SUMMARY
Oncogene-induced senescence is an important tumor-suppressing defense mechanism. However, relatively little is known about the signaling pathway mediating the senescence response. Here, we demonstrate that a multifunctional acetyltransferase Tip60 plays an essential role in oncogenic ras-induced senescence. Further investigation reveals a novel cascade of posttranslational modifications involving p38, Tip60 and PRAK, three proteins that are essential for ras-induced senescence. Upon activation by ras, p38 induces the acetyltransferase activity of Tip60 through phosphorylation of Thr158; activated Tip60 in turn directly interacts with and induces the protein kinase activity of PRAK through acetylation of K364 in a manner that depends on phosphorylation of both Tip60 and PRAK by p38. These posttranslational modifications are critical for the pro-senescent function of Tip60 and PRAK, respectively. These results have defined a novel signaling pathway that mediate oncogene-induced senescence, and identified novel posttranslational modifications that regulate the enzymatic activity and biological functions of Tip60 and PRAK.
INTRODUCTION
Activation of oncogenes such as ras triggers a stable proliferative arrest, known as oncogene-induced senescence, in normal mammalian cells (Courtois-Cox et al., 2008; Serrano et al., 1997). Like apoptosis, oncogene-induced senescence serves as an anti-tumorigenic defense mechanism. Recent studies have demonstrated that oncogene-induced senescence occurs in vivo and provides a bona fide barrier to tumorigenesis (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005; Narita and Lowe, 2005; Sun et al., 2007).
The signaling pathway mediating ras-induced senescence has yet to be fully understood. Oncogenic ras-induced senescence depends on activation of the Raf-MEK-ERK MAPK pathway (Lin et al., 1998; Zhu et al., 1998), and is marked by upregulation of inhibitors of cell proliferation, including p16INK4A, p53, p21WAF1, and p14/p19ARF (Ferbeyre et al., 2002; Serrano et al., 1997), and increased secretion of inflammatory cytokines (Coppe et al., 2010). We and others have shown that ras-induced senescence requires activation of the p38 MAPK (Haq et al., 2002; Iwasa et al., 2003; Kwong et al., 2009; Nicke et al., 2005; Wang et al., 2002). We further demonstrated that the function of p38 in senescence and tumor suppression is mediated by its downstream substrate kinase PRAK (Sun et al., 2007). Inactivation of PRAK leads to disruption of senescence response and enhanced tumorigenesis both in cell culture and in mouse cancer models (Sun et al., 2007), suggesting that PRAK has a tumor suppressing activity.
In an attempt to delineate the signaling pathway mediating the tumor suppressing function of PRAK, we identified Tip60 as a PRAK-interacting protein through a yeast 2-hybrid screen in the current study. Further investigation revealed that like p38 and PRAK, Tip60 is essential for oncogenic ras-induced senescence. Tip60 is a member of the MYST family of histone acetyltransferase (HAT), which has been implicated in multiple cellular processes (Fisher et al., 2012; Gorrini et al., 2007; Sapountzi et al., 2006; Squatrito et al., 2006). Tip60 has been shown to function as a tumor suppressor (Gorrini et al., 2007). The functions of Tip60 is mediated either by a transcriptional mechanism in which Tip60 is recruited to target promoters and participates in histone acetylation, or by a transcription-independent mechanism in which Tip60 alters the activity or expression of non-histone proteins via direct interaction and acetylation (Sapountzi et al., 2006; Squatrito et al., 2006; Sykes et al., 2006; Tang et al., 2006). Despite mounting evidence indicating the important roles of Tip60 in multiple cellular processes, mechanisms that regulate the activity of Tip60 are poorly understood. Recent studies identified Ser86 as the GSK3 phosphorylation site on Tip60 (Charvet et al., 2011; Lin et al., 2012). Upon phosphorylation of Ser86 by GSK3, Tip60 participates in growth factor deprivation-induced autophage by acetylating ULK1, or promotes apoptosis by acetylating p53. However, more comprehensive analysis of posttranslational regulation of Tip60 functions is needed.
In the current study, we have identified a novel posttranslation modification cascade involving p38, Tip60 and PRAK, 3 signaling components in the pathway mediating oncogene-induced senescence, which regulate the enzymatic activity and biological functions of these proteins. In response to oncogenic ras, p38 induces the acetyltransferase activity of Tip60 by phosphorylating its Thr158 residue; and activated Tip60 in turn acetylates PRAK at Lys364, thereby stimulating the protein kinase activity of PRAK. These modifications are required for the ability of Tip60 and PRAK to mediate senescence. Our results have thus defined a novel signaling pathway that plays a key role in oncogene-induced senescence, and identified novel post-translational modifications that regulate the enzymatic activity and biological functions of Tip60 and PRAK proteins.
