Abstract
Apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), an adaptor protein for inflammasome receptors, is essential for inducing the caspase-1 activaton and the consequent secretion of IL-1β, which associates with local inflammation during liver ischemia/reperfusion injury (IRI). However, little is known on mechanisms by which ASC/Caspase-1/IL-1β axis exerts its function in hepatic IRI. This study was designed to explore the functional roles and molecular mechanisms of ASC/Caspase-1/IL-1β signaling in the regulation of inflammatory responses in vitro and in vivo. Using a partial lobar liver warm ischemia (90min) model, ASC-deficient and WT mice (C57BL/6) were sacrificed at 6h of reperfusion. Separate animal cohorts were treated with anti-IL-1β Ab or control IgG (10mg/kg, day −1, i.p.). We found that ASC deficiency inhibited Caspase-1/IL-1β signaling, leading to the protection against liver IR-damage, local enhancement of antiapoptotic functions, and downregulation of HMGB1-mediated TLR4-driven inflammation. Interestingly, treatment of ASC-deficient mice with rHMGB1 recreated liver IRI. Moreover, neutralization of IL-1β ameliorated the hepatocellular damage by inhibiting NF-κB/COX2 signaling in IR-stressed livers. In parallel in vitro studies, knockout of ASC in LPS-stimulated bone marrow derived-macrophages depressed HMGB1 activity via p38 MAPK pathway, leading to the inhibition of TLR4/NF-κB, and ultimate depression of proinflammatory cytokine programs. Conclusion: ASC-mediated Caspase-1/IL-1β signaling promotes HMGB1 to produce TLR4-dependent inflammatory phenotype, leading to hepatocellular injury. Hence, the ASC/Caspase-1/IL-1β signaling mediates inflammatory response by triggering HMGB1 induction in hepatic IRI. Our findings provide the rationale for a novel therapeutic strategy to manage liver injury due to IR.
Keywords: TLR4, Inflammation, Liver, injury
Ischemia and reperfusion-elicited injury (IRI) in the liver remains a major complication of hemorrhagic shock, liver resection and transplantation (1). Despite improved preservation and surgical techniques, IRI resulting from donor organ retrieval, cold storage and warm ischemia during the surgery often leads to primary organ non-function, predisposes to chronic rejection, and contributes to the acute shortage of donor organs available for transplantation. Liver IRI represents an exogenous antigen-independent inflammatory process that includes Kupffer cell/neutrophil activation, cytokine release, followed by hepatocyte and sinusoidal endothelial cell death (1). We and others have documented Toll-like receptor (TLR) 4 -dependent innate immune mechanisms that initiate liver IRI cascade, transcript NF-κB-mediated cytokine/chemokine and cell adhesion genes, leading to the development of local inflammation and apoptosis (2–4).
IL-1β, a pro-inflammatory cytokine produced mainly by macrophages, has many biological functions that are essential in sterile inflammation initiated by endogenous danger signals, such as the upregulation of endothelial adhesion molecules for the recruitment of innate immune cells (5) and the development of inflammatory phenotype (6). The secretion of IL-1β by inflammatory cells is largely dependent on a multiprotein complex termed the inflammasome, which consists of a nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) molecule and procaspase-1, and mediates the activation of caspase-1 (7–10).
Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) plays a critical role in the activation of inflammasomes as an adaptor protein that bridges procaspase-1 and inflammasome receptors, such as NLR family pyrin domain containing 3 (NLRP3) and absent in melanoma 2 (AIM2) (11–13). Indeed, ASC contributes to immune response through the assembly of inflammasome complexes that activate downstream effector cysteine protease caspase-1, resulting in the generation of active IL-1β and IL-18 from inactive pro-IL-1β and pro-IL-18 precursors.
High mobility group box-1 (HMGB1), an evolutionarily conserved and ubiquitously expressed DNA-binding protein in the nucleus of almost all eukaryotic cells, stabilizes nucleosome formation, facilitates gene transcription, repair and recombination (14). In addition to its nuclear role, extracellular HMGB1, known as one of key endogenous damage-associated molecular pattern (DAMP) molecules, can activate inflammatory pathways. Indeed, macrophage-derived HMGB1 was shown to mediate delayed endotoxin lethality and acute lung injury in mice (15–17). HMGB1 can also be released by ischemia-stressed cells (18, 19), suggesting its role as an endogenous danger signal or alarmin that may engage diverse receptor repertoire, including TLR2, TLR4, TLR9 and advanced glycation end products (RAGE) for the initiation of an array of inflammatory responses (20–22). Although ASC/Caspase-1/IL-1β axis is essential in triggering the inflammation cascade, little is known on its crosstalk with HMGB1. In the present study, we show that ASC mediates caspase-1/IL-1β signaling and promotes HMGB1 to trigger TLR4-driven inflammation. Our results identify previously unrecognized HMGB1-dependent ASC/Caspase-1/IL-1-mediated inflammation response in the mechanism of liver IRI.
Materials and Methods
Animals
Male C57BL/6 wild-type (WT) mice (The Jackson Laboratory, Bar Harbor, ME) and ASC KO mice (bred at UCLA) at 8–10 weeks of age were used. Animals were housed in UCLA animal facility under specific pathogen-free conditions, and received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
Liver IRI and treatment
We used a well-established mouse model of partial warm hepatic IRI (2, 23, 24). Separate groups of WT and ASC KO mice were injected with heparin (100U/kg) and an atraumatic clip was used to interrupt the artery/portal venous blood supply to the left/middle liver lobes. After 90min of ischemia, the clip was removed and mice were sacrificed at 6h after reperfusion. Some of ASC KO recipients received a single injection of rHMGB1 (0.8mg/kg, i.p., a gift from Dr. A. Tsung, University of Pittsburgh) immediately at reperfusion. Additional WT cohorts were given anti-mouse IL-1β mAb (10mg/kg, i.p.; Novartis Inc., Basel, Switzerland) or control IgG one day prior to ischemia. An equivalent of anti-human-IL-1β (canakinumab), this mAb binds to mouse IL-1β and neutralizes its activity by blocking interaction with IL-1 receptors. Sham-operated controls underwent the same procedure but without vascular occlusion.
