Abstract
Rationale
Prior research has found that adults with attention-deficit/hyperactivity disorder (ADHD) show increased sensitivity to the impairing effects of alcohol (Weafer et al. 2009). However, these studies have focused exclusively on the ascending limb of the blood alcohol concentration (BAC) curve, and it is unclear whether these adults continue to show increased sensitivity during the later phase of the dose as BAC is declining.
Objective
This study tested the hypothesis that those with ADHD would display increased response to alcohol during the ascending limb of the BAC curve and less recovery from the impairing effects during the descending limb.
Methods
Adult social drinkers with ADHD and control adults completed measures of motor coordination, reaction time, and subjective intoxication twice following 0.64 g/kg alcohol and placebo. The measures were administered during the ascending limb of the BAC curve and again during the descending limb.
Results
During the ascending limb, alcohol reduced motor coordination, slowed reaction time (RT), and increased self-reports of subjective intoxication. Those with ADHD displayed greater impairment of motor coordination compared with controls. During the descending limb, controls reported diminished subjective intoxication and showed recovery from the impairing effects of alcohol on both their motor coordination and their RT. Those with ADHD showed reduced subjective intoxication and faster RT during this time, but they did not recover motor control.
Conclusions
The protracted time course of motor impairment in adults with ADHD despite reductions in subjective intoxication may contribute to poor decision making and diminished behavioral control in this group.
Keywords: acute recovery, alcohol impairment, motor control, response time, ADHD, at-risk drinkers
Introduction
A single dose of alcohol produces a time-dependent change in blood alcohol concentration (BAC) known as the blood alcohol curve. BAC initially rises rapidly to a peak and then begins to decline gradually. Laboratory studies of the acute behavioral effects of alcohol often measure the response to the drug early after drinking during the ascending limb. However, it is now well recognized that a dose of alcohol can exert biphasic effects on behavior (Martin et al. 1993). As BAC rises, drinkers report stimulation and euphoria and display pronounced behavioral impairment (e.g., reduced motor coordination, slowed response time [RT]). By contrast, as BAC begins to decline, behavioral and subjective effects can abate even at elevated BACs (70–80 mg/100 ml) (Fillmore et al. 2005; King et al. 2002; Portans et al. 1989). Diminished perceptions of intoxication during this time might lead drinkers to perceive themselves as “sober” despite lingering impairments, possibly resulting in risky decision-making, such as deciding to drive or engage in other hazardous activities.
Such disagreement between perceived intoxication and actual impairment might be particularly important to individuals who are more vulnerable to the impairing effects of alcohol. For example, we have previously shown that adults with attention-deficit/hyperactivity disorder (ADHD) displayed greater behavioral impairment following a dose of alcohol than do their nonclinical peers (Weafer et al. 2009, 2008). However, these studies tested the effects of alcohol in those with ADHD early after alcohol administration as BAC was rising and did not examine behavioral responses during the declining limb of the BAC curve. As such, it is not clear if their increased behavioral response to alcohol on the ascending limb might also be accompanied by less recovery from impairment as BAC begins to decline. In fact, no study to date has examined the behavioral effects of alcohol in adults with ADHD during the later phase of the dose when BAC is declining. In the current study, we compared the behavioral and subjective effects of alcohol in groups of adults with and without ADHD during the descending limb of the BAC curve.
Much has been learned about how the effects of alcohol change over the time-course of a dose, particularly after drinking stops and BAC begins to decline. Researchers have sought to identify factors that influence the rate and consistency with which people acutely recover from alcohol impairment, and prior work has focused on the types of behaviors being assessed (e.g., motor control versus inhibitory control) and characteristics of the drinker (i.e., drinking habits). Investigations examining acute recovery from alcohol have shown that as BAC begins to decline some functions recover more quickly than others. In a review of the literature, Schweizer and Vogel-Sprott (2008) found that as BAC declines humans typically show early recovery on measures of RT, but recovery from alcohol impairment of response accuracy is less consistent. Several studies have shown evidence for early recovery of motor control (Haubenreisser and Vogel-Sprott 1987; Post et al. 1998). In terms of subjective impairment, there is evidence that drinkers are subjectively less intoxicated during the descending limb, even when those drinkers continue to show behavioral impairment (Fillmore et al. 2005; Ostling and Fillmore 2010).
Other studies have examined how drinkers’ characteristics can influence their rate of recovery. Fillmore and Weafer (2012) found that as BAC began to decline binge drinkers showed recovery on measures of RT and motor control, whereas infrequent drinkers did not display recovery on either task. Interestingly, both groups showed acute reductions in subjective intoxication. Unlike behavioral impairment, acute reduction in subjective intoxication appears to occur consistently in drinkers regardless of their drinking habits. Indeed, Ostling and Fillmore (2010) found that moderate social drinkers experienced acute reductions in subjective intoxication soon after BAC began to decline. Numerous other studies have demonstrated similar reductions in subjective alcohol effects in groups with a range of drinking habits (Ekman et al. 1964; King et al. 2002; Portans et al. 1989). Individuals who feel less impaired during the descending limb despite their continued behavioral impairment may misjudge their own sobriety due to their lack of perceived intoxication. Under these circumstances, such individuals could make decisions (e.g., deciding to drive) that are risky given their level of behavioral impairment, but they may be unaware of the risk associated with these decisions due to their lowered subjective intoxication. Indeed, several studies have demonstrated that subjective intoxication, rather than BAC or behavioral impairment, informs a drinker’s decision to drive (Beirness 1987; Marczinski and Fillmore 2009; Quinn and Fromme 2012).
