Abstract
Pain sometimes has a throbbing, pulsating quality, particularly when it is severe and disabling. We recently challenged the presumption that this throbbing quality is a sensory experience of arterial pulsations, but were unable to offer an alternative explanation for its rhythmic character. Here we report a case study of a woman with a history of daily headache consistent with the diagnosis of chronic migraine, but whose throbbing quality persisted long after the resolution of the headache. This chronic, daily, and persistent throbbing sensation, in the absence of headache pain, prompted closer examination for its neurophysiological correlate. By simultaneously recording the subjective report of the throbbing rhythm, arterial pulse, and high-density electroencephalogram (EEG), we found that the subjective throbbing rate (48 ± 1.7 bpm) and heart rate (68 ± 2 bpm) were distinct, in accord with our previous observations that the two are unrelated. On spectral analysis of the EEG we found that the overall amount of activity in the alpha range (8 to 12 Hz), or alpha power, increased in association with greater throbbing intensity. In addition, we also found that the rhythmic oscillations of overall alpha power, the so-called modulations of alpha power, coincided with the timing of the throbbing rhythm, and that this synchrony, or coherence, was proportional to the subjective intensity of the throbbing quality. This index case will motivate further studies whose aim is to determine whether modulations of alpha power could more generally represent a neurophysiological correlate of the throbbing quality of pain.
Introduction
Descriptions of pain quality, such as throbbing, aching, or sharp pain, provide invaluable clues to the underlying diagnosis. However, we know very little about the neurophysiological events underlying these clinically relevant pain qualities. Throbbing, pulsatile pain is one such quality that has long been presumed to arise in the periphery, as a sensory experience of arterial pulsations. Accordingly, throbbing pain that is frequently associated with tissue injury and inflammation, such as post-surgical pain [3], bone fracture [12], cervical artery dissection [2], giant cell arteritis [40], and dental pain [44] is often taken as evidence in support of this presumption.
However, a throbbing quality is also highly prevalent in a range of other pain conditions more specifically associated with nerve injury, such as carpal tunnel syndrome [22] and post-herpetic neuralgia [37]. Even isolated lesions affecting the central nervous system, such as post-spinal cord injury pain [13], post-traumatic brain injury pain [38], multiple sclerosis [19] and late-onset chronic pain after a thalamic stroke [27] are also associated with throbbing pain. Isnard and colleagues recently described another compelling example of a purely central lesion related to throbbing pain in a patient whose episodic sensory seizures were manifested by a throbbing pain sensation, and whose clinical and electrographic abnormalities resolved with the ablation of a focal area of cortical dysplasia within the right posterior insula [21].
The presence of throbbing pain in such a wide range of physiologically distinct conditions should, by itself, undermine the widely held presumption that the throbbing quality of pain is a sensory experience of arterial pulsations. In fact, our recent studies of the throbbing rhythm in patients with migraine [1] and dental pain [34] formally excluded any association between the rhythm of throbbing pain and the timing of any rhythms that could be derived from hemodynamic activity, such as venous flow or cerebrospinal fluid pressure. However, we were unable to offer any evidence for an alternative source for this rhythm. In the present case report we describe a woman with a resolved history of chronic migraine and chronic daily headache due to medication overuse, but whose throbbing sensations persisted chronically, long after the resolution of the chronic daily headache.
In considering the possibility that there is a neurophysiological representation of throbbing pain, the high temporal resolution of the EEG is ideally suited to follow these rhythmic events. Among the most consistent neurophysiological correlates of pain are changes in spectral power at lower frequencies, including the alpha rhythm (8–12 Hz), in clinical pain [41] as well as in experimental models of pain [4,5,45]. It was also of particular interest that the subject’s throbbing rate (1 Hz or less) closely approximated the rate of the rhythmic modulation of alpha power, which has been implicated as having a role in brain signaling in health and disease, and may also be relevant to the processing of pain [18,48].