RESULTS
Identification of Tip60 as a PRAK-binding protein
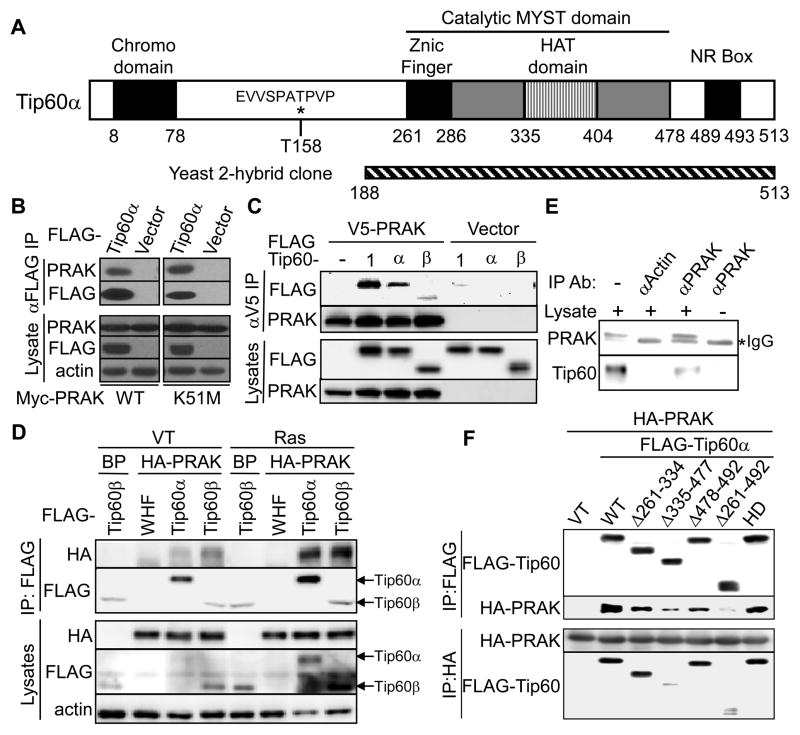
To elucidate the mechanisms underlying the role of PRAK in senescence induction and tumor suppression, we screened a HeLa cell cDNA library to search for PRAK-interacting proteins in a yeast-2-hybrid screen using PRAK as bait. The screen identified a clone representing a C-terminal fragment (amino acid 188–513) of a multifunctional histone acetyltransferase (HAT) Tip60 (Fig. 1A). To confirm PRAK-Tip60 interaction, FLAG-tagged Tip60α and Myc-tagged PRAK were transfected into 293T cells, and immunoprecipitated with an anti-FLAG antibody. PRAK was readily detected in the FLAG-Tip60 complex (Fig. 1B). In a reciprocal experiment, all 3 Tip60 isoforms (Tip60-1, α and β) co-precipitated with V5-tagged PRAK (Fig. 1C). Tip60 also bound to a kinase-dead mutant (K51M) of PRAK (Fig. 1B), indicating that the interaction does not require the kinase activity of PRAK. Moreover, HA-tagged PRAK was co-immunoprecipitated with FLAG-tagged Tip60α and Tip60β from primary BJ human fibroblast cells (Fig. 1D). Interaction between endogenous Tip60 and PRAK was also observed in the nuclear extract of senescent BJ cells (Fig. 1E). These results confirm that Tip60 physically interacts with PRAK in cells. In a GST pull down assay, recombinant His-Tip60α was retained on GST-PRAK beads, but not on GST-beads (Fig. S1A), indicating that the Tip60-PRAK interaction is direct. Furthermore, using reciprocal co-immunoprecipitation (Fig. 1F) and GST pull down (Fig. S1B) assays, we found that PRAK interaction was nearly abrogated when the HAT domain of Tip60 was deleted (Δ335–477 and Δ261–492), demonstrating that PRAK mainly binds to the HAT-domain of Tip60. Since the conserved HAT domain of Tip60 binds to acetyl Co-A and substrates, such as histone H2AX (Sapountzi et al., 2006; Yamagata and Kitabayashi, 2009), this finding raises a possibility that PRAK is a Tip60 substrate.
Fig. 1.
PRAK interacts with Tip60 in vitro and in senescent cells.
(A) Schematic diagram of the Tip60α protein. p38 phosphorylation site is denoted by *. The hatched bar represents the PRAK-interacting Tip60 fragment identified from the yeast 2-hybrid screen using PRAK as bait.
(B) FLAG-Tip60α were immunoprecipitated from 293T cells transfected with Myc-PRAK or -PRAK-K51M, FLAG-Tip60α or vector, and MKK3E or vector, and subjected to Western blotting along with lysates.
(C) V5-tagged PRAK was immunoprecipitated from 293T cells transfected with V5-PRAK or vector and FLAG-Tip60 isoforms (1, a or b) or vector using an anti-V5 antibody, and subjected to Western blotting along with lysates.
(D) FLAG-Tip60 were immunoprecipitated from BJ cells transduced with HA-PRAK or vector (BP), FLAG-Tip60α-Tip60β or vector (WHF), and HaRasV12 or vector, and subjected to Western blotting along with lysates.
(E) Endogenous PRAK was immunoprecipitated from BJ cells transduced with HaRasV12 using an anti-PRAK or anti-actin antibody, and subjected to Western blotting along with lysates.
(F) FLAG-Tip60α or HA-PRAK were immunoprecipitated from 293T cells transfected with HA-PRAK, and wild type or indicated deletion mutants of FLAG-Tip60α or vector, and subjected to Western blotting.
See also Figure S1.