The dissociation between acute reductions in subjective intoxication and behavioral impairment may confer greater risk for individuals who might be vulnerable to the impairing effects of alcohol, such as adults with ADHD (e.g., Roberts et al. 2012). This increased sensitivity to alcohol in adults with ADHD is of clinical interest because these individuals are at an increased risk to abuse alcohol (Molina et al. 2003; Glass and Flory 2012). These adults show increased sensitivity to alcohol’s impairing effects on inhibitory control (Weafer et al. 2009), driving ability (Weafer et al. 2008), and attentional control (Barkley et al. 2006). These studies only examined responses to alcohol during the ascending limb of the BAC curve, so it is currently unclear whether adults with ADHD might also display less recovery from impairment later under the dose as BAC begins to decline. In addition to increased responses to alcohol, adults with ADHD appear to underestimate their own impairment. We found that despite displaying greater impairment of driving ability from alcohol relative to controls, adults with ADHD rated themselves as being less impaired and more willing to drive (Weafer et al. 2008). Thus, it appears that adults with ADHD may underestimate the effects of alcohol on their performance. Moreover, this dissociation between subjective and behavioral effects of alcohol could become more pronounced during the declining limb of the BAC curve when subjective intoxication diminishes.
In the current study, we tested acute recovery from alcohol impairment in adults with ADHD and a group of control adults. Participants’ motor control, reaction time, and subjective intoxication were tested under two doses of alcohol (i.e., placebo, 0.64 g/kg). To assess acute reductions in response to alcohol, participants completed the battery twice during each dose: once as BAC ascended (i.e., Test 1) and again during the descending limb of the BAC curve (i.e., Test 2). Consistent with previous findings, we predicted that that both groups would report diminished subjective intoxication as BAC declined (Weafer et al. 2008, 2009). However, given evidence that drinkers with ADHD might be more vulnerable to the behavior-impairing effects of alcohol, we predicted that this group may not show recovery from alcohol impairment during the time-course studied.
Method
Participants
Participants were 19 adults with ADHD and 19 adults with no history of ADHD. Participants were recruited through advertisements seeking adults with and without ADHD for a study of the effects of alcohol on computer tasks. Participation was limited to individuals who were between the ages of 21 and 29 and had no uncorrected vision problems. Individuals who reported taking psychotropic medication other than psychostimulant medication for ADHD did not participate. Similarly, individuals with past or current severe psychiatric diagnoses (e.g., bipolar disorder, schizophrenia), as determined by their self report, were not invited to participate. Those who reported infrequent drinking or symptoms of alcohol dependence, as determined by a score of 5 or higher on the Short Michigan Alcoholism Screening Test (Selzer et al. 1975), were not invited to participate. Recent drug use was assessed through urine analysis, and those who tested positive for the presence of drug metabolites (excluding metabolites of tetrahydrocannabinol and, in the ADHD group, amphetamine) were discontinued. One participant in the control group and three participants in the ADHD group reported smoking regularly (i.e., more than 1 cigarette/day). Female participants were tested for HCG to verify that they were not pregnant. The University of Kentucky Medical Institutional Review Board approved the study.
To ensure that members of the ADHD group experienced symptomatology severe enough to necessitate medication, only volunteers who were currently prescribed medication for ADHD were invited to participate. Members of the ADHD group reported several different prescriptions, including mixed amphetamine salts (n = 10), methylphenidate (n = 5), lisdexamphetamine (n = 3), and dexmethylphenidate (n = 1). Prescription status was visually confirmed by the experimenter during the first session. Participants were asked to abstain from taking their medication for at least 24 hours prior to each session to ensure that they were unmedicated during the testing sessions. Participants verbally confirmed their compliance with this requirement at the beginning of each session.
Materials
Diagnostic method
ADHD diagnosis was also validated by self-report measures of ADHD symptomatology. Participants in the ADHD group were required to meet symptoms-based criteria on two measures of ADHD symptomatology, including the Conners Adult ADHD Rating Scale—Long Form (CAARS—S:L; Conners et al. 1999) and the Barkley Adult ADHD Rating Scale (BAARS; Barkley 2011). The CAARS is a 66-item questionnaire that measures the core symptoms and associated features of adult ADHD. The BAARS is an 18-item questionnaire that measures the core symptoms of adult ADHD according to the DSM-IV criteria. The diagnostic criteria was a T score greater than 65 on either DSM-IV symptom total subscale of the CAARS as well as a symptom count of four or more in the corresponding symptom cluster on the BAARS, as recommended by Barkley (2010). Both of these measures are sufficiently reliable and valid instruments for identifying adults with ADHD (Barkley 2011; Erhardt et al. 1999). Information about functional impairment, childhood history of ADHD symptomatology, and alternative sources of ADHD symptomatology (e.g., history of traumatic brain injury) was gathered in a clinical interview with a master’s level clinician (WR). Complex cases were reviewed by a licensed clinical psychologist (RM) with over 30 years experience diagnosing ADHD. A similar method of diagnostic confirmation has been successfully used by this research group in other studies (Roberts et al 2011a, 2011b). Participants in the control group who met criteria on either measure were discontinued from the study.