Methods
Recording session
The subject refrained from taking any acute pain medications during the 72 hours prior to the evaluation day. As was customary for the subject, the throbbing intensity increased markedly as the day progressed. She recorded the psychophysical properties of the throbbing quality, while we simultaneously recorded the arterial pulse. In addition, we performed high-density EEG recordings in two sessions each lasting 5 minutes, one at mid-day and the other at the end of the afternoon. Each of these recording sessions was primarily a resting-state session with her fixing her gaze at a visual fixation target. A short task period was inserted into each session during which she pressed a key on an instrument panel to indicate the throbbing rhythm. Based on the relative intensity of the throbbing intensity experienced by the subject, the two EEG recording sessions were referred to as “weak” and “strong” intensity throbbing sessions, respectively.
EEG recordings
The electroencephalogram (EEG) was recorded using a 128-channel BioSemi Active System at a sampling rate of 1024Hz. The visual fixation target was delivered using E-Prime software and the psychophysical responses were registered using a Berisoft EXKEY microprocessor logic pad.
Data preprocessing and spectral analysis
EEG data were preprocessed as follows: (1) bandpass filtering with cutoffs set at 0.53 and 50Hz and downsampling to a frequency of 250Hz, (2) re-referencing each channel against the average reference, (3) using independent component analysis (ICA - EEGLAB 7.2 tutorial, sccn.ucsd.edu/eeglab) to remove muscle artifacts, movement artifacts and excessive eye blinks. To mitigate the impact of volume conduction, we used BESA 5.2 to transform voltage time series into current source density (CSD) time series.
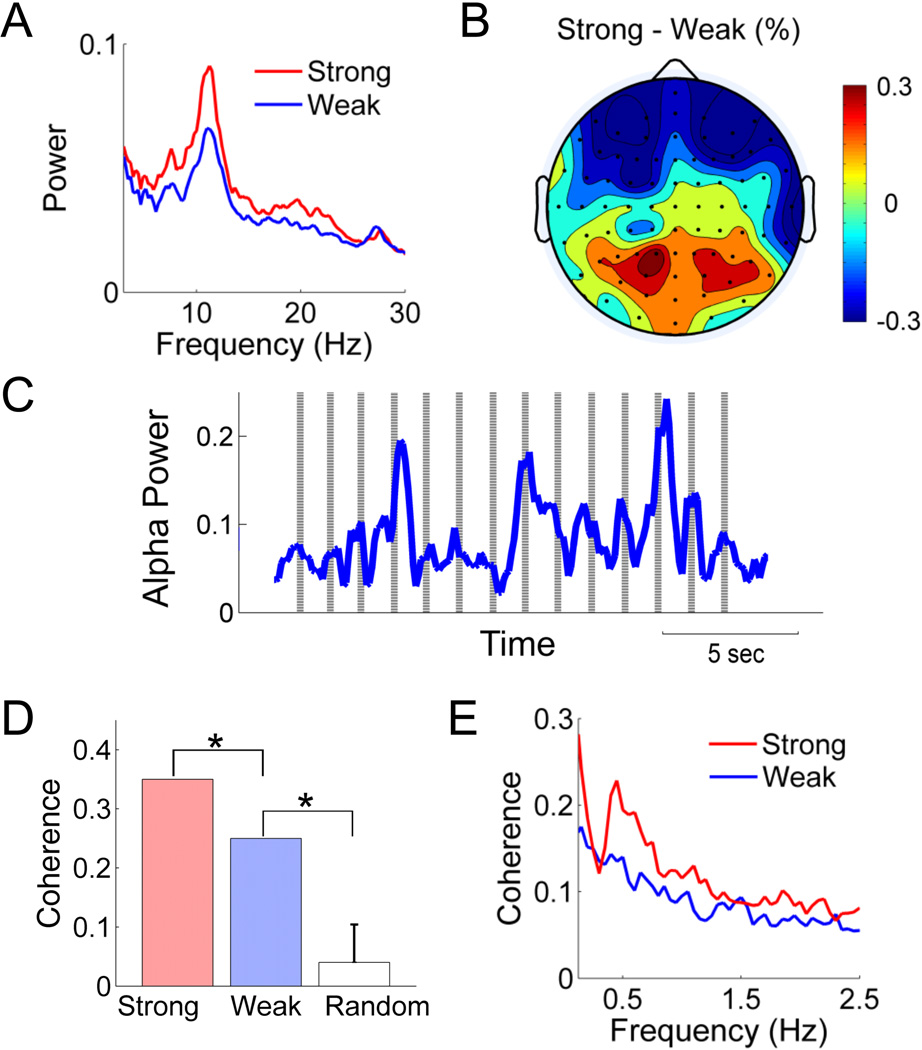
The overall power spectra associated with weak or strong throbbing (Figure 1A) is the average power spectra from all channels for both recording sessions. We also created a topographic map of the channel-by-channel difference in average alpha power (Figure 1B) using EEGLAB software. The statistical significance of the difference was assessed by paired t-test.