Tip60 is essential for ras-induced senescence
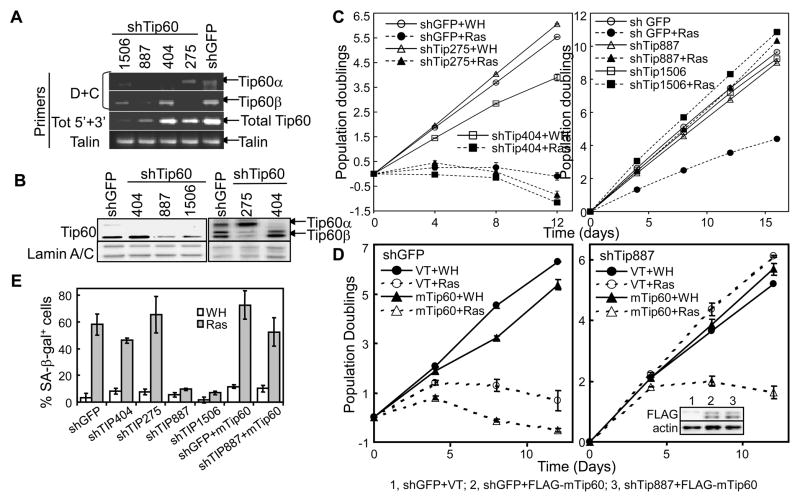
The Tip60 gene encodes 3 isoforms resulted from alternative splicing, Tip60-1, Tip60α and Tip60β (Sapountzi et al., 2006). 4 sets of primers were designed to detect these isoforms by PCR. Primers A and C detect Tip60α and β; B and C detect Tip60-1; C and D amplify the variable regions of all 3 isoforms; and tot5′ and tot3′ amplify a region common to all isoforms (Fig. S2A, B). While Tip60α and β were readily detected by primers A+C and C+D in primary BJ human fibroblasts, fragments of proper sizes from Tip60-1 were not amplified by C+D or B+C (Fig. S2C). (Only a non-specific fragment of smaller size was amplified by B+C.) In Western blot analysis of BJ cell lysates, an anti-Tip60 antibody detected protein bands co-migrating with Tip60α and β ectopically expressed in 293T cells, but not that co-migrating with Tip60-1 (Fig. S2D). These results demonstrate that both Tip60α and β, but not Tip60-1, are expressed in BJ cells. Similar results were obtained in WI38 primary human fibroblast cells (data not shown).
To investigate the role of Tip60 in senescence induction, Tip60 shRNAs were constructed and examined for their effect on ras-induced senescence in BJ cells. shRNAs that effectively silenced both Tip60α and β expression (shTip887 and 1506, Fig. 2A, 2B, S2D) prevented ras-induced proliferative arrest and accumulation of SA-β-gal (Fig. 2C, right panel, and 2E). In addition, we designed an shRNA (shTip404) that knocks down Tip60-1 and α but not β by targeting exon 5, and an shRNA (shTip275) that knocks down Tip60β but not -1 or α by targeting the junction of exons 4 and 6 (Fig. S2A). As expected, shTip404 and shTip275 silenced the expression of Tip60α and β, respectively, in BJ cells (Fig. 2A, 2B, S2D). However, these constructs failed to block ras-induced senescence (Fig. 2C, left panel, and 2E), suggesting that Tip60α and β are functionally redundant.
Fig. 2.
Tip60 is essential for oncogenic ras-induced senescence.
(A) Semi-quantitative RT-PCR analysis of Tip60 mRNA levels in BJ cells transduced with GFP or indicated Tip60 shRNA using isoform-specific (D+C) or universal (Tot 5′+3′) primers (Fig. S2A–C).
(B) Western blot analysis of BJ cells transduced with GFP or indicated Tip60 shRNA.
(C) Population doubling of BJ cells transduced with GFP (shGFP) or Tip60 (shTip) shRNA and Ha-RasV12 or vector (WH).
(D) Population doubling of BJ cells transduced with GFP (shGFP) or Tip60 (shTIP887) shRNA, mouse Tip60 (mTip60) or vector (VT), and Ha-RasV12 or vector (WH). Insert, Western blotting of BJ cells transduced with GFP or Tip60 shRNA (shTip887) and FLAG-mTip60 or vector (VT).
(E) BJ cell populations in (C) and (D) were stained for SA-β-gal on day 18 post ras transduction.
(C–E) Values are mean ± SD for triplicates.
See also Figure S2.
Furthermore, ectopic expression of a murine Tip60 (mTip60) that could not be silenced by human Tip60 shRNA (Fig. 2D, insert) restored ras-induced senescence in BJ cells expressing shTip887 (Fig. 2D, E), indicating that the bypass of senescence induction in BJ cells was due to specific knockdown of Tip60, but not off-target effects of shRNA. Taken together, these results demonstrate that Tip60 is required for ras-induced senescence.
p38 phosphorylates Tip60 at Thr158/106 in vitro and in cells
The interaction between Tip60 and PRAK prompted us to investigate whether ras-induces Tip60 phosphorylation through PRAK and/or its upstream kinase p38. When co-expressed in 293T cells together with p38α and active MKK3 (MKK3E), Tip60 displayed a slower mobility on SDS-PAGE (Fig. S3A). The shift in mobility was due to phosphorylation, because it was abolished when isolated Tip60 was treated with λ phosphatase (Fig. S3A). The mobility shift was also eliminated when either p38 or MKK3 was rendered inactive by mutations (p38αAF or MKK3AA) (Fig. S3B, top panels), indicating that Tip60 phosphorylation relies on active MKK3 and p38. The same observations were made in H1299 cells (Fig. S3B, bottom panels). Thus, Tip60 is phosphorylated by activated p38 in cells. In contrast, co-transfection of PRAK together with MKK3E and p38α failed to further enhance Tip60 phosphorylation (Fig. S3B), suggesting that PRAK did not contribute to Tip60 phosphorylation in cells.