Hepatocellular damage assay
Serum alanine transaminase (sALT) levels, an indicator of hepatocellular injury, were measured in blood samples with an autoanalyzer (ANTECH Diagnostics, Los Angeles, CA).
Liver histology and immunohistochemistry
Liver paraffin sections (5-μm) were stained with hematoxylin and eosin (H&E). The severity of liver IRI was graded blindly using Suzuki’s criteria on a scale from 0 to 4 (25). No necrosis, congestion/centrilobular ballooning is given a score of 0, while severe congestion and >60% lobular necrosis is given a value of 4.
Liver infiltrating macrophages and neutrophils were detected using primary mAb against CD11b and Ly6G (BD Biosciences, San Jose, CA), respectively. HMGB1 was detected in hepatocytes and liver-infiltrating macrophages/monocytes with rabbit anti-HMGB1 Ab (Cell Signaling Technology, Danvers, MA). The secondary, biotinylated goat anti-rat IgG or goat anti-rabbit IgG (Vector, Burlingame, CA) were incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section (×400).
Myeloperoxidase assay
Neutrophil influx in the liver tissue was assessed by the enzymatic activity of myeloperoxidase (MPO) (26). One unit of MPO activity (U/g) was defined as the quantity of enzyme degrading 1μmol peroxide per minute at 25°C per gram of tissue.
Quantitative RT-PCR
Total RNA was extracted from frozen livers using RNAse Mini Kit (Qiagen, Valencia, CA); RNA concentration was determined by a spectrophotometer. A total of 5.0μg of RNA was reverse-transcribed into cDNA. Quantitative PCR was performed using DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA). In a final reaction volume of 20μl, the following were added: 1 X SuperMix (Platinum SYBR Green qPCR Kit, Invitrogen, Carlsbad, CA), complementary DNA, and 10μM of each primer. Amplification conditions were: 50°C (2min), 95°C (5min), followed by 40 cycles of 95°C (15sec), 60°C (30sec). Primer sequences for the amplification of HMGB1, TNF-α, IL-1β, MCP-1, CXCL-1, CXCL-10, IL-18, IL-20p40, iNOS, COX2, and HPRT are shown in Supplementary Table I. Target gene expressions were calculated by their ratios to the housekeeping gene HPRT.
Apoptosis assay
Apoptosis in formalin-fixed paraffin-embedded liver sections was detected by TUNEL staining kit (Calbiochem, Gibbstown, NJ) (26). Negative controls were prepared through omission of terminal transferase. Positive controls were generated by treatment with DNase. TUNEL-positive cells were counted in 10 HPF/section (×400).
In vitro cell cultures
Bone marrow derived-macrophages (BMMs) were generated, as described (24). Cells (1×106/well) were cultured for 7 days, followed by incubation with LPS (100ng/ml) for 6h or rHMGB1 (1μg/ml) for 24h (both from Sigma-Aldrich Corp., St. Louis, MO).
Western blots
Proteins (30μg/sample) from livers or cell cultures were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Polyclonal rabbit anti-mouse cleaved caspase-3, Bcl-2, Bcl-xL, HMGB1, COX2, phospho-p38 MAPK, β-actin (Cell Signaling Technology, Danvers, MA), TLR4 (IMGENEX, San Diego, CA), NF-κB and polyclonal goat anti-mouse cleaved caspase-1 (Santa Cruz Biotechnology, Santa Cruz, CA) were used. Relative protein quantities were determined by densitometer and expressed in absorbance units (AU).
Caspase-1 enzymatic activity assay
Caspase-1 enzymatic activity was determined by a colorimetric assay kit (R&D System, Minneapolis, MN). Briefly, BMMs were cultured with rTNF-α (100ng/ml) for 24h and cellular protein was extracted with cold protein lysis buffer. A 50μl of cell lysate was added to 50μl of caspase-1 reaction buffer in a 96-well flat bottom microplate. Each sample was then added to 200mM caspase-1 substrate, WEHD-pNA, followed by 2h of incubation at 37°C. The enzymatic activity of caspase-1 was measured on an ELISA reader at 405nm wavelength.
ELISA assay
Mouse ELISA kit was used to measure IL-1β levels in BMM culture supernatants (eBioscience, San Diego, CA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Differences between experimental groups were analyzed by Student-t test. All differences were considered statistically significant at the p-value of <0.05.
Results
Disruption of ASC signaling ameliorates liver IRI
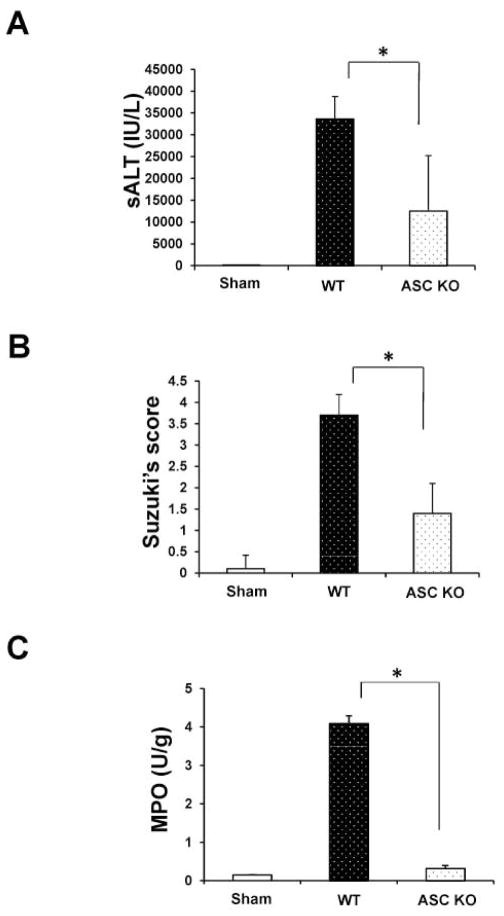
We analyzed the hepatocellular function in mouse livers subjected to 90min of warm ischemia followed by 6h reperfusion. As shown in Fig. 1A, sALT levels (IU/L) were decreased in ASC KO mice as compared with WT controls (12506.8±12717 vs. 32812±5133; p<0.01). These data correlated with Suzuki’s grading of histological liver IR-damage. Indeed, ASC-deficient mice showed minimal sinusoidal congestion, vacuolization without edema or necrosis (Fig. 1B: score= 1.4±0.6). Similar findings were recorded in ASC-deficient livers subjected to 90min of warm ischemia only (Supplementary Fig. 2A,B; Suzuki score=1.2±0.4). In contrast, ASC proficient (WT) livers revealed moderate to severe edema and extensive hepatocellular necrosis at 6h of reperfusion (Fig. 1B; score=3.7±0.5; p<0.0001). Liver MPO activity (U/g), an index of neutrophil accumulation, was suppressed in ASC KO mice, as compared with WT controls (Fig. 1C: 0.32±0.076 vs. 4.1±0.2; p<0.005).