Grooved pegboard task
The grooved pegboard task (Lafayette Instruments, Lafayette, IN) was used to measure motor coordination. The pegboard task consists of a 5 × 5 in. metal surface that contains 25 keyhole-shaped slots arranged in five rows of five holes. Each of these holes has a large rounded side and a smaller square side. The orientation of the groove in each hole varies such that no two adjacent holes have the same orientation. Each peg is 3 mm in diameter and 2.5 cm long. Each peg also has a rounded side and a grooved side (with the groove running down the peg vertically). Pegs fit into the holes of the board as a key would fit into a lock. Participants were required to pick up the pegs one at a time and place them into the holes, filling in one row at a time from left to right before moving to the next row. Participants completed the pegboard task four times during each test. This task required approximately 5 minutes to complete.
Two-choice response time (Choice RT) task
RT was measured by a two-choice response time task that was operated using E-prime Experimental Generation software (Schneider et al. 2002) and performed on a personal computer. Participants were instructed to respond as quickly and as accurately as possible to the presentation of the letter X or O during each trial. They were instructed to press the (“) and (/) keys in response to the letter O and X, respectively.
Each test included 90 trials. Each trial consisted of the following sequence of events: (a) a fixation point ( + ) displayed for 800 ms; (b) a blank white screen displayed for one of three stimulus onset asynchronies (SOAs = 100 ms, 400 ms, 900 ms); (c) the stimulus presented for 1000 ms or until the response occurred; (d) a feedback screen that presented the RT in ms, and (e) an intertrial interval of 700 ms separating the trials. Trials were presented in a random order. The different SOAs between the targets encouraged participants to pay attention and prevented participants from anticipating the exact onset of the targets. A test required approximately 5 minutes to complete.
Subjective Intoxication
Subjective intoxication was measured on a visual analogue scale. Participants rated their degree of subjective intoxication by clicking the position on a vertical line representing the extent to which they “feel intoxicated” on a scale ranging from one (not at all) to nine (very much). This scale required approximately 1 minute to complete.
Assessment of Drinking Habits
Participants’ drinking habits were assessed using the timeline follow-back procedure (Sobell and Sobell 1992), which assessed daily drinking patterns over the past 3 months. Participants’ also completed the personal drinking habits questionnaire (Vogel-Sprott 1992). This measure assessed how many times each week they typically consume alcohol (frequency) and the number of drinks consumed during a typical drinking session (quantity). This measure is commonly used in research (Vogel-Sprott, 1992), and it was included in the current study to allow potential comparisons with other studies reporting quantity and frequency measures of alcohol consumption.
Procedure
Eligible participants made appointments to come to the laboratory for three sessions, including a familiarization session and two dose-challenge sessions. Participants were required to fast for 4 hours prior to each dose-challenge session. They were instructed to abstain from consuming alcohol or using other psychoactive drugs, including their stimulant medication, during the 24 hours preceding each session.
Familiarization session
All participants completed a familiarization session during which they became acquainted with laboratory procedures, completed questionnaires, provided informed consent for participation, and completed the Kaufman Brief Intelligence Test (Kaufman and Kaufman 2004). Volunteers who did not meet criteria for participation in the study were paid $10 and discontinued.
Test sessions
Task performance was tested under two doses of alcohol: 0.0 g/kg (placebo) and 0.64 g/kg. Doses were administered on separate days in a counterbalanced order. Sessions were separated by a minimum of one day and a maximum of one week. Alcohol doses were calculated on the basis of body weight and administered as absolute alcohol mixed with three parts carbonated soda. The 0.64 g/kg alcohol dose produces an average peak BAC of 80 mg/100 ml at approximately 65 min and begins to decline at about 75 min (Fillmore et al. 2005; Ostling and Fillmore 2010). The placebo dose (0.0 g/kg) consisted of a volume of carbonated mix that matched the total volume of the 0.64 g/kg alcohol drink. A small amount (3 ml) of alcohol was floated on the surface of the beverage. It was sprayed with an alcohol mist that resembled condensation and provided a strong alcoholic scent as the beverage was consumed.
All drinks were consumed in six minutes. Participants performed the 25 min test battery (choice RT task, pegboard task, and subjective intoxication scale) twice after each dose. Test 1 occurred 45–70 minutes post administration. This time interval corresponded to the late ascending portion of the BAC curve in the active dose condition. Test 2 occurred 105–130 min post administration. In the active dose condition, this time interval corresponded to the early phase of the descending limb, beginning approximately 30 min after the peak BAC. The tasks were completed at standardized times, and the grooved pegboard, choice RT, and visual analogue scale were completed 40, 60, and 70 minutes post administration for Test 1 and 100, 120, and 130 minutes post administration for Test 2, respectively. Each testing session required 150 minutes to complete.
BACs were determined from expired air samples measured by an Intoxilyzer, Model 400 (CMI, Inc., Owensboro, KY). BACs were measured at 30, 50, 70, 90, 110, 130, and 150 min after drinking began. Breath samples were also obtained at these times during the placebo session, ostensibly to measure BACs. Once the testing was finished, participants remained at leisure in a lounge area until their BACs, which were monitored at 20-minute intervals, reached 20 mg/100 ml or below. Participants received a meal during this leisure time and were allowed to watch movies and read magazines. Transportation home was provided as needed. Upon completing the final session, participants were paid and debriefed.