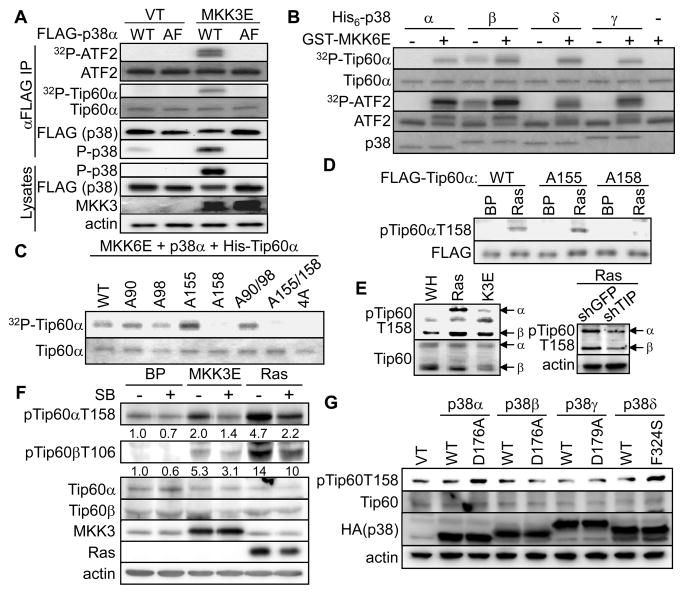
To directly assess Tip60 phosphorylation by p38, we performed in vitro kinase assays with recombinant or immunoprecipitated p38. When immunoprecipitated from BJ cells co-transduced with MKK3E, FLAG-p38α but not its activation site-mutant (AF), phosphorylated Tip60 in vitro (Fig. 3A). Four p38 isoforms, p38α, β, γ and δ, exist in mammals (Shi and Gaestel, 2002). Tip60 was phosphorylated by all 4 recombinant p38 isoforms in a MKK6E-dependent manner (Fig. 3B). As a positive control, immunoprecipitated and recombinant p38 isoforms phosphorylated a known substrate ATF2 (Fig. 3A, B). Thus, Tip60 is a direct substrate of p38.
Fig. 3.
p38 phosphorylates Tip60 at Thr158/106 in vitro and during ras-induced senescence.
(A) FLAG-p38α was immunoprecipitated from 293T cells expressing FLAG-p38αWT or -p38αAF, and MKK3E or vector, using an agarose-conjugated anti-FLAG antibody M2, and subjected to kinase assay toward recombinant ATF2 or Tip60α in the presence of [γ-32P]ATP. Western blotting was performed with part of the IPs and whole cell lysates.
(B) Recombinant His-Tip60α or ATF2 was incubated with recombinant His-p38α, β, γ, δ or buffer and GST-MKK6E (+) or buffer (−) in the presence of [γ-32P]ATP. Phosphorylation of Tip60 and ATF2 were detected by autoradiography.
(C) Wild type or indicated mutant of His-Tip60α was incubated with MKK6E and p38α in the presence of [γ-32P]ATP and resolved on SDS-PAGE. The phosphorylation of Tip60 is detected by autoradiography.
(A–C) Substrate input was stained with Coomassie Brilliant Blue R.
(D) Western blotting of BJ cells transduced with wild type or indicated mutant of FLAG-Tip60α and HaRasV12 or vector (BP) on day-8 post ras transduction.
(E) Western blotting of BJ cells on day-8 post transduction with HaRasV12, MKK3E (K3E) or vector (WH) (left panel) or with shRNA for GFP or Tip60 (shTIP887) and HaRasV12 (right panel).
(F) Western bloting of BJ cells on day-8 post transduction with HaRasV12, MKK3E or vector (BP) and treated with 10 μM SB203581 for 7 days. Numbers are relative levels of pTip60αT158 and pTip60βT158 after normalizing to the levels of total Tip60α and Tip60β, respectively.
(G) Western blotting of BJ cells on day-8 post transdcution with wild type or indicated active mutant of p38 isoforms.
See also Figure S3.
When Tip60α phosphorylated by recombinant p38α and MKK6E was analyzed by mass spectrometry, we identified a phospho-peptide containing phosphorylated Ser155 or Thr158 (corresponding to Ser103 or Thr106 on Tip60β, respectively). However, mass spectrometry was unable to resolve these 2 sites due to their close proximity. We thus examined phosphorylation of recombinant Tip60α with S155A, T158A or both mutations in vitro. T158A, but not S155A, abolished Tip60α phosphorylation by p38 (Fig. 3C). Similarly, T106A, but S103A, disrupted p38-mediated phosphorylation of Tip60β (Fig. S3C). Replacement of Ser90, a cyclin B/Cdc2 phosphorylation site (Lemercier et al., 2003), and Ser98 also failed to impact p38-mediated Tip60 phosphorylation (Fig. 3C). Furthermore, upon cotransfected into 293T cells, the p38/MKK3E-induced, phosphorylation-dependent mobility shift of Tip60 was abolished by T158/106A, but not by S155/103A mutation (Fig. S3D). These results thus identified Thr158/106 as the p38 substrate site on Tip60 both in vitro and in cells. Thr158/106 is located in the hinge region between the chromo domain and zinc finger, and matches the consensus of p38 phosphorylation sites (S/T-P) (Fig. 1A).