Figure 1.
ASC knockout ameliorates the hepatocellular damage and prevents liver IRI. Mice were subjected to 90min of partial liver warm ischemia, followed by 6h of reperfusion.
(A) Hepatocellular function evaluated by sALT (IU/L). Decreased sALT levels in ASC-deficient group as compared with controls. *p<0.01, n=5–6/group. Mean±SD are shown.
(B) Suzuki’s histological grading of liver IRI. *p<0.0001, n=5–6/group. Mean±SD are shown.
(C) MPO neutrophil activity (U/gm). *p<0.005. n=2–3/group. Mean±SD are shown.
ASC deficiency inhibits HMGB1 in liver IRI
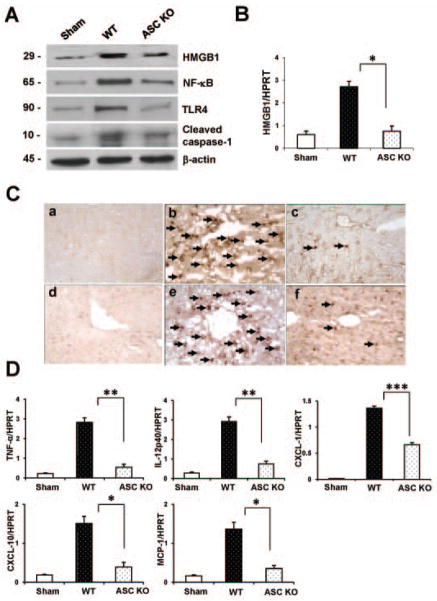
As shown in Fig. 2A, Western-assisted expression (AU) of HMGB1 (2.0–2.2), NF-κB (2.6–2.8), TLR4 (1.7–1.9), and cleaved caspase-1 (1.5–1.7) proteins were consistently increased in WT, as compared with ASC-deficient livers (0.8–1.0, 1.0–1.2, 0.2–0.4 and 0.5–0.7, respectively). We further analyzed mRNA levels coding for HMGB1 by qRT-PCR (Fig. 2B). ASC KO mice showed decreased hepatic expression of HMGB1, compared with controls (p<0.05). Unlike in ASC-proficient (WT) controls, the baseline HMGB1 levels were decreased in ASC-deficient mice, as evidenced both in vitro (BMM cultures) and in vivo (normal livers) (Supplementary Fig. 4A,B). To determine whether ASC signaling may have influenced macrophage and neutrophil trafficking patterns, we performed immunohistochemical staining in IR-stressed livers. As shown in Fig. 2C, ASC-deficient livers were devoid of both CD11b+ macrophages (panel a,b,c) and Ly6G+ neutrophils (panel d,e,f), as compared with WT controls (CD11b+ macrophages/HPF: 7.4±4.4 vs. 38.1±18.6; p<0.005; LY6G+ neutrophils/HPF: 10.7±5.6 vs. 42.5±18.5, p<0.0001). Consistent with these immunostaining data, qRT-PCR-assisted detection of mRNA coding for TNF-α/IL-12p40 (p<0.01), CXCL-10/MCP-1 (p<0.05) and CXCL-1 (p<0.0005) were reduced in ASC-deficient livers, compared with WT controls (Fig. 2D).
Figure 2.
Knockout of ASC signaling reduces HMGB1, TLR4-mediated inflammation, macrophage sequestration and innate cytokine/chemokine programs in IR-stressed livers.
(A) Western blot-assisted detection of HMGB1, NF-κB, TLR4, and cleaved caspase-1. β-actin expression serves as an internal control. Representative of three experiments is shown.
(B and D) Quantitative RT-PCR-assisted detection of cytokines (HMGB1 [B], TNF-α/IL-12p40 [D]), chemokines (MCP-1/IP-10/CXCL-1 [D]). Data were normalized to HPRT gene expression. *p<0.05, **p<0.01, ***p<0.005, n=3/group. Mean±SD are shown.
(C) Representative ischemic liver lobes stained for CD11b (panel a,b,c) and Ly6G (panel d,e,f), magnification ×400, n=3/group.