Criterion Measures and Data Analyses
Motor Coordination
The grooved pegboard task measured participants’ motor coordination as the time (sec) required to insert the pegs into the board averaged across the four trials. Faster mean completion times indicated better motor control.
Response time
The choice RT task measured participants’ RT as the mean RT of accurate responses and response accuracy. Responses with RTs less than 100 ms and greater than 1000 ms were excluded. These outliers were infrequent and occurred on less than 1% of trials.
All dependent measures were analyzed by a 2 group (ADHD versus control) × 2 dose (0.0 g/kg vs. 0.64 g/kg) × 2 test (Test 1 – ascending limb vs. Test 2 – descending limb) mixed-design analysis of variance (ANOVA) in which group was the between-subjects factor and dose and test were within subject factors. A priori t tests were used to directly test for recovery from impairment from Test 1 to Test 2 under 0.64 g/kg alcohol within each group.
Results
Drinking Habits and Demographics
Participants’ self-reported drinking habits and demographic information are presented in Table 1. There were no significant differences in drinking habits or demographic variables between groups.
Table 1.
Group comparisons on demographic characteristics, diagnostic information, and self-reported drinking habits
| Group
|
t | d | ||||
|---|---|---|---|---|---|---|
| Control (n = 19) | ADHD (n = 19) | |||||
|
| ||||||
| Mean | SD | Mean | SD | |||
|
|
|
|
|
|||
| Demographic | ||||||
| Age | 23.4 | 2.1 | 22.5 | 1.7 | 1.5 | 0.5 |
| Gender (% male) | 78.9 | 78.9 | ||||
| Education | 16.7 | 0.9 | 16.7 | 1.5 | 0.1 | 0.03 |
| IQ: Verbal | 106.5 | 12.6 | 107.0 | 14.0 | 0.1 | 0.03 |
| IQ: Nonverbal | 107.1 | 13.0 | 102.9 | 15.2 | 0.9 | 0.30 |
| IQ: Composite | 107.3 | 11.3 | 106.0 | 15.5 | 0.3 | 0.09 |
| Diagnostic | ||||||
| CAARS | ||||||
| DSM-IA | 45.4 | 8.8 | 78.4 | 7.9 | 12.1* | 4.03 |
| DSM-HI | 41.6 | 7.3 | 71.4 | 12.2 | 9.1* | 3.03 |
| DSM-Tot | 42.6 | 8.6 | 80.3 | 7.7 | 14.2* | 4.73 |
| BAARS | ||||||
| IA | 0.3 | 0.8 | 4.8 | 2.3 | 8.0* | 2.67 |
| HI | 0.5 | 0.7 | 5.1 | 2.6 | 7.4* | 2.47 |
| Drinking Habits | ||||||
| TLFB | ||||||
| Drinking Days | 30.7 | 12.8 | 28.3 | 15.0 | 0.5 | 0.17 |
| Total Drinks | 143.2 | 92.5 | 192.3 | 189.7 | 1.0 | 0.33 |
| Total Drinks (log) | 4.7 | 0.7 | 4.9 | 0.9 | 0.5 | 0.17 |
| Drunk Days | 9.4 | 7.6 | 13.9 | 13.4 | 1.3 | 0.43 |
| Binge Days | 13.7 | 11.6 | 17.9 | 15.7 | 0.9 | 0.30 |
| PDHQ | ||||||
| Frequency | 2.5 | 1.1 | 2.3 | 1.3 | 0.4 | 0.13 |
| Quantity | 4.5 | 2.4 | 5.5 | 2.2 | 1.3 | 0.43 |
| Dose | 1.0 | 0.6 | 1.3 | 0.5 | 1.8 | 0.60 |
Note. Age is reported in years. CAARS scores are T-scores; DSM-IA is DSM-IV Inattentive Symptoms, DSM-HI is DSM-IV Hyperactive-Impulsive Symptoms; and DSM-Tot is DSM-IV ADHD Symptoms Total. BAARS scores are symptom counts. TLFB refers to the Timeline follow-back. PDHQ refers to the Personal Drinking Habits Questionnaire.
p < .05
Blood Alcohol Concentrations
Group differences in BAC were examined using a 2 (group) × 8 (time: 30, 50, 70, 90, 110, 130, and 150 min) mixed-design ANOVA. Neither the main effect of group, F (1, 36) = 2.3, p = .139, η2 = .060, nor the group X time interaction, F (1, 36) = 1.5, p = .185, η2 = .018 was significant. There was a significant main effect of time, F (1, 36) = 54.7, p < .001, η2 = .594, owing to the rise and decline in BAC over the time course of the testing session. BACs for both groups at each time point are presented in Table 2. The table shows that BACs during the beginning of Test 1 (77.4 mg/100 ml) were higher than during Test 2 (62.3 mg/100 ml). No detectable BACs were observed in the placebo condition.
Table 2.