Oncogenic ras induces p38-mediated phosphorylation of Tip60 at Thr158/106 in senescent cells
To facilitate the analysis of Tip60 phosphorylation in senescent cells, we raised a phospho-specific antibody (pTip60T158) that specifically recognized Tip60 phosphorylated at Thr158 (Fig. S3E). In an IP-coupled Western blot analysis using this antibody, oncogenic ras induced phosphorylation of Thr158/106 on both FLAG-tagged Tip60α and Tip60β stably transduced into BJ cells (Fig. S3F). Ras-induced Tip60α phosphorylation as detected by the pTip60T158 antibody was abrogated by the T158A mutation, but not by the S155A mutation (Fig. 3D), further confirming the identity of the ras-induced phosphorylation site as Thr158. The pTip60T158 antibody also detected ras- and MKK3E-induced signals on endogenous Tip60α and Tip60β in BJ cells, which were reduced by Tip60 shRNA (Fig. 3E). Thus both ectopically expressed and endogenous Tip60 proteins are phosphorylated at Thr158/106 upon activation of ras and p38 in senescent cells.
Ras- and MKK3E-induced Thr158/106 phosphorylation on both Tip60α and Tip60β was greatly reduced when BJ cells were treated with SB203580, a chemical inhibitor of p38α and p38β, suggesting that p38 is required for Tip60-T158/106 phosphorylation in senescent cells (Fig. 3F). To further explore the p38 isoforms that mediate ras-induced Tip60 phosphorylation, we examined the effects of constitutively active mutants of p38α, β, γ and δ (Askari et al., 2007; Avitzour et al., 2007). These mutants have acquired spontaneous protein kinase activity in vitro and in vivo while maintaining specificity toward substrates and inhibitors similar to that of the wild type p38 isoforms. As compared to the wild type counterparts, the active mutants of p38α and p38δ, but not those of the other isoforms, stimulated Tip60α-T158 phosphorylation when stably expressed in BJ cells (Fig. 3G), suggesting that only p38α and p38δ contributes to the phosphorylation of Tip60-T158. Moreover, shRNA-mediated knockdown of p38α or p38δ greatly reduced oncogenic ras-induced T158-phosphorylation of ectopically expressed Tip60α in BJ cells (Fig. 4D). In contrast, shRNAs targeting p38γ had no effect on ras-induced Tip60-T158 phosphorylation (Fig. S3G). These findings indicate that upon activation by oncogenic ras in senescent cells, p38α and p38δ, but not p38γ, mediate the phosphorylation of Tip60 at Thr158/106, despite the fact that all 4 p38 isoforms could phosphorylate Tip60 in vitro (Fig. 3B). It is possible that p38α and p38δ, but not p38γ, are accessible to the Tip60 protein due to differential subcellular localization. Alternatively, the p38 isoforms may interact with different cofactors that regulate their ability to phosphorylation Tip60 in cells.
Fig. 4.
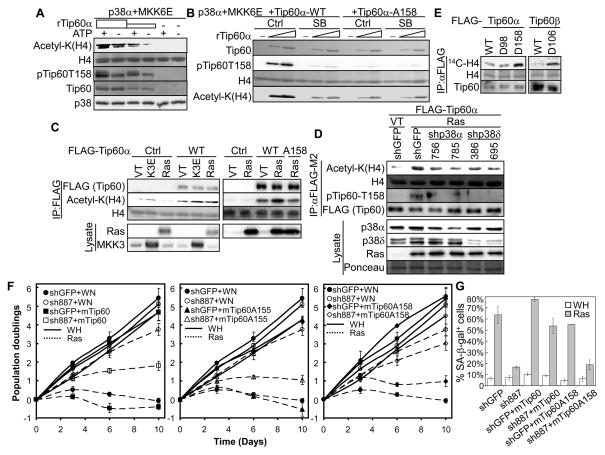
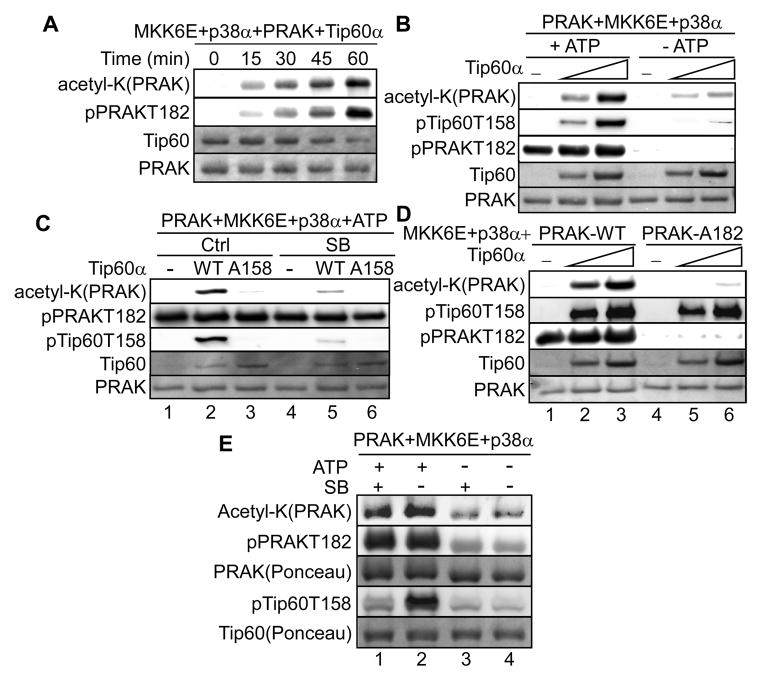
Phosphorylation of Tip60-T158 by p38 induces the acetyltransferase activity of Tip60 and is required for the ability of Tip60 to mediate senescence.