ASC deficiency promotes anti-apoptotic function and decreases apoptosis in IR-stressed livers
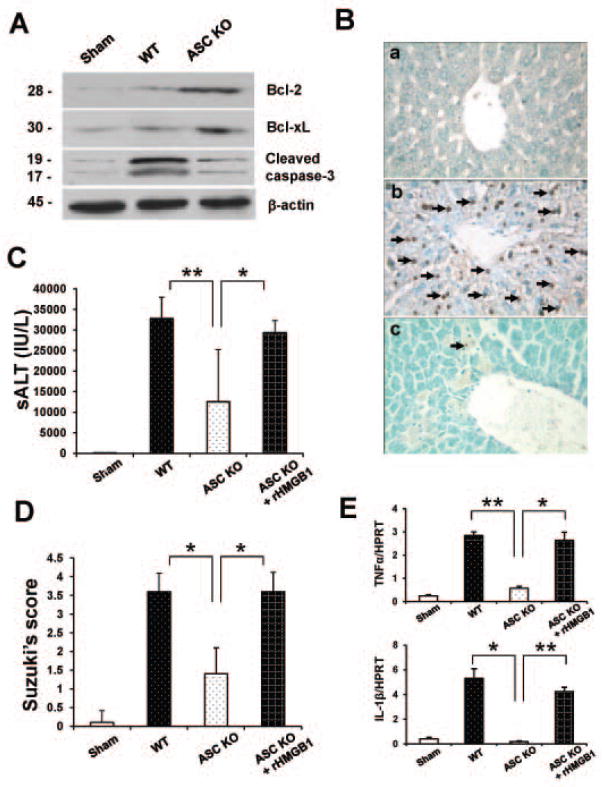
To determine whether ASC affects IR-induced apoptosis, we performed Western blots to detect anti-apoptotic genes. The expression (AU) of Bcl-2/Bcl-xl was upregulated in ASC KO (Fig. 3A: 1.8–2.0/1.5–1.7) as compared with WT (0.2–0.4) livers. Moreover, ASC deficiency inhibited the expression of cleaved caspase-3 (0.3–0.5), compared with controls (1.9–2.1). Consistent with Western analysis, the frequency of TUNEL+ cells/HPF in the ischemic liver lobes was diminished inASC KO mice as compared with WT counterparts (Fig. 3B[c]: 7.9±15.22 and Fig. 3B[b]: 75.4±15.12, resp.; p<0.001).
Figure 3.
ASC knockout promotes anti-apoptotic function and reduces IR-induced apoptosis, whereas adjunctive treatment with rHMGB1 recreates liver IRI.
(A) Western blot-assisted analysis of Bcl-2, Bcl-xl, and cleaved caspase-3. β-actin was used as an internal control. Representative of three experiments.
(B) TUNEL-assisted detection of apoptosis. Representative staining of TUNEL+ apoptotic cells (magnification ×400). n=3/group.
(C) Hepatocellular function evaluated by sALT (IU/L). Decreased sALT levels in ASC KO group, compared with WT or ASC-deficient group conditioned with rHMGB1. *p<0.05, **p<0.01, n=3–6/group. Mean±SD are shown.
(D) Suzuki’s histological grading of liver IRI. *p<0.0001, n=3–6/group. Mean±SD are shown.
(E) Quantitative RT-PCR-assisted detection of TNF-α and IL-1β. Data were normalized to HPRT gene expression. *p<0.05, **p<0.005, n=3/group. Mean±SD are shown.
Exogenous rHMGB1 recreates liver IRI in ASC-deficient mice
To clarify the function of HMGB1 in ASC-mediated inflammatory response, we administered rHMGB1 to ASC KO mice immediately at reperfusion after 90min of warm ischemia. As shown in Fig. 3C, rHMGB1 infusion increased sALT levels (IU/L) at 6h of reperfusion, as compared with untreated ASC-deficient mice (29354.3±2971 vs. 12506.8±12717; p<0.05). These data correlated with Suzuki’s grading of histological liver damage (Fig. 3D). Hence, unlike ASC-deficient but otherwise untreated livers (score=1.5±0.7), those conditioned with adjunctive rHMGB1 revealed moderate to severe edema, sinusoidal congestion, and hepatocellular necrosis (score=3.6±0.51; p<0.0001). Similar effect was displayed in rHMBG1-treated WT livers subjected to 90min ischemia and 6h reperfusion (Supplementary Fig. 5A,B). In contrast, rHMGB1 did not affect well-preserved histological architecture in sham controls. Furthermore, adjunctive rHMGB1 significantly increased expression of mRNA coding for TNF-α (p<0.05) and IL-1β (p<0.005) in ASC KO livers, as compared with otherwise untreated ASC-deficient livers (Fig. 3E). These findings were confirmed in vitro where addition of rHMGB1 into either WT or ASC-deficient BMM cultures increased cleaved caspase-1/TLR4, and IL-1β expression (Supplementary Fig. 3A,B).
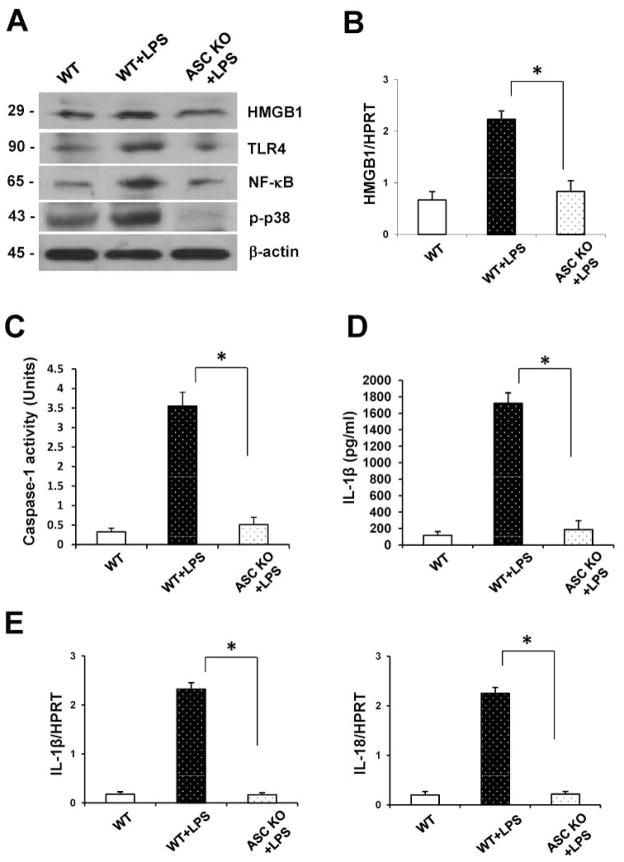
ASC promotes HMGB1/TLR4-mediated inflammation
To further elucidate the molecular mechanisms of HMGB1- and ASC-mediated inflammatory response in liver IRI, we used LPS-stimulated BMM cell culture system. As shown in Fig. 4A, Western-assisted expression (AU) of HMGB1 was reduced in ASC-deficient BMMs (0.9–1.2), compared with WT controls (2.0–2.2). Unlike in LPS-stimulated WT BMMs (2.1–2.3), the expression of TLR4 (0.2–0.4)/NF-κB (0.3–0.5) was decreased in ASC-deficient BMMs. Moreover, LPS-stimulated ASC-deficient BMMs showed reduced expression of phospho-p38 MAPK (0.1–0.2), compared with WT (2.7–2.9). Next, we analyzed HMGB1 gene expression by qRT-PCR (Fig. 4B). The mRNA levels coding for HMGB1 were decreased in LPS-stimulated BMMs of ASC KO mice, compared with LPS-stimulated WT cells (p<0.05). These findings were confirmed by decreased caspase-1 activity in ASC-deficient but not WT BMM (Fig. 4C: 0.51±0.357 vs. 3.55±0.19; p<0.0005). To investigate the role of ASC/Caspase-1/IL-1- mediated inflammatory responses, we analyzed the production of IL-1β in BMMs by ELISA. As shown in Figure 4D, LPS-stimulated ASC-deficient BMMs revealed decreased IL-1β levels, compared with WT BMMs (186.5±108.7 vs. 1722.7±125.9; p<0.005). Furthermore, our qRT-PCR showed that IL-1β and IL-18 decreased in LPS-stimulated ASC-deficient as compared with WT BMMs (Fig. 4E; p<0.005).