Mean blood alcohol concentrations (mg/100 ml)
| Group | Time after drinking (min)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 50 | 70 | 80 | 90 | 110 | 130 | 150 | |
| Control | ||||||||
| M | 60.5 | 72.8 | 76.8 | 70.9 | 68.5 | 60.1 | 57.8 | 52.7 |
| SD | 14.8 | 12.1 | 9.8 | 10.8 | 10.7 | 10.4 | 12.4 | 11.2 |
| ADHD | ||||||||
| M | 67.7 | 81.9 | 79.8 | 75.6 | 71.1 | 64.6 | 59.9 | 52.4 |
| SD | 16.3 | 12.1 | 10.1 | 7.7 | 9.3 | 8.1 | 8.8 | 7.4 |
| Whole Sample | ||||||||
| M | 64.1 | 77.4 | 78.3 | 73.3 | 69.8 | 62.3 | 58.9 | 52.5 |
| SD | 15.8 | 12.8 | 9.9 | 9.5 | 9.9 | 9.5 | 10.6 | 9.4 |
Note. BACs were estimated from expired air samples.
Grooved Pegboard Task
Mean completion times for each group are plotted in Figure 1. The three-way ANOVA found a significant main effect of dose, F (1, 36) = 52.4, p < .001, η2 = .593, and test, F (1, 36) = 10.6, p = .002, η2 = .227, as well as a significant interaction between dose and group, F (1, 36) = 7.1, p = .012, η2 = .164. The three-way interaction was not significant, F (1, 36) = 1.9, p = .178, η2 = .047. As seen in Figure 1, alcohol slowed completion time in both groups compared to placebo, and the ADHD group was more impaired by alcohol relative to the control group. The figure shows group differences in acute recovery. The ADHD group showed no significant difference in their completion time between Test 1 and Test 2 under 0.64 g/kg, t (18) = 0.8, p = .215, d = .19. In contrast, the control group showed a significant reduction in completion time from Test 1 to Test 2, t (18) = 4.2, p < .001, d = .96.
Fig. 1.
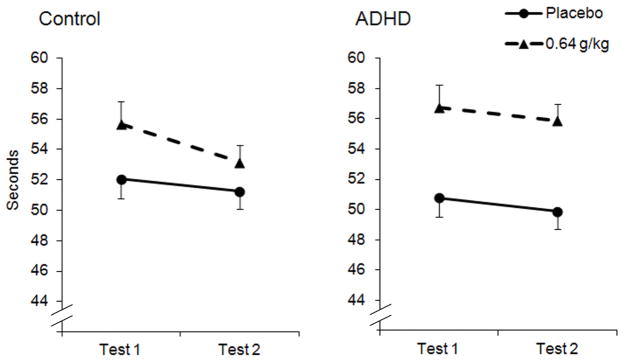
Mean (+ SE) completion times on the grooved pegboard task following 0.64 g/kg alcohol and placebo in the control and ADHD groups. Test 1 corresponds to the ascending limb and Test 2 corresponds to the descending limb.
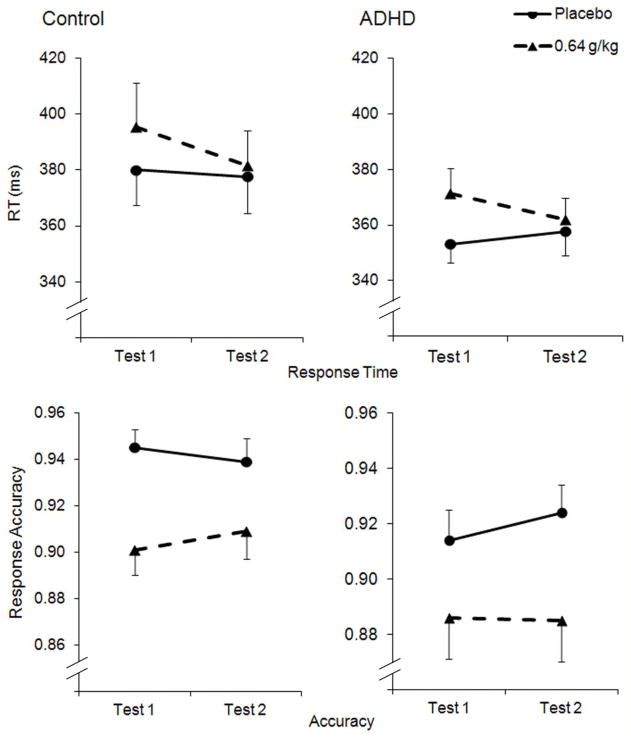
Choice RT Task
3.4.1 Response time
Figure 2 plots the mean RTs for each group at each test. The three-way ANOVA on RT found a significant main effect of dose, F (1, 36) = 6.9, p = .013, η2 = .160, owing to a general slowing in RT following 0.64 g/kg alcohol, and a significant dose X test interaction, F (1, 36) = 6.9, p = .014, η2 = .156, owing to recovery from impairment during Test 2 under 0.64 g/kg alcohol. Neither the main effect of group nor any interaction effect involving group reached significance, ps > .348. As seen in Figure 2, RT returned to sober levels during Test 2 of the 0.64 g/kg dose. The a priori t tests found a marginally significant speeding of RT between Test 1 and Test 2 under the 0.64 g/kg in the ADHD group, t (18) = 1.6, p = .064, d = 0.37, and a significant speeding of RT between the same tests in the control group, t (18) = 2.0, p = .032, d = 0.45.