(A) Increasing amounts of Tip60α or buffer (−) was incubated first with p38α and MKK6E with (+) or without (−) ATP and then with histone and acetyl-CoA.
(B) Increasing amounts of wild type or indicated mutant of Tip60α or buffer (−) was incubated first with p38α, MKK6E and SB203580 (SB) or vehicle control (Ctrl) and then with histone and acetyl-CoA.
(C) FLAG-Tip60 was immunoprecipitated from BJ cells transduced with FLAG-Tip60α (WT) or -Tip60α-A158 or vector (Ctrl) and HaRasV12 (Ras), MKK3E (K3E) or vector (VT), and incubated with histone and acetyl-CoA.
(D) FLAG-Tip60 was immunoprecipitated from BJ cells transduced with FLAG-Tip60α; shRNA for GFP or p38α (756, 785) or p38δ (386, 695); and HaRasV12 (Ras) or vector (VT), and incubated with histone and acetyl-CoA.
(A–D) Acetylated histone H4 was detected by Western blotting using an anti-acetyl-Lys antibody. Substrate input and recombinant proteins were stained with Ponceau. Part of the IPs and lysates were analyzed by Western blotting (C, D).
(E) FLAG-Tip60 was immunoprecipitated from BJ cells transduced with FLAG-Tip60α (WT), -Tip60α-D98, -Tip60α-D158, -Tip60β (WT) or -Tip60β-D106 and incubated with histone and [14C]acetyl-CoA. Reactions were resolved by SDS-PAGE. Acetylation of histone H4 was detected by autoradiography. Histone input was stained with Coomassie Brilliant Blue. Part of the IPs were analyzed by Western blotting.
(F) Population doublings of BJ cells transduced with shRNA for GFP or Tip60 (sh887); mTip60 (left panel), mTip60-A155 (middle panel), mTip60-A158 (right panel) or vector (WN); and HaRasV12 (Ras) or vector (WH) over 10 days, starting on day-4 post ras transduction.
(G) % of SA-β-gal positive cells in cell populations described in (F).
(F–G) Values are mean ± SD for triplicates.
See also Figure S4.
P38-mediated phosphorylation of Tip60-T158 is essential for the ras-induced enzymatic activity and pro-senescent function of Tip60
We next investigated the possibility that phosphorylation by p38 regulates the acetyltransferase activity and biological function of Tip60. When incubated in vitro with p38α and MKK6E in the absence of ATP (a condition in which Tip60-T158 was not phosphorylated), Tip60α acetylated histone H4 in a dose-dependent manner (Fig. 4A). Inclusion of ATP into the reactions led to phosphorylation of Tip60-T158 and an increase in histone acetylation at every concentration of Tip60 (Fig. 4A). In addition, p38α and MKK6E failed to enhance histone acetylation by Tip60 in the presence of SB203580, which inhibited Tip60-T158 phosphorylation, or when Thr158 was mutated to Ala (Fig. 4B). Thus, phosphorylation of Thr158 by p38 stimulates the HAT activity of Tip60 in vitro.
Using an IP-coupled HAT assay, we analyzed the effect of p38 and oncogenic ras on Tip60 activity in senescent cells. FLAG-Tip60α immunoprecipitated from BJ cells transduced with Ha-RasV12 or MKK3E displayed an increased HAT activity towards histones as compared to that from control cells; and ras-induced HAT activity of Tip60 was abrogated by the T158A mutation (Fig. 4C), suggesting that oncogenic ras and activated p38 induce Tip60 activity through phosphorylation of Thr158. Further supporting the essential role of p38 in Tip60 activation by ras, shRNA-mediated knockdown of p38α or p38δ abrogated ras-induced Tip60α-T158 phosphorylation, and reduced ras-induced HAT activity of Tip60α in BJ cells as determined in an IP-HAT assay using histone H4 as substrate (Fig. 4D). Furthermore, the Tip60 mutants containing phosphomimetic mutations at Thr158/106, Tip60α-D158 and Tip60β-D106, displayed increased HAT activity as compared to the wild type Tip60 proteins, when expressed in and immunoprecipitated from BJ cells (Fig. 4E). These data indicate that during senescence induction, oncogenic ras stimulates the enzymatic activity of Tip60 through p38-mediated phosphorylation of Thr158.