Figure 4.
ASC-mediated caspase-1 signaling induces HMGB1 via MAPKs pathway and NF-κB activation in LPS-stimulated BMMs in vitro.
(A) Western blot-assisted analysis of HMGB1, NF-κB, TLR4, and phospho-p38. β-actin was used as an internal control. Representative of three experiments.
(B) Quantitative RT-PCR-assisted detection of HMGB1. Data normalized to β-actin gene expression. *p<0.05, n=3/group. Mean±SD are shown.
(C) Caspase-1 activity (U). Mean±SD; n=3/group. *p<0.005.
(D) ELISA-assisted detection of IL-1β. Mean±SD; n=3/group. *p<0.005.
(E) Quantitative RT-PCR-assisted detection of IL-1β and IL-18. Data were normalized to β-actin gene expression. *p<0.005, n=3/group. Mean±SD are shown.
Neutralization of IL-1β mitigatesliver IRI in WT mice
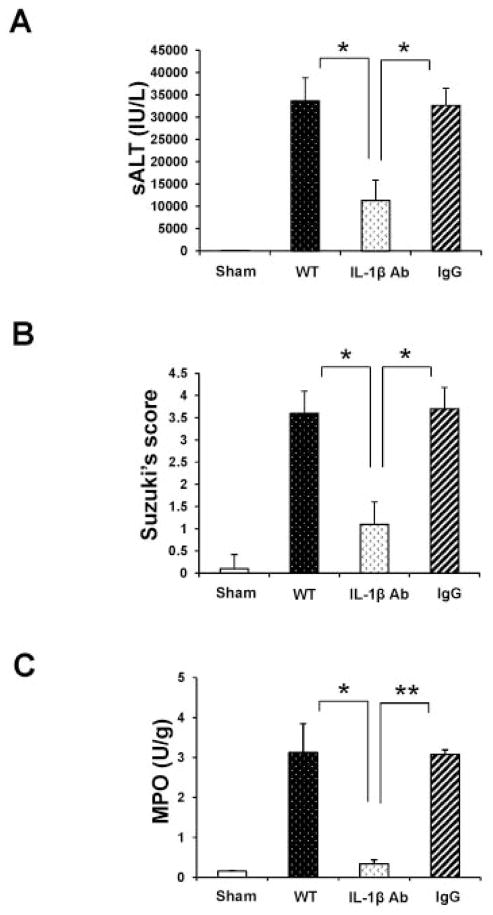
Having demonstrated that ASC/Caspase-1/IL-1β contributes to IR-inflammation response, we next investigated the role of IL-1β by using a neutralizing anti-IL-1β mAb in our model. Disruption of IL-1β signaling alleviated IR-liver damage, evidenced by diminished sALT (Fig. 5A: 11300±4595.5 vs. 33626±5156.6/32617±3859.4 respectively; p<0.0001) and well-preserved liver histology (Supplementary Fig. 1; Fig. 5B: Suzuki’s score=1.1±0.5). In contrast, livers in PBS or IgG groups revealed moderate to severe edema and extensive hepatocellular necrosis (Fig. 5B: Suzuki’s score=3.6±0.5 and 3.7±0.48, respectively; p<0.0001). Consistent with these data, MPO activity was suppressed in anti-IL-1β mAb treated group, compared with PBS/IgG controls (Fig. 5C: 0.34±0.1 vs. 3.13±0.72/3.08±0.11; p<0.05 and p<0.005, respectively).
Figure 5.
Treatment of WT mice with anti-IL-1β mAb mitigates the hepatocellular damage and liver IRI (6h of reperfusion following 90min of warm ischemia).
(A) The sALT levels (IU/L). *p<0.0001, n=5–7/group. Mean±SD are shown.
(B) Suzuki’s histological grading of liver IRI. *p<0.0001, n=5–7/group. Mean±SD are shown.
(C) MPO neutrophil activity (U/gm). *p<0.05, **p<0.005. n=2–3/group. Mean±SD are shown.
