nerve-evoked contractions in guinea pig DSM isolated strips suggesting that in this particular species β3-adrenoceptors play a minor to no role at all (Afeli et al., 2012). Here, we found that contrarily to guinea pig DSM, the β3-adrenoceptor agonist, BRL37344, caused a significant decrease of the nerve-evoked contraction amplitude and force in rat DSM isolated strips in the presence or absence of atropine (Figs. 2–3). BRL37344 (10 and 100 µM) caused a ~26% and ~52% decrease in rat DSM nerve-evoked contractions force, respectively. The BRL37344 inhibitory effect reported here is similar to that in previous reports on human DSM (Takeda et al., 1999). The aforementioned study showed that BRL37344 (pEC50=6.25) caused a 22% and a 47% inhibition of human DSM carbachol-induced tone when used at concentrations of 10 µM and 100 µM, respectively (Takeda et al., 1999). Similar to Takeda et al. study, the BRL37344 inhibitory effects that we report here at 10 and 100 µM could also be mediated via activation of β2-adrenoceptors, as recently suggested by Baker (Baker, 2010). We further studied the purinergic and the cholinergic components separately by using inhibitors of these two components. In the presence of atropine, which was used to block the cholinergic component of the nerve-evoked contractions, we found that BRL37344 decreased the amplitude of the nerve-evoked contractions at all stimulation frequencies tested suggesting that BRL37344 inhibits the purinergic component of the nerve-evoked contractions (Fig. 3A, C). This BRL37344 inhibitory effect was completely blocked at all EFS stimulation frequencies by SR59230A, which suggests that BRL37344 inhibits the purinergic component of the nerveevoked contractions. Our data also showed that in the presence of α,β-methylene-ATP and suramin which were used to block the purinergic component of the nerve-evoked contractions, BRL37344 decreased the amplitude of the EFS-induced contractions at all stimulation frequencies tested, suggesting that BRL37344 inhibits the cholinergic component of the nerve-evoked contractions. However, the BRL37344 inhibitory effect on the cholinergic component was not completely antagonized by SR59230A suggesting that BRL37344 might exhibit some non-selective effects by also activating the β2-adrenoceptors (Baker, 2010). Taken together, these data suggest that BRL37344 inhibitory effects on rat DSM nerve-evoked contractions were mediated via inhibition of both purinergic and cholinergic pathways.
4.2 Functional BK channels regulate β3-adrenoceptor-mediated relaxation of rat DSM neurogenic contractions
Previous studies, both in human and rodents, have shown that the BK channel plays an important regulatory role in DSM physiology (Brown et al., 2008; Herrera and Nelson, 2002; Hristov et al., 2011; Hristov et al., 2012; Petkov, 2012; Petkov et al., 2001; Petkov and Nelson, 2005; Soder and Petkov, 2011; Xin et al., 2012a; Xin et al., 2012b). Activation of BK channels hyperpolarizes the cell membrane and prevents Ca2+ entry through L-type voltage-gated Ca2+ channels, which ultimately causes DSM relaxation (Hristov et al., 2011; Hristov et al., 2008; Petkov, 2012; Petkov and Nelson, 2005; Soder and Petkov, 2011). This BK channel-mediated DSM relaxation has made BK channel-targeting compounds potential drug candidates for the treatment of bladder dysfunction (Chang et al., 2010; Hristov et al., 2012; Layne et al., 2010; Petkov, 2012; Soder and Petkov, 2011). Along with BK channels, β3-adrenoceptors have also been demonstrated to be promising targets for the pharmacological treatment of overactive bladder (Bhide et al., 2012; Brown et al., 2008; Gras, 2012; Leon et al., 2008; Tyagi and Tyagi, 2010). Our group has recently provided evidence that there is a functional link between β3- adrenoceptors and BK channels (Hristov et al., 2008). We have previously demonstrated that the pharmacological blockage of the BK channels with iberiotoxin opposes β3-adrenoceptor-mediated inhibition of rat DSM myogenic contractions (Hristov et al., 2008). In the present study, the β3-adrenoceptor agonist, BRL37344, attenuates the contraction amplitude and muscle force of the 20 Hz EFS-induced contractions in a concentration-dependent manner (Fig. 5). We further observed that pharmacological blockade of BK channels with iberiotoxin antagonized the β3-adrenoceptor-mediated inhibition of rat DSM nerve-evoked contraction amplitude and force (Fig. 5). Using immunohistochemical analysis, Dr. Mark Nelson’s group has demonstrated that BK channels are expressed in DSM but not in the bladder nerves that innervate DSM (Werner et al., 2007). These findings clearly suggest that all of the iberiotoxin effects reported in our study were not mediated via activation of efferent nerve terminals to increase neurotransmitter release but rather solely through direct inhibition of BK channels which are highly expressed in DSM (Hristov et al., 2011; Petkov et al., 2001). Collectively, our data suggest that BK channel activity is critical for the β3-adrenoceptor-induced attenuation of the nerve-evoked DSM contractions.
5. CONCLUSION
The present study reveals that the β3-adrenoceptor agonist, BRL37344, inhibited both the purinergic and cholinergic components of the nerve-evoked contractions in rat DSM. We also found that functional BK channels play a critical role in the BRL37344-induced inhibition of the nerve-evoked contractions in rat DSM.