Fig. 2.
Mean (+SE) response times and accuracy on the choice response time task follow 0.64 g/kg and placebo in the control and ADHD groups. Test 1 corresponds to the ascending limb and Test 2 corresponds to the descending limb.
Accuracy
Response accuracy is plotted separately for each group in Figure 2. The three-way ANOVA of accuracy scores found a main effect of dose, F (1, 36) = 37.5, p < .001, η2 = .505, owing to reduced accuracy following 0.64 g/kg alcohol. There was no other significant main effect or interaction. The a priori t tests did not find a significant difference in accuracy between Test 1 and Test 2 under 0.64 g/kg in either group, ps > .146.
Subjective Intoxication
Ratings of subjective intoxication are plotted in Figure 3. The three-way ANOVA found significant main effects of dose, F (1, 36) = 95.2, p < .001, η2 = .726, and test, F (1, 36) = 117.9, p < .001, η2 = .766, and a significant dose X test interaction, F (1, 36) = 36.6, p < .001, η2 = .498. Both groups showed increased subjective intoxication following 0.64g/kg alcohol relative to placebo. Further, under 0.64 g/kg alcohol, subjective intoxication declined considerably during Test 2 in both groups, and this degree of decline was similar between groups. Neither the main effect of group nor any interaction effect involving group reached significance, ps > .376. A priori t tests found that the reduction in subjective intoxication between tests under 0.64 g/kg was significant in the control group, t (18) = 8.7, p < .001, d = 2.00, and the ADHD group, t (18) = 8.5, p < .001, d = 1.95.
Fig. 3.
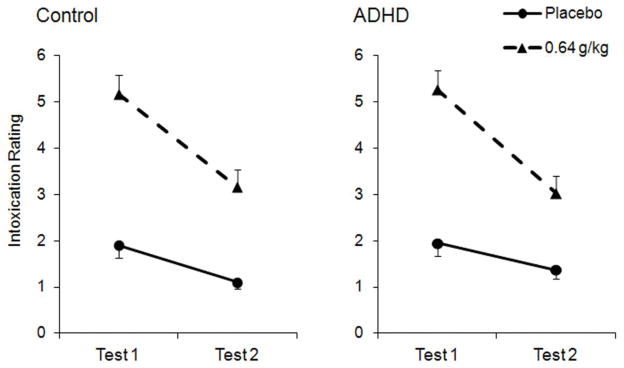
Mean (+ SE) subjective intoxication ratings following 0.64 g/kg alcohol and placebo in the control and ADHD groups. Test 1 corresponds to the ascending limb and Test 2 corresponds to the descending limb.
Covariate Analyses
Although there were no significant group differences in BACs or drinking habits, we reanalyzed each measure (i.e., motor coordination, RT, subjective intoxication) using a series of 2 (group) × 2 (dose) × 2 (limb) ANCOVAs that controlled for recent drinking habits (i.e., total drinks on the TLFB) and peak BAC (i.e., BAC 70 minutes post administration) to ensure that the group differences in alcohol response occurred independently of these variables. Total drinks was log transformed due to its positively skewed distribution, and the transformed variable is reported in Table 1. Results of the ANCOVAs were consistent with the ANOVAs reported. Specifically, inclusion of the covariates did not alter any main effects or interactions involving the group factor.
Discussion
This study measured changes in the behavioral and subjective effects of a single dose of alcohol as BAC ascended and began to decline in adults with and without ADHD. We hypothesized that control participants would recover from the impairing effects of alcohol on motor coordination and RT during the early phase of the descending limb, whereas the ADHD group would show no such recovery. The results were generally consistent with our hypothesis. Both groups showed alcohol impairment of motor control, and those with ADHD were more impaired than controls. The control group showed acute recovery from impairment of motor control during the descending limb, whereas those with ADHD were impaired to a similar degree during both limbs. The lack of recovery in the ADHD group is particularly noteworthy considering the reduction in BAC (i.e., 15.1mg/100ml) that occurred between Test 1 and Test 2. These group differences in response to alcohol and in recovery from impairment cannot be attributed to differences in drinking habits or BACs during testing, because the pattern of effects was retained even when controlling for these variables.
On the choice RT task, both groups showed alcohol impairment during the ascending limb of the active dose and acute recovery from impairment during the descending limb. Acute recovery of RT was particularly robust because performance returned to near sober levels during Test 2, despite participants’ elevated BACs. With regard to response accuracy on the choice RT task, both groups showed impairment in response to alcohol on the ascending limb, and neither group recovered from this impairment during the descending limb. Alcohol also increased self-rated levels of subjective intoxication compared with placebo, and this effect was similar in both groups. Likewise, subjective intoxication levels diminished during the declining limb in both groups.