Neutralization of IL-1β inhibits NF-κ B/COX2 in lR-stressed livers
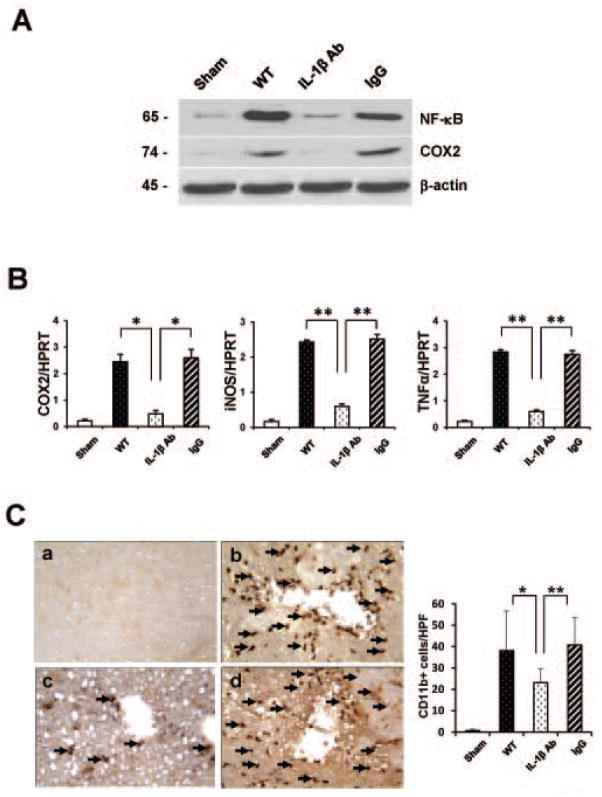
To investigate the mechanism by which anti-IL-1β mAb treatment may exert anti-inflammatory effects, we analyzed the expression of NF-κB, COX2 and inflammatory mediators in IR-livers. As shown in Fig. 6A, anti-IL-1β Ab depressed Western-assisted expression (AU) of NF-κB (0.4–0.6) and COX2 (0.1–0.2) proteins, as compared with WT (2.6–2.8 and 1.4–1.6, respectively) and IgG controls (2.0–2.2 and 1.6–1.8, respectively). Moreover, treatment with anti-IL-1β mAb decreased mRNA expression coding for COX2 (p<0.05), iNOS (p<0.005) and TNF-α (p<0.005), as compared with controls (Fig. 6B). Neutralization of IL-1β reduced the frequency of CD11b+ macrophages sequestered in the ischemic liver lobes (Fig. 6C), compared with those in WT or IgG-treated controls (23.1±6.44 vs. 38.1±18.64/40.8±12.91, respectively; p<0.05, p<0.005).
Figure 6.
Disruption of IL-1β signaling reduces cytokine/chemokine programs and macrophage traffic in IR-livers.
(A) Western blot-assisted expression of NF-κB and COX2; β-actin was used as an internal control. Representative of three experiments are shown.
(B) Quantitative RT-PCR-assisted detection of TNF-α, iNOS, and COX2.*p<0.05, **p<0.005, n=3/group. Mean±SD are shown.
(C) Left panel: Representative liver sections stained for CD11b (magnification×400). Right panel: Quantification of CD11+ cells/HPF. *p<0.05, **p<0.005, n=3/group.
Discussion
The adaptor protein ASC contributes to immune responses through activation of the cysteine protease caspase-1-dependent IL-1β (27). Although under normal conditions ASC-associated inflammasomes are autorepressed, they become activated by a wide range of pathogen stimuli, including oxidative stress, ischemia, and damage signals. As an endogenous danger signal or alarmin, HMGB1 released from activated macrophages/necrotic cells may bind immune receptors, including TLRs and RAGE, to trigger immune responses (22). This study identifies the essential role of HMGB1 in ASC/Caspase-1/IL-1β-dependent inflammatory responses in hepatic IRI. Indeed, global ASC knockout decreased sALT levels, depressed local macrophage/neutrophil sequestration, reduced hepatocellular apoptosis, and mitigated proinflammatory cytokine/chemokine programs in IR-stressed livers. Moreover, ASC deficiency diminished the induction of HMGB1 and alleviated IR-triggered liver damage through negative regulation of TLR4.
The molecular mechanisms of ASC/Caspase-1/IL-1β signaling to program inflammatory phenotype might involve activation of multiple intercellular pathways. We found disruption of ASC inhibited HMGB1/TLR4 expression, leading to decreased induction of inflammatory mediators, suggesting ASC/Caspase-1/IL-1β plays an important role in triggering local inflammation. In fact, the adaptor ASC was initially believed to exert its effects by bridging the interaction between NLRs and caspase-1 in inflammasome complexes (28). Activation of ASC within inflammasomes leads to the maturation of caspase-1 and processing of its IL-1β and IL-18 substrates. Our in vitro data demonstrate knockout of ASC decreased caspase-1 activity and IL-1β/IL-18 production in LPS-stimulated BMMs, implying the role of ASC in caspase-1/IL-1β-mediated inflammation.
Although ASC/Caspase-1/IL-1β axis is essential for the initiation of inflammatory response, the molecular pathways involved in cross talk with HMGB1 have not been elucidated. Our data demonstrates that treatment of ASC KO mice with rHMGB1 increased IR-induced hepatocellular damage, whereas disruption of ASC without exogenous rHMGB1 prevented the hepatic inflammatory development. These results are consistent with the ability of endogenous HMGB1 to promote liver IR-damage (19) and suggest HMGB1 might have a distinct role during ASC/IL-1β-mediated inflammation in hepatic IRI.