Figure 1.
Spontaneous alpha power (power between 8–12Hz) is modulated by the intensity of the throbbing sensation. (A) Power spectra from a parietal-occipital channel. (B) Topography of the percentage change in alpha power calculated at each electrode by (Astrong−Aweak)/Aweak, where A stands for alpha power. (C) The point process of reported throbbing onset is plotted as vertical lines on which alpha power time series in PO3 channel was superimposed during stronger throbbing session. (D) Coherence around 0.7 to 1Hz between the alpha power time course and the throbbing reporting events in (C). The coherence is significant compared to random permutation (p< 0.05) under either weak or strong throbbing session. (E) Coherence of amplitude envelop of alpha oscillation was calculated between pairwise posterior channels, and then averaged. The temporal dynamic of alpha powers is more synchronous when stronger throbbing sensation was reported. The difference between two conditions is significant under paired t-test across channels (p<0.01).
Rhythmic modulation of alpha power
In order to calculate the dynamic changes in alpha power, we applied a moving window of 500 ms in duration to the CSD time series, stepping forward in 4 ms increments. The spectral power within each window was calculated using the multitaper method (Mitra & Pesaran 1999) and integrated between 8 to 12Hz to yield alpha power. The resulting calculations describe a time series of alpha power with a sampling rate of 250 Hz, whose rhythmic fluctuations we term the modulation of alpha power (Figure 1C).
Coherence between rhythmic modulation of alpha power and throbbing events
In order to compare the rhythmic modulation of alpha power to the sequence of reported throbbing events, we transformed the record of reported throbbing events from a series of discrete key presses into a continuous waveform by convoluting the sequence of discrete events with a Gaussian kernel. The synchrony between the alpha power time series and the reported throbbing events was determined by measuring the coherence between these two waveforms (Figure 1D), where the window length was set at 10 s and the overlap was half the window length. We tested the significance of the coherence between the throbbing rhythm and the rhythmic modulation of alpha power, by comparing it to the null hypothesis, which was comprised of a large distribution of coherence values derived from pairing the recorded sequence of throbbing events with random samples of alpha power time series of equal length from the recording of resting state.
We assessed the change in alpha synchrony at the cortical network level by computing the coherence between the modulations of alpha power from all pairwise combinations of posterior channels during each of the two 5-minute resting state sessions (Figure 1E). The window length was 25 s and the overlap was half the window length.
Case History and Results
Case History
The subject was a 56-year old clinical psychologist with a prior headache history consistent with the ICHD-II diagnosis of migraine without aura [20], consisting of episodic throbbing frontal head pain accompanied by nausea, photophobia and phonophobia. The usual triggers included stress, lack of sleep, wine, and skipped meals, and she obtained substantial and consistent relief from oral sumatriptan. Her present clinical history began at the age of 49 when she was disabled by chronic low back pain due to multi-level degenerative disc disease, obtaining only partial pain relief on a daily regimen of oxycodone. However, concurrent with this chronic back pain and opioid use, the subject developed a chronic daily headache, consisting of dull holocephalic pressure, from which she no longer obtained complete relief from sumatriptan, NSAIDS, and caffeine containing combination analgesics.