The findings support our hypothesis that adults with ADHD would show protracted alcohol impairment on some measures of behavioral control (i.e., motor control). In understanding how this persistent motor impairment might act as a risk factor for those with ADHD, it is important to consider this finding in the context of drinkers’ subjective intoxication. Specifically, drinkers with ADHD showed pronounced declines in subjective intoxication during the descending limb. They reported approximately 60% less subjective intoxication during Test 2 compared with Test 1, despite their prolonged impairment of motor control. Given this lack of recovery of motor control in the ADHD group, their reduced subjective intoxication may lead them to overestimate their level of sobriety. Thus, drinkers with ADHD may feel as if they have reached a point of sobriety following a drinking session and decide to drive or engage in other activities contraindicated by alcohol intoxication. Indeed, subjective intoxication appears to be an important determinant of drinkers’ willingness to drive (Quinn and Fromme 2012). Further, motor control is one of many functions that contribute to driving ability (Groeger 2000; Weafer and Fillmore 2011), and the protracted impairment experienced by adults with ADHD may result in increased incidence of alcohol-related accidents and injuries. Young adults with ADHD symptoms are more likely than nonclinical adults to drive while intoxicated (Woodward et al. 2000) and to be involved in accidents (Barkley et al. 2002). Although sober-state deficits likely contribute to this increased risk of traffic accidents in adults with ADHD, it is also possible that protracted alcohol impairment may act as an additional risk factor.
It is interesting that the ADHD group showed acute recovery of RT under alcohol, despite their lack of recovery of motor control. Evidence that the behaviors might recover from impairment at different rates as BAC declines fits with other recent evidence for the non-uniform recovery of behavioral functions under alcohol. For example, we have recently shown that binge drinkers display rapid recovery from alcohol impairment on measures of response activation (i.e., reaction time) but not on measures of response inhibition (Fillmore and Weafer 2012). Evidence that adults with ADHD show rapid and robust recovery from alcohol impairment with respect to their RT, but not their motor control, provides additional support for the notion that recovery from impairment might differ depending on the population studied and the behavior examined.
Task characteristics may have contributed to the differences in acute recovery between RT and motor control in the ADHD group. Performance feedback has been shown to improve performance in individuals with ADHD (Slusarek et al. 2001). In the present study, the tasks differed in the degree of performance feedback that was available to participants. The choice RT task provided participants with explicit information about their RT and accuracy after each trial. In contrast, no explicit feedback was provided about the time required to complete trials on the pegboard task. This raises the possibility that performance feedback on the choice RT task facilitated recovery in the ADHD group — a level of recovery that otherwise might not have been evident if no feedback was provided. Indeed, performance feedback can facilitate the development of acute tolerance (Post et al. 1998; Vogel-Sprott 1992). This task difference in feedback availability may explain the more robust acute recovery from impairment on the choice RT task in both groups. As such, it is important to better understand how recovery from alcohol impairment can be influenced by the characteristics of the task, as well as the characteristics of the drinkers.
Another possibility is that acute recovery is associated with a drinker’s initial degree of impairment. In the current study, participants with ADHD only showed protracted impairment of motor control, the only function for which they showed heightened sensitivity during the ascending limb. On the choice RT task, the ADHD group was no more impaired than the control group during the ascending limb, and their recovery on this measure was comparable to that of controls. A similar pattern of increased initial sensitivity and reduced acute recovery has been shown in light social drinkers (Fillmore and Weafer 2012). This raises the possibility that increased initial sensitivity and reduced acute recovery occur due to a shared underlying mechanism that predisposes an individual to experience heightened impairment under the drug.
The current research contributes to our understanding of alcohol sensitivity in adults with ADHD; however, results should be interpreted in light of some limitations. First, the sample included only individuals who were between 21 and 29 years old, so generalizing these findings to adolescents and older adults with ADHD may not be appropriate. Second, our small sample size may have limited our ability to detect group differences. However, the pattern of effects did not change substantially when we statistically controlled for group differences in variables that are associated with sensitivity to alcohol (i.e., BAC, drinking habits). Third, we only observed participants’ responses to alcohol at two time points under each dose, and both of these time points occurred early in the time course. Although this allowed us to assess impairment at similar time points on the ascending and descending limbs of the BAC curve, we were not able to determine when adults with ADHD would return to sober levels of performance. Additional research will be required to determine at what point in the time course adults with ADHD fully recover from impairment. Fourth, we were not able to determine the reason why the adults with ADHD did not show recovery of motor control during the descending limb. An important future research direction will be to explore neuropharmacological mechanisms contributing to this group difference. Fifth, although we used a multi-step diagnostic method to identify adults with ADHD, our diagnostic method could have been strengthened by informant report data and a structured clinical interview. Sixth, although there were not any differences between groups in terms of subjective intoxication, it is possible that participants in the ADHD group would have shown differences if they were asked about their level of impairment. Future studies should use a wider range of questions to gather information on subjective alcohol effects. Finally, participants with ADHD were tested in an unmedicated state. It is possible that these adults would show acute recovery if they had taken their medication, and testing this possibility will be an important question for future research.
In conclusion, this study provides evidence that adults with ADHD experience protracted impairment of motor control after consuming alcohol. Despite this protracted impairment, these adults report diminished subjective intoxication over the same time period, which may lead to underestimation of impairment during the descending limb of the BAC curve. Thus, increased susceptibility to the impairing effects of alcohol in drinkers with ADHD is not only evident early under the dose as BAC ascends, but they may also require additional time to recover from some effects of the drug.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism grant R01 AA018274.