As an intracellular protein, HMGB1 translocates to the nucleus where it binds DNA to regulate gene transcription (29). However, the extracellular HMGB1 was shown to act as a cytokine mediator in response to inflammatory stimuli due to infection (15), whereas HMGB1 promotes TLR4-mediated inflammation in hepatic IRI (3, 4). Both hepatocytes and infiltrating macrophages/monocytes (including Kupffer cells) did express HMGB1 in IR-stressed WT and ASC-deficient livers. However, HMGB1 levels selectively increased in the ischemic WT but not ASC-deficient liver infiltrate (Supplementary Fig. 1A–D). Consistent with our findings, enhanced HMGB1 activated TLR4/NF-κB, leading to increased macrophage sequestration along with expression of proinflammatory cytokines (TNF-α/IL-12p40) and chemokines (MCP-1/CXCL-10). Using well-controlled in vitro culture system, we found ASC deficiency decreased mRNA and protein HMGB1 expression in LPS-stimulated BMMs and that TLR4 and NF-κB expression were diminished in ASC-deficient BMMs. Furthermore, rHMGB1 increased cleaved caspase-1 and IL-1β levels in BMM cultures (Supplementary Fig. 3A,B), suggesting HMGB1 was at least in part responsible for activation of caspase-1/IL-1β signaling in IR-stressed livers. Interestingly, knockout of ASC resulted in inhibition of phosphorylated p38 MAPK. Consistent with the essential role of MAPKs in IL-1β-mediated inflammation (30) our data imply HMGB1 induction in ASC/Caspase-1/IL-1β-mediated inflammation cascade in hepatic IRI is p38 MAPK-dependent.
IL-1β is an important cytokine that targets inflammatory injury due to hepatic IR. IL-1β can only express its biological activity from pro- to mature-IL-1β through proteolytic cleavage by the protease caspase-1 (31), and induce the release of HMGB1 from monocytes and macrophages (15). Moreover, IL-1β can form complexes with HMGB1 to enhance immune responses (32, 33). Our results demonstrate IL-1β blockade reduced IR-induced hepatocellular damage and improved liver function. Blocking IL-1β decreased expression of NF-κB and COX-2. Indeed, COX-2 overexpression has been associated with hypoxia/ischemia and inflammatory chronic diseases (34), whereas COX2 inhibition improved liver transplant function (35). It is plausible that IL-1β stimulates COX2 production through NF-κB in IR-stressed livers. Our findings support recent clinical data on the efficacy of anti-human IL-1β (canakinumab) therapy in type-2 diabetes (36) and auto-immune inflammatory disorders caused by mutations in the NLRP-3 nucleotide-binding domain, such as cryopyrin-associated periodic syndromes (CAPS) (37) or Schnitzler syndrome (38).
ASC, originally identified as a protein that mediates apoptosis in human leukemia cells (39), interacts with Bax to induce apoptosis via p53-Bax pathway (40). In addition, HMGB1 release can occur during the process of apoptotic cell death (41). Our data demonstrates that ASC knockdown decreased HMGB1 and caspase-3 but increased antiapoptotic Bcl-2/Bcl-xL expression. These findings were further supported by increased frequency of apoptotic cells in WT ischemic livers, whereas ASC deficiency markedly decreased hepatocellular apoptosis. Hence, as ASC-mediated apoptosis is essential in the mechanism of hepatic IRI, blocking ASC-mediated signaling may represent a novel strategy to regulate apoptotic pathways in the ischemia-stressed liver.
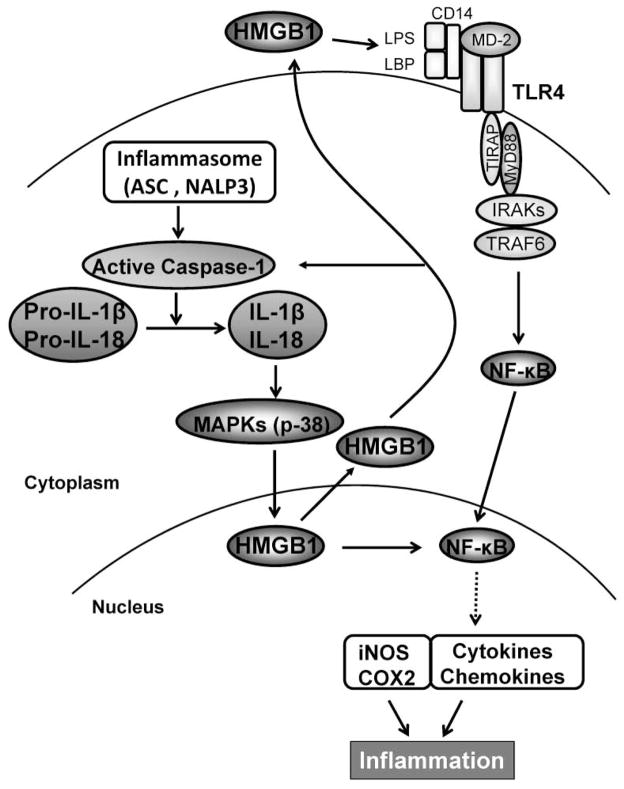
Figure 7 depicts molecular mechanisms by which ASC/Caspase-1/IL-1β - HMGB1 axis may regulate liver IRI immune cascade. ASC contributes to inflammatory responses by activation of inflammasomes, which in turn activates caspase-1 and catalyses pro-IL-1β/pro-IL-18 to mature IL-1β/IL-18. IL-18 is closely related to and shares a similar dimensional structure with IL-1β. ASC/Caspase-1/IL-1 promotes HMGB1 induction through activation of p38 MAPK, which triggers TLR4 and NF-κB to program proinflammatory mediators. In addition, HMGB1 might provide a positive feedback mechanism to regulate caspase-1 activation. ASC/caspase-1-mediated elaboration of IL-1β and its downstream COX2 are required for the inflammatory development in the course of hepatic IRI.
Figure 7.
ASC/Caspase-1/IL-1β - HMGB1 cross talk in the regulation of innate inflammation response in IR-stressed livers.
In conclusion, ASC/Caspase-1/IL-1β signaling promotes HMGB1 induction to facilitate TLR4-dependent inflammatory phenotype leading to IR-hepatocellular damage. By identifying HMGB1 as a novel mediator in ASC/Caspase-1/IL-1β-triggered inflammation, our findings provide the rationale for refined therapeutic strategies against liver IRI.
Supplementary Material
Suppl. Figure 1: Representative immunohistochemical staining for HMGB1 in hepatocytes (A/B) or infiltrating macrophages/monocytes (C/D) harvested from WT vs. ASC KO livers (6h of reperfusion after 90min of warm ischemia). Magnification ×400, n=3–4/group (*p<0.0001).