At age 53, she underwent a multi-level laminectomy and stabilization of the L2–3, L3–4, and L4–5 intervertebral spaces, which together with continued physical therapy, resulted in a successful outcome for her chronic back pain. However the patient’s headache and daily opioid use continued until age 54, when the presumptive diagnosis of Medication Overuse Headache [20] prompted the withdrawal from all abortive medications, including opioids, triptans, NSAIDs, and caffeine. She also discontinued estrogen replacement therapy. The chronic daily headache gradually remitted over months, and by age 55 she returned to a stable baseline of episodic migraine attacks at a frequency of less than two per month, with a full or near-complete treatment response to oral sumatriptan.
However, despite the remission of her chronic daily headache, she noted the persistence of a chronic throbbing, pulsatile sensation located symmetrically in the frontal and bitemporal regions of her head. This sensation was present at all times, though the sensation became more intense at the end of the workday. At those times especially, the throbbing sensation was intrusive and distracting. There were no other provocative maneuvers, such as bending, coughing, valsalva, or physical exertion, though the throbbing sensation was aggravated by agitation and strong emotions. Leading up to and during the episodic migraine attacks, the throbbing sensation also became much more intense, but had the same rhythmic qualities. The only known palliative technique that she could find was through distraction, such as with vigorous physical activity. She reports that on occasion, should she pause from physical activity to note it, the rate and rhythm of the throbbing quality did not change appreciably.
An extensive imaging workup failed to reveal evidence for an arteriovenous fistula, cerebral sinus stenosis, or other abnormality that could account for a pulsatile tinnitus. Medication trials that included naproxen, sumatriptan, atenolol, verapamil, topiramate, valproic acid, and gabapentin provided no meaningful benefit or relief from the throbbing sensation. It was notable that atenolol produced a symptomatic bradycardia without any effect on the rate or quality of the throbbing sensation. At one year follow up from the time of evaluation and two years from the onset of symptoms, the subject continues to experience this throbbing sensation daily.
The subject expressed a strong interest in the neurophysiological basis of her present condition, and was offered the opportunity to characterize her throbbing experiences through traditional psychophysical and electrophysiological recording methods.
Psychophysical recording
The experience of throbbing on the day of the recording was typical for the subject, in which the throbbing intensity was relatively modest in the morning and had much greater intensity at the end of the afternoon. The subject practiced recording the rhythm of the throbbing perceptions into a digital recording device in the morning prior to the simultaneous recording of the EEG (see Methods). During the two afternoon EEG recording sessions, there was an interval increase in the subjective intensity of the throbbing experience, yet there was only modest variation in the rate of throbbing, at 44 and 48 bpm, respectively. In addition, the overall subjective throbbing rate of 48 ± 1.7 bpm was significantly slower than the subject’s regular heart rate of 68 ± 2 bpm (p<0.005, paired t-test).
Throbbing intensity influences alpha power
We compared the overall EEG spectral power between weak and strong throbbing conditions. Figure 1A shows the power spectra from a representative parietal-occipital channel, which demonstrates that the overall alpha power (8–12Hz) was higher in the session associated with the stronger throbbing pulsations. When represented topographically as the percent change in alpha power over the whole scalp, the increase in alpha power was significant (p<0.01), and most prominent over the posterior channels (Figure 1B). Concurrent with an increase posterior alpha power, we observed a decrease in alpha power over frontal electrodes (p< 0.01).
Throbbing pulsations synchronize with rhythmic modulation of alpha power
We also noted that the magnitude, or power, of alpha oscillations fluctuated over time in a rhythmic fashion, and that these fluctuations were in synchrony with the reported throbbing pulsations (Figure 1C). The coherence between the reported throbbing events (see Methods) and the simultaneously recorded rhythmic fluctuations of alpha power from the parietal-occipital lead (PO3) was significantly higher during the period associated with a strong subjective experience of throbbing (coherence=0.35), compared to that associated with the subjectively weak experience of throbbing (coherence=0.25, p<0.05). In addition, the coherence values from both of these states were stronger than those derived from a large sample of randomly chosen epochs from the resting state recording overlaid onto the record of throbbing events (p<0.05). Other electrodes over both posterior and frontal cortices exhibited similar effects.