Ectopic expression of murine Tip60 restored ras-induced senescence in BJ cells expressing human Tip60 shRNA (Fig. 2D, 2E, 4F-left panel, 4G), indicating that murine Tip60 can functionally replace human Tip60 in senescence. The S155A mutation had no effect on murine Tip60-mediatd senescence in this system (Fig. 4F-middle panel); however, the T158A mutant failed to restore senescence in the presence of the human Tip60 shRNA (Fig. 4F-right panel, 4G). Thus, phosphorylation of Tip60-Thr158 is required for the ability of Tip60 to mediate ras-induced senescence. Taken together, our findings demonstrate that phosphorylation of Thr158 by p38 plays an essential role in the induction of both enzymatic activity and biological function of Tip60 during ras-induced senescence.
Although the Tip60-D158 mutation enhances the HAT activity of Tip60, this mutant failed to induce senescence (Fig. S4), suggesting that phosphorylation and activation of Tip60 by p38 alone is not sufficient for senescence induction.
Tip60 acetylates PRAK in a phosphorylation-dependent manner
We investigated whether PRAK-Tip60 interaction leads to acetylation of PRAK by Tip60. Indeed, incubation of Tip60α with PRAK in the presence of MKK6E and p38α led to acetylation of PRAK in a time-dependent (Fig. 5A) and dose-dependent (Fig. 5B) manner. Acetylation of PRAK by Tip60 depends on phosphorylation, as PRAK acetylation was reduced in the absence of Tip60 and PRAK phosphorylation when ATP was omitted (Fig. 5B).
Fig. 5.
Tip60 acetylates PRAK in a phosphorylation dependent manner.
(A) His-PRAK was incubated with Tip60α, p38α, MKK6E, ATP and acetyl-CoA for an indicated period.
(B) His-PRAK was incubated with increasing amounts of Tip60α or buffer (−), p38α, MKK6E and acetyl-CoA with or without ATP.
(C) His-PRAK was incubated first with p38α, MKK6E and ATP, and then with wild type Tip60α (WT), Tip60α-A158 or buffer (−) and acetyl-CoA with or without SB203580.
(D) Wild type PRAK or PRAK-A182 was incubated with increasing amounts of Tip60α or buffer (−), p38α, MKK6E, ATP and acetyl-CoA.
(E) PRAK was incubated first with p38α and MKK6E with or without ATP, and then with Tip60α and acetyl-CoA with or without SB203580.
(A–E) Acetylation of PRAK and phosphorylation of PRAK and Tip60 were detected by Western blotting using anti-acetyl-Lys, anti-pPRAK-T182 and anti-pTip60-T158 antibodies, respectively. Input of recombinant proteins was stained by Ponceau.
See also Figure S5.
Since both PRAK and Tip60 are substrates of p38, we determined the phosphorylation of which protein contributes to PRAK acetylation by Tip60. To analyze the effect of Tip60 phosphorylation, we first incubated PRAK with MKK6E and p38α in the presence of ATP, following which SB203580 was added to inhibit p38 before incubation with Tip60α. Compared to the vehicle treatment control, treatment with SB203580 effectively prevented phosphorylation of wild type Tip60α without affecting PRAK phosphorylation, and at the same time, greatly inhibited PRAK acetylation (Fig. 5C, compare lanes 2 and 5). In addition, the T158A mutation that abolished Tip60α phosphorylation by p38 also reduced the ability of Tip60α to acetylate PRAK (Fig. 5C, compare lanes 2 and 3). Thus, the acetyltransferase activity of Tip60 towards PRAK is stimulated by phosphorylation of Tip60 by p38. Moreover, the T182A mutation of the p38 phosphorylation site on PRAK, which rendered PRAK unphosphorylatable by p38, significantly reduced acetylation of PRAK by Tip60 despite intact Tip60 phosphorylation by p38 (Fig. 5D, compare lanes 2 and 5, and lanes 3 and 6), suggesting that PRAK phosphorylation by p38 is also required for the optimal acetylation of PRAK by Tip60. Thus, Tip60 acetylates PRAK in a manner that is dependent on the phosphorylation of both Tip60 and PRAK by p38.
To compare the effects of Tip60 and PRAK phosphorylation in parallel, we incubated PRAK first with MKK6E and p38α with or without ATP, and then with Tip60α in the presence or absence of SB203580 (Fig. 4E). The absence of ATP prevented phosphorylation of both Tip60α and PRAK (lanes 3 and 4). Inclusion of both ATP and SB203580 allowed phosphorylation of PRAK but not Tip60α, and resulted in a moderate increase in PRAK acetylation (lane 1), while inclusion of ATP in the absence of SB203580 led to phosphorylation of both PRAK and Tip60α, and a further increase in PRAK acetylation (lane 2) as compared to when PRAK alone was phosphorylated (lane 1). These results indicate that the contribution of PRAK phosphorylation and that of Tip60 phosphorylation by p38 are additive to the acetyltransferase activity of Tip60 towards PRAK.
Furthermore, when cotransfected into 293T cells in the presence of MKK3E and p38α, wild type Tip60α, but not its HAT-defective Q377E/G380E mutant (HD), acetylated PRAK (Fig. S5C, compare lanes 2 and 4; and Fig. 6B, compare lanes 2 and 3), indicating that Tip60 is a PRAK acetyltransferase in cells. Efficient PRAK acetylation by Tip60α was only observed when MKK3E and p38α were cotransfected (Fig. S5C, compare lanes 2 and 3), demonstrating that p38-mediated phosphorylation also contributes to PRAK acetylation by Tip60 in cells.