Suppl. Figure 2: Histopathology of ASC-deficient livers at 90min of warm ischemia only (A) vs. 90min ischemia plus 6h reperfusion (B). Representative of n=3–5/group; Magnification x200.
Suppl. Figure 3: BMMs from ASC KO or WT mice were cultured with rHMGB1 (1μg/ml) for 24h. The expression of cleaved caspase-1 and TLR4 was evaluated by Western blots. rHMGB1 supplement increased the expression of cleaved caspase-1/TLR4 (A), and IL-1β (B, p<0.01) in both ASC-deficient and WT groups, compared with BMM controls without rHMGB1 adjunct. Representative of three experiments.
Suppl. Figure 4: BMMs and liver tissue proteins were isolated from ASC KO and WT mice. HMGB1 expression was evaluated by Western blots. The baseline HMGB1 levels were decreased both in vitro (A) and in vivo (B) in ASC KO group, as compared with WT controls. Representative of three experiments.
Suppl. Figure 5: Liver histopathology (A) and Suzuki’s scores (B) in WT mice infused with rHMGB1 immediately at reperfusion (0.8mg/kg, i.p. after 90min of ischemia). Sham controls underwent the same procedure without vascular occlusion. Unlike in sham controls, treatment with rHMGB1 augmented hepatocellular injury in WT mice subjected to liver IR. Magnification x200. Representative of n=4/group. (*p<0.0001).
Primers used in qRT-PCR studies.
Acknowledgments
Financial Support: NIH Grants RO1 DK062357 (JWKW); AI056154 (GH); The Diann Kim and The Dumont Research Foundations.
List of Abbreviations
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- BMMs
bone marrow derived-macrophages
- HMGB1
high mobility group box 1
- IRI
ischemia/reperfusion injury
- MPO
myeloperoxidase
- sALT
serum alanine aminotransferase
- TLR4
Toll-like receptor 4
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling
- WT
wild-type
References
- 1.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 3.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 4.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis. 1995;115:89–98. doi: 10.1016/0021-9150(94)05503-b. [DOI] [PubMed] [Google Scholar]
- 6.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 7.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 12.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S, Yamamoto T, et al. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol. 2011;187:4890–4899. doi: 10.4049/jimmunol.1100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaherian K, Liu JF, Wang JC. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978;199:1345–1346. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 18.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 19.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, Busuttil RW, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kamo N, Shen XD, Ke B, Busuttil RW, Kupiec-Weglinski JW. Sotrastaurin, a protein kinase C inhibitor, ameliorates ischemia and reperfusion injury in rat orthotopic liver transplantation. Am J Transplant. 2011;11:2499–2507. doi: 10.1111/j.1600-6143.2011.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 28.Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 30.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. 25. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-κB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol. 2007;211:759–770. doi: 10.1002/jcp.20992. [DOI] [PubMed] [Google Scholar]
- 31.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 32.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 33.Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, Erlandsson-Harris H, Tarkowski A. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003;48:1693–1700. doi: 10.1002/art.11028. [DOI] [PubMed] [Google Scholar]
- 34.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 35.Oshima K, Yabata Y, Yoshinari D, Takeyoshi I. The effects of cyclooxygenase (COX)-2 inhibition on ischemia-reperfusion injury in liver transplantation. J Invest Surg. 2009;22:239–245. doi: 10.1080/08941930903040080. [DOI] [PubMed] [Google Scholar]
- 36.Rissanen A, Howard CP, Botha J, Thuren T for the Global Investigators. Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes Metab. 2012 doi: 10.1111/j.1463-1326.2012.01637.x. (In press) [DOI] [PubMed] [Google Scholar]
- 37.Koné-Paut I, Piram M. Targeting interleukin-1β in CAPS (cryopyrin-associated periodic) syndromes: What did we learn? Autoimmun Rev. 2012;12:77–80. doi: 10.1016/j.autrev.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Vanderschueren S, Knockaert D. Canakinumab in Schnitzler Syndrome. Semin Arthritis Rheum. 2012 doi: 10.1016/j.semarthrit.2012.06.003. (In press) [DOI] [PubMed] [Google Scholar]
- 39.Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, Aaronson SA, et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 41.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1: Representative immunohistochemical staining for HMGB1 in hepatocytes (A/B) or infiltrating macrophages/monocytes (C/D) harvested from WT vs. ASC KO livers (6h of reperfusion after 90min of warm ischemia). Magnification ×400, n=3–4/group (*p<0.0001).
Suppl. Figure 2: Histopathology of ASC-deficient livers at 90min of warm ischemia only (A) vs. 90min ischemia plus 6h reperfusion (B). Representative of n=3–5/group; Magnification x200.
Suppl. Figure 3: BMMs from ASC KO or WT mice were cultured with rHMGB1 (1μg/ml) for 24h. The expression of cleaved caspase-1 and TLR4 was evaluated by Western blots. rHMGB1 supplement increased the expression of cleaved caspase-1/TLR4 (A), and IL-1β (B, p<0.01) in both ASC-deficient and WT groups, compared with BMM controls without rHMGB1 adjunct. Representative of three experiments.
Suppl. Figure 4: BMMs and liver tissue proteins were isolated from ASC KO and WT mice. HMGB1 expression was evaluated by Western blots. The baseline HMGB1 levels were decreased both in vitro (A) and in vivo (B) in ASC KO group, as compared with WT controls. Representative of three experiments.
Suppl. Figure 5: Liver histopathology (A) and Suzuki’s scores (B) in WT mice infused with rHMGB1 immediately at reperfusion (0.8mg/kg, i.p. after 90min of ischemia). Sham controls underwent the same procedure without vascular occlusion. Unlike in sham controls, treatment with rHMGB1 augmented hepatocellular injury in WT mice subjected to liver IR. Magnification x200. Representative of n=4/group. (*p<0.0001).
Primers used in qRT-PCR studies.