In another critical control of whether the coupling between the EEG and the subjective report was an artifact of the motor task, we applied the same analysis of the rhythmic modulations of alpha power to the somatosensory/premotor region (electrodes C3/C5), where we found a coherence value of 0.08, which was not significantly higher than the null hypothesis (p>0.3).
To further examine the synchrony between the rhythmic fluctuations of alpha power and the simultaneous record of throbbing events, we measured the variability of the time lag between each throbbing event and the nearest peak of the alpha power time series, and found that the mean time lag was smaller during the strong throbbing session (0.24 sec) compared to the weak session (0.43 sec), which matched the patient’s own subjective report that the accuracy of her reports were greater during the strong throbbing session. In the session associated with strong throbbing, the average interval between adjacent throbbing events was 1.25 +/− 0.05 sec, which is close to the average interval between adjacent peaks in the rhythmic modulations of alpha power (1.20 +/− 0.49 sec), whereas in the session associated with the weak experience of throbbing, the average interval between adjacent throbbing events was 1.44 +/−0.11 sec, which is larger compared to the average interval between adjacent alpha power peaks of 1.18 + /− 0.45 sec.
Throbbing intensity modulates large-scale network synchrony
Concurrent with the increase in overall alpha power seen in Figure 1B, the pairwise coherence among the posterior EEG channels is also enhanced in the session with intense throbbing across a wide range of frequencies (Figure 1E). The increase in coherence is significant under the paired t-test (p<0.01). A similar increase in coherence was also found among the frontal channels.
Discussion
Sufferers of migraine headache often describe a range of somatosensory experiences that persist well after the resolution of the acute head pain. Those with chronic migraine also report migrainous symptoms that persist beyond the episode of head pain, such as photophobia, nausea, vertigo, or tinnitus [10], but a persistent throbbing, pulsatile sensation in the absence of pain is uncommon. However, the history of chronic daily headache associated with several well-recognized risk factors for chronic migraine, followed by its resolution following the effective management of these risk factors, provides a compelling clinical context for this unusual symptom.
Another term that could be used to describe this symptom might be “pulsatile tinnitus”, the term strongly implying the auditory perception of rhythmic, turbulent blood flow. However, extensive radiographic studies failed to find a vascular etiology, and in the present case there was no correspondence to arterial pulse. However, it may be of interest to hypothesize a clinical distinction between this migrainous throbbing experience, which is unrelated to arterial pulse, and pulsatile tinnitus, which is generally understood to have a vascular etiology. However, this operational distinction needs to be formally tested in a broader clinical context.
There are two other persistent rhythmic somatosensory symptoms that we are aware of with a close association with migraine. The first is a report of oscillating hyperacusis occurring prior to the onset of a migraine attack, termed “oscillocusis” by Whitman and Lipton [49], though this was a transient, self-limited event, best characterized as migraine aura. Second is the so-called mal de debarquement, a chronic illusory perception of a swaying, rocking or bobbing motion triggered by a prolonged exposure to passive rhythmic movement, such as riding on a boat [8]. However, the physiological basis for this chronic, rhythmic, somatosensory disorder could be of great relevance in that mal de debarquement is associated with changes in resting state metabolic activity and functional connectivity in areas of the brain associated with self-perception and somatosensory processing, such as the insula, amygdala, and middle temporal gyrus [9].
Non-invasive neurophysiological approaches such as EEG (as well as magnetoencephalography – MEG) represent important, clinically accessible, opportunities to understand fundamental aspects of somatosensory processing, including pain [18]. Investigators have reported complex changes in neuronal activity in those with chronic neurogenic pain [41] as well as in association with acute migraine [6,11,14,47], many features of which have been replicated in studies of acute experimental pain in healthy subjects [5,45]. Recent studies have found that local somatosensory region activity at gamma (30–100 Hz) frequencies correlate with pain intensity [42,50], while complex changes at lower frequencies may suppress resting activity in the alpha and beta (8–25 Hz) frequencies [39] or at least contribute differentially to differences in these ratings of pain intensity [43]. However, these studies have not investigated whether there are distinct neurophysiological properties underlying the individual perceptual qualities of pain.