ACKNOWLEDGEMENTS
We would like to thank Drs. John Malysz, Wenkuan Xin, Kiril Hristov, Shankar Parajuli, Ms. Amy Smith, Mr. Qiuping Cheng and Mr. Ning Li for the critical evaluation of the manuscript.
GRANTS
This study was supported by a grant from the National Institutes of Health DK084284 to Georgi V. Petkov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afeli SA, Hristov KL, Petkov GV. Do beta3-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility? Am J Physiol Renal Physiol. 2012;302:F251–F263. doi: 10.1152/ajprenal.00378.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010;160:1048–1061. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide AA, Digesu GA, Fernando R, Khullar V. Use of mirabegron in treating overactive bladder. Int Urogynecol J. 2012;23:1345–1348. doi: 10.1007/s00192-012-1724-0. [DOI] [PubMed] [Google Scholar]
- Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int. 2006;98:1310–1314. doi: 10.1111/j.1464-410X.2006.06564.x. [DOI] [PubMed] [Google Scholar]
- Brading AF, Williams JH. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha,beta-methylene ATP. Br J Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol. 2008;295:F1149–F1157. doi: 10.1152/ajprenal.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol. 2010;298:F1416–F1423. doi: 10.1152/ajprenal.00595.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, Kobayashi M, Yamaguchi O. Expression and possible functional role of the beta3-adrenoceptor in human and rat detrusor muscle. J Urol. 1999;161:680–685. [PubMed] [Google Scholar]
- Gras J. Mirabegron for the treatment of overactive bladder. Drugs Today (Barc) 2012;48:25–32. doi: 10.1358/dot.2012.48.1.1738056. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol. 2005;564:201–212. doi: 10.1113/jphysiol.2004.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol. 2009;587:5275–5288. doi: 10.1113/jphysiol.2009.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks A, McCafferty GP, Riedel E, Aiyar N, Pullen M, Evans C, Luce TD, Coatney RW, Rivera GC, Westfall TD, Hieble JP. GW427353 (solabegron), a novel, selective beta3-adrenergic receptor agonist, evokes bladder relaxation and increases micturition reflex threshold in the dog. J Pharmacol Exp Ther. 2007;323:202–209. doi: 10.1124/jpet.107.125757. [DOI] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol. 2011;301:C903–C912. doi: 10.1152/ajpcell.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2008;295:C1344–C1353. doi: 10.1152/ajpcell.00001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2012;302:C1632–C1641. doi: 10.1152/ajpcell.00417.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanie S, Otsuka A, Yoshikawa S, Morimoto T, Hareyama N, Okazaki S, Kobayashi R, Hasebe K, Nakao K, Hayashi R, Mochizuki H, Matsumoto R, Ozono S. Pharmacological effect of TRK-380, a novel selective human beta3-adrenoceptor agonist, on mammalian detrusor strips. Urology. 2012;79:744, e741–e747. doi: 10.1016/j.urology.2011.08.080. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D, Wos J, Rosenbaum JS, de Groat WC. Effects of beta3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther. 2009;330:704–717. doi: 10.1124/jpet.109.155010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R378–R384. doi: 10.1152/ajpregu.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LA, Hoffman BE, Gardner SD, Laping NJ, Evans C, Lashinger ES, Su X. Effects of the beta3-adrenergic receptor agonist disodium 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL-316243) on bladder micturition reflex in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008;326:178–185. doi: 10.1124/jpet.108.138651. [DOI] [PubMed] [Google Scholar]
- Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl 2):S88–S119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol. 2012;9:30–40. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1255–C1263. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- Sato M, Horinouchi T, Hutchinson DS, Evans BA, Summers RJ. Ligand-directed signaling at the beta3-adrenoceptor produced by 3-(2-Ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propan ol oxalate (SR59230A) relative to receptor agonists. Mol Pharmacol. 2007;72:1359–1368. doi: 10.1124/mol.107.035337. [DOI] [PubMed] [Google Scholar]
- Soder RP, Petkov GV. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol. 2011;670:252–259. doi: 10.1016/j.ejphar.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, Sasamata M, Miyata K, Uchida H, Yamaguchi O. Effect of (R)-2-(2-aminothiazol-4-yl)-4'-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther. 2007;321:642–647. doi: 10.1124/jpet.106.115840. [DOI] [PubMed] [Google Scholar]
- Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, Hatano A, Takahashi K, Nomura S. Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther. 1999;288:1367–1373. [PubMed] [Google Scholar]
- Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005;289:F604–F610. doi: 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- Tyagi P, Thomas CA, Yoshimura N, Chancellor MB. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol. 2009;35:76–83. doi: 10.1590/s1677-55382009000100012. [DOI] [PubMed] [Google Scholar]
- Tyagi P, Tyagi V. Mirabegron, a beta(3)-adrenoceptor agonist for the potential treatment of urinary frequency, urinary incontinence or urgency associated with overactive bladder. IDrugs. 2010;13:713–722. [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol. 2007;292:R616–R624. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- Xin W, Cheng Q, Soder RP, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol. 2012a;302:C1361–C1370. doi: 10.1152/ajpcell.00432.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Cheng Q, Soder RP, Rovner ES, Petkov GV. Constitutively active phosphodiesterase activity regulates urinary bladder smooth muscle function: critical role of KCa1.1 channel. Am J Physiol Renal Physiol. 2012b;303:F1300–F1306. doi: 10.1152/ajprenal.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]