Footnotes
The authors have no conflict of interest to declare.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. Text Revision. [Google Scholar]
- Barkley RA. Barkley Adult ADHD Rating Scale. The Guilford Press; New York: 2011. [Google Scholar]
- Barkley RA. ADHD in adulthood: What the science says. The Guilford Press; New York: 2010. [Google Scholar]
- Barkley RA, Murphy KR, Dupaul GJ, Bush T. Driving in young adults with attention deficit hyperactivity disorder: Knowledge, performance, adverse outcomes, and the role of executive functioning. J Int Neuropsychol Soc. 2002;8:655–672. doi: 10.1017/s1355617702801345. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, O’Connell T, Anderson D, Connor DF. Effects of two doses of alcohol on simulator driving performance in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:77–87. doi: 10.1037/0894-4105.20.1.77. [DOI] [PubMed] [Google Scholar]
- Beirness DJ. Self-estimates of blood alcohol concentration in drinking-driving context. Drug Alcohol Depend. 1987;19:79–90. doi: 10.1016/0376-8716(87)90089-5. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow EP. Conners’ Adult ADHD Rating Scales. Multi-Health Systems; Toronto: 1999. [Google Scholar]
- Ekman G, Frankenhaeuser M, Goldberg L, Hagdahl R, Myrsten AL. Subjective and objective effects of alcohol as functions of dosage and time. Psychopharmacologia. 1964;6:399–409. doi: 10.1007/BF00429567. [DOI] [PubMed] [Google Scholar]
- Erhardt D, Epstein JN, Conners CK, Parker JDA, Sitarenios G. Self-ratings of ADHD symptoms in adults: II. Reliability, validity, and diagnostic sensitivity. J Attention Disord. 1999;3:153–158. [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Acute tolerance to alcohol in at-risk binge drinkers. Psychol Addict Behav. 2012;26:693–702. doi: 10.1037/a0026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass K, Flory K. Are symptoms of ADHD related to substance use among college students? Psychol Addict Behav. 2012;26:124–132. doi: 10.1037/a0024215. [DOI] [PubMed] [Google Scholar]
- Groeger JA. Understanding driving: applying cognitive psychology to a complex everyday task. Psychology Press; New York: 2000. [Google Scholar]
- Haubenreisser T, Vogel-Sprott M. Reinforcement reduces behavioral impairment under an acute dose of alcohol. Pharmacol Biochem Behav. 1987;26:29–33. doi: 10.1016/0091-3057(87)90528-4. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. American Guidance Service, Inc; Circle Pines: 2004. [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Acute alcohol tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychol Addict Behav. 2009;23:238–247. doi: 10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcohol Clin Exp Res. 2007;31:643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Mortzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: Application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 2006;22:202–210. [PubMed] [Google Scholar]
- Ostling EW, Fillmore MT. Tolerance to the impairing effects of alcohol on the inhibition and activation of behavior. Psychopharmacology. 2010;212:465–473. doi: 10.1007/s00213-010-1972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J of Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Portans I, White JM, Staiger PK. Acute tolerance to alcohol: changes in subjective effects among social drinkers. Psychopharmacology. 1989;97:365–369. doi: 10.1007/BF00439452. [DOI] [PubMed] [Google Scholar]
- Post RB, Tavano LA, Maddock RJ. Role of feedback in formation of acute tolerance to alcohol. J Stud Alcohol. 1998;59:723–730. doi: 10.15288/jsa.1998.59.723. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Event-level associations between objective and subjective alcohol intoxication and driving after drinking across the college years. Psychol Addict Behav. 2012;26:384–392. doi: 10.1037/a0024275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlow R. A quantitative theory of acute tolerance to alcohol. Psychopharmacology. 1994;114:1–8. doi: 10.1007/BF02245438. [DOI] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Separating automatic and intentional inhibitory mechanisms of attention in adults with attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2011a;120:223–233. doi: 10.1037/a0021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Linking impulsivity and inhibitory control using manual and oculomotor response inhibition tasks. Acta Psychol. 2011b;138:419–428. doi: 10.1016/j.actpsy.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Drinking to distraction: does alcohol increase attentional bias in adults with ADHD? Exp Clin Psychopharmacol. 2012;20:107–117. doi: 10.1037/a0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime User’s Guide. Pittsburgh, PA: Psychology Software Tools Inc; 2002. [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press; Totowa: 1992. pp. 41–72. [Google Scholar]
- Vogel-Sprott M. Alcohol tolerance and social drinking: learning the consequences. Guilford Press; New York: 1992. [Google Scholar]
- Weafer J, Camarillo D, Fillmore MT, Milich R, Marczinski CA. Simulated driving performance of adults with ADHD: comparisons with alcohol intoxication. Exp Clin Psychopharmacol. 2008;16:251–263. doi: 10.1037/1064-1297.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Acute tolerance to alcohol impairment of behavioral and cognitive mechanisms related to driving: drinking and driving on the descending limb. Psychopharmacology. 2012;220:697–706. doi: 10.1007/s00213-011-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, Milich R. Increased sensitivity to the disinhibiting effects of alcohol in adults with ADHD. Exp Clin Psychopharmacol. 2009;17:113–121. doi: 10.1037/a0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM, Horwood LJ. Driving outcomes of young people with attentional difficulties in adolescence. J Am Acad Child Adolesc Psychiatry. 2000;39:627–634. doi: 10.1097/00004583-200005000-00017. [DOI] [PubMed] [Google Scholar]