The mechanisms underlying alpha rhythm are not fully understood, but are thought to involve the intrinsic bursting properties of neurons supporting thalamocortical signaling [17,32], and whose disruption, or dysrhythmia, may be the basis for certain neurogenic pain disorders [18,48]. In turn, slow modulations of EEG activity in the alpha band are thought to be of great relevance to brain function because these large-scale, brain-wide network oscillations appear to be the basis for communication between distant but functionally related regions of the brain [28–30,36]. Accordingly, a functional role for these slow oscillatory activities in somatosensory processing is emerging, as they predict perceptual acuity and performance [31] by a mechanism that appears to involve the synchronization of local fast neuronal activity between different brain regions, so-called cross-frequency coupling [7,24]. Some of this data suggests that the amplitude of the alpha rhythm can be modulated by the phase of the delta rhythm (0 to 3 Hz) [16,24], though we did not find evidence for the synchronization between the delta rhythm and the throbbing rhythm in this subject.
Recent simultaneous fMRI-EEG studies demonstrate that alpha power can be modulated by various large-scale brain networks [25,26,33] and suggest its potential as a biomarker for some clinical conditions [35,46]. Our finding that alpha power fluctuations are synchronized with the rhythm of throbbing events, and that the degree of synchrony correlates with throbbing intensity, is significant not only because these rhythmic modulations of alpha power may represent a neuronal signature of the experience of throbbing pain, but also because it affirms a role for these large-scale network properties in the processing of pain.
The general relevance of these observations can only be addressed with further confirmation in more typical cases of migraine, as well as in other throbbing pain conditions. This is of particular relevance in this case as the association between throbbing and either pain or migraine remains circumstantial. In addition to this, there are two other notable limitations of this case report. First, the subject reported rhythmic throbbing events by pressing an instrument key, which raises the possibility of an artifact due to motor activation. Although we are unable to completely exclude this possibility, it is unlikely to be significant because the synchrony between the sensorimotor channels and throbbing rhythm is low and is not correlated with throbbing intensity. The second limitation is that, because the EEG technique has limited spatial resolution, it is difficult to resolve the contribution from individual brain regions, especially those regions that are not in close proximity to the surface electrodes.
Future work will focus on reproducing these findings and on overcoming these methodological and technological hurdles, which we hope will reveal a more detailed picture of the neurophysiological events underlying the experience of throbbing pain. It is hoped that these insights may one day lead to clinically meaningful benefits, such as the development of neurophysiological markers identifying subpopulations most amenable to existing therapies, or novel neuromodulatory strategies with clinical applications to chronic pain [23].
Summary.
This patient with migraine developed a persistent sense of throbbing, without pain. Her EEG describes what may be a neurophysiological correlate of the throbbing quality.
Acknowledgments
Acknowledgements and Attestations. This work was supported in part by funds from a grant from the NIH MH097320 (MD), NS066091 (AHA) and the Facial Pain Research Foundation. No funding source had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest. The authors report no conflicts with the work presented in this manuscript.
References
- 1.Ahn AH. On the temporal relationship between throbbing migraine pain and arterial pulse. Headache. 2010;50:1507–1510. doi: 10.1111/j.1526-4610.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77:1021–1024. doi: 10.1136/jnnp.2006.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslan FE, Badir A, Arli SK, Cakmakci H. Patients' experience of pain after cardiac surgery. Contemp Nurse. 2009;34:48–54. doi: 10.5172/conu.2009.34.1.048. [DOI] [PubMed] [Google Scholar]
- 4.Babiloni C, Brancucci A, Del Percio C, Capotosto P, Arendt-Nielsen L, Chen AC, Rossini PM. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain. 2006;7:709–717. doi: 10.1016/j.jpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Backonja M, Howland EW, Wang J, Smith J, Salinsky M, Cleeland CS. Tonic changes in alpha power during immersion of the hand in cold water. Electroencephalogr Clin Neurophysiol. 1991;79:192–203. doi: 10.1016/0013-4694(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 6.Bjork MH, Stovner LJ, Nilsen BM, Stjern M, Hagen K, Sand T. The occipital alpha rhythm related to the "migraine cycle" and headache burden: a blinded, controlled longitudinal study. Clin Neurophysiol. 2009;120:464–471. doi: 10.1016/j.clinph.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha YH. Mal de Debarquement. Semin Neurol. 2009;29:520–527. doi: 10.1055/s-0029-1241038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha YH, Chakrapani S, Craig A, Baloh RW. Metabolic and functional connectivity changes in mal de debarquement syndrome. PLoS One. 2012;7:e49560. doi: 10.1371/journal.pone.0049560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha YH, Lee H, Santell LS, Baloh RW. Association of benign recurrent vertigo and migraine in 208 patients. Cephalalgia. 2009;29:550–555. doi: 10.1111/j.1468-2982.2008.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppola G, Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep. 2012;16:93–100. doi: 10.1007/s11916-011-0231-1. [DOI] [PubMed] [Google Scholar]
- 12.Cottalorda J, Bourelle S. The often-missed Kocher-Lorenz elbow fracture. Orthop Traumatol Surg Res. 2009;95:547–550. doi: 10.1016/j.otsr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Almeida Y, Felix ER, Martinez-Arizala A, Widerstrom-Noga EG. Pain symptom profiles in persons with spinal cord injury. Pain Med. 2009;10:1246–1259. doi: 10.1111/j.1526-4637.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 14.de Tommaso M, Sciruicchio V, Guido M, Sasanelli G, Specchio LM, Puca FM. EEG spectral analysis in migraine without aura attacks. Cephalalgia. 1998;18:324–328. doi: 10.1046/j.1468-2982.1998.1806324.x. [DOI] [PubMed] [Google Scholar]
- 15.Fisher CM. Late-life migraine accompaniments--further experience. Stroke. 1986;17:1033–1042. doi: 10.1161/01.str.17.5.1033. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Ramirez M, Kelly SP, Molholm S, Sehatpour P, Schwartz TH, Foxe JJ. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. J Neurosci. 2011;31:18556–18567. doi: 10.1523/JNEUROSCI.2164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauck M, Lorenz J, Engel AK. Role of synchronized oscillatory brain activity for human pain perception. Rev Neurosci. 2008;19:441–450. doi: 10.1515/revneuro.2008.19.6.441. [DOI] [PubMed] [Google Scholar]
- 19.Houtchens MK, Richert JR, Sami A, Rose JW. Open label gabapentin treatment for pain in multiple sclerosis. Mult Scler. 1997;3:250–253. doi: 10.1177/135245859700300407. [DOI] [PubMed] [Google Scholar]
- 20.IHS CS. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 21.Isnard J, Magnin M, Jung J, Mauguiere F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152:946–951. doi: 10.1016/j.pain.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MP, Gould EM, Victor TW, Gammaitoni AR, White RE, Galer BS. The relationship of changes in pain quality to pain interference and sleep quality. J Pain. 2010;11:782–788. doi: 10.1016/j.jpain.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Jensen MP, Hakimian S, Sherlin LH, Fregni F. New insights into neuromodulatory approaches for the treatment of pain. J Pain. 2008;9:193–199. doi: 10.1016/j.jpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 25.Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 26.Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leijon G, Boivie J, Johansson I. Central post-stroke pain--neurological symptoms and pain characteristics. Pain. 1989;36:13–25. doi: 10.1016/0304-3959(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 28.Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 29.Linkenkaer-Hansen K, Nikouline VV, Palva JM, Ilmoniemi RJ. Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci. 2001;21:1370–1377. doi: 10.1523/JNEUROSCI.21-04-01370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linkenkaer-Hansen K, Nikulin VV, Palva JM, Kaila K, Ilmoniemi RJ. Stimulusinduced change in long-range temporal correlations and scaling behaviour of sensorimotor oscillations. Eur J Neurosci. 2004;19:203–211. doi: 10.1111/j.1460-9568.2004.03116.x. [DOI] [PubMed] [Google Scholar]
- 31.Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci. 2004;24:10186–10190. doi: 10.1523/JNEUROSCI.2584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 33.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza AF, Mo J, Holt JL, Kairalla JA, Heft MW, Ding M, Ahn AH. Is there a relationship between throbbing pain and arterial pulsations? J Neurosci. 2012;32:7572–7576. doi: 10.1523/JNEUROSCI.0193-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montez T, Poil SS, Jones BF, Manshanden I, Verbunt JP, van Dijk BW, Brussaard AB, van Ooyen A, Stam CJ, Scheltens P, Linkenkaer-Hansen K. Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:1614–1619. doi: 10.1073/pnas.0811699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikulin VV, Brismar T. Long-range temporal correlations in alpha and beta oscillations: effect of arousal level and test-retest reliability. Clin Neurophysiol. 2004;115:1896–1908. doi: 10.1016/j.clinph.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Niv D, Ben-Ari S, Rappaport A, Goldofski S, Chayen M, Geller E. Postherpetic neuralgia: clinical experience with a conservative treatment. Clin J Pain. 1989;5:295–300. [PubMed] [Google Scholar]
- 38.Ofek H, Defrin R. The characteristics of chronic central pain after traumatic brain injury. Pain. 2007;131:330–340. doi: 10.1016/j.pain.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Pain suppresses spontaneous brain rhythms. Cerebral cortex. 2006;16:537–540. doi: 10.1093/cercor/bhj001. [DOI] [PubMed] [Google Scholar]
- 40.Rozen TD. Brief sharp stabs of head pain and giant cell arteritis. Headache. 2010;50:1516–1519. doi: 10.1111/j.1526-4610.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- 41.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 42.Schulz E, Tiemann L, Witkovsky V, Schmidt P, Ploner M. Gamma oscillations are involved in the sensorimotor transformation of pain. J Neurophysiol. 2012;108:1025–1031. doi: 10.1152/jn.00186.2012. [DOI] [PubMed] [Google Scholar]
- 43.Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M. Decoding an individual's sensitivity to pain from the multivariate analysis of EEG data. Cerebral Cortex. 2012;22:1118–1123. doi: 10.1093/cercor/bhr186. [DOI] [PubMed] [Google Scholar]
- 44.Seymour RA, Charlton JE, Phillips ME. An evaluation of dental pain using visual analogue scales and the Mcgill Pain Questionnaire. J Oral Maxillofac Surg. 1983;41:643–648. doi: 10.1016/0278-2391(83)90017-4. [DOI] [PubMed] [Google Scholar]
- 45.Shao S, Shen K, Yu K, Wilder-Smith EP, Li X. Frequency-domain EEG source analysis for acute tonic cold pain perception. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.02.084. [DOI] [PubMed] [Google Scholar]
- 46.Smit DJ, de Geus EJ, van de Nieuwenhuijzen ME, van Beijsterveldt CE, van Baal GC, Mansvelder HD, Boomsma DI, Linkenkaer-Hansen K. Scale-free modulation of resting-state neuronal oscillations reflects prolonged brain maturation in humans. J Neurosci. 2011;31:13128–13136. doi: 10.1523/JNEUROSCI.1678-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dijk JG, Dorresteijn M, Haan J, Ferrari MD. Visual evoked potentials and background EEG activity in migraine. Headache. 1991;31:392–395. doi: 10.1111/j.1526-4610.1991.hed3106392.x. [DOI] [PubMed] [Google Scholar]
- 48.Walton KD, Llinas RR. Central Pain as a Thalamocortical Dysrhythmia: A Thalamic Efference Disconnection? In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: 2010. [PubMed] [Google Scholar]
- 49.Whitman BW, Lipton RB. Oscillocusis: an unusual auditory aura in migraine. Headache. 1995;35:428–429. doi: 10.1111/j.1526-4610.1995.hed3507428.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J Neurosci. 2012;32:7429–7438. doi: 10.1523/JNEUROSCI.5877-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]