Fig. 6.
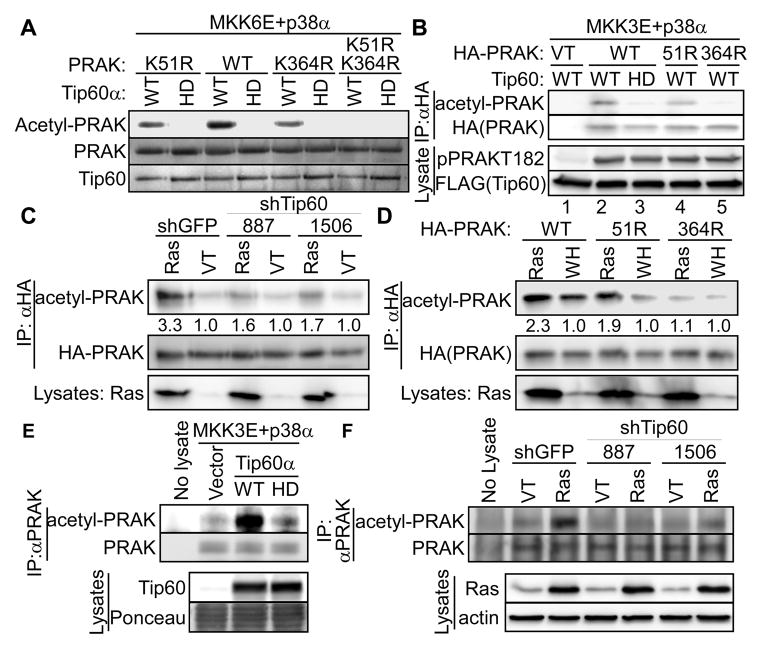
Tip60 acetylates PRAK at K364 in vitro and during oncogenic ras-induced senescence.
(A) Wild type (WT) or indicated mutant of recombinant PRAK was incubated with wild type (WT) or HAT-defective (HD) Tip60α and acetyl-CoA in the presence of p38α and MKK6E. Acetylation of PRAK was detected by Western blotting using an anti-acetyl-Lys antibody. Recombinant proteins were stained by Ponceau.
(B) HA-PRAK was immunoprecipitated from 293T cells transfected with wild type (WT) or indicated mutant of HA-PRAK or vector (VT) and wild type (WT) or HAT-defective (HD) Tip60α.
(C) HA-PRAK was immunoprecipitated from BJ cells transduced with HA-PRAK, shRNA for GFP or Tip60 (887, 1506), and HaRasV12 or vector (VT).
(D) HA-PRAK was immunoprecipitated from BJ cells transduced with wild type (WT) or indicated mutant of HA-PRAK, and HaRasV12 or vector (WH).
(E) Endogenous PRAK was immunoprecipitated from 293T cells transfected with wild type (WT) or HAT-defective (HD) Tip60α or vector, MKK3E and p38α.
(F) Endogenous PRAK was immunoprecipitated from BJ cells transduced with shRNA for GFP (shGFP) or Tip60 (887 or 1506) and HaRasV12 (Ras) or vector (VT).
(B–F) Acetylated PRAK and total immunoprecipitated PRAK were detected by Western blotting using an anti-acetyl-Lys antibody and an anti-HA (B–D) or anti-PRAK (E–F) antibody, respectively. Part of the lysates was also analyzed by Western blotting.
(C–D). Numbers are fold induction of acetyl-PRAK signals by ras, after normalizing to the levels of total HA-PRAK.
See also Figure S5.
Tip60 acetylates PRAK at K364 in vitro, in 293T cells and during ras-induced senescence
From mass spectrometry analysis of PRAK acetylated in vitro by Tip60, we identified 2 PRAK peptides containing acetylated K51 and K364, respectively. Confirming K51 and K364 as Tip60 acetylation sites in vitro, mutation of either K51R or K364R alone greatly reduced, while the double mutation abolished, acetylation of PRAK by Tip60α (Fig. 6A). However, when Tip60α and PRAK were cotransfected into 293T cells in the presence of MKK3E and p38α, K51R only slightly reduced, while K364R completely abolished, Tip60-mediated acetylation of PRAK (Fig. 6B), suggesting that K364 is the major Tip60 acetylation site on PRAK in cells.
We next investigated the effect of Tip60 on PRAK acetylation during oncogenic ras-induced senescence. HA-tagged PRAK was stably transduced into primary BJ cells, and immunoprecipitated after induction of senescence by oncogenic ras. PRAK acetylation was assessed by Western blotting using an anti-acetyl-Lys antibody. Indeed, ras induced PRAK acetylation, which was abrogated in cells stably expressing Tip60 shRNA (shTip60–887 and 1506) (Fig. 6C). In addition, while the K364R mutation essentially abolished ras-induced acetylation of PRAK, K51R had little effect (Fig. 6D). Thus, during senescence induction, oncogenic ras induces Tip60-dependent acetylation of PRAK mainly on K364.
We further investigated the effect of Tip60 on acetylation of endogenous PRAK using IP-Western analysis. When cotransfected into 293T cells with MKK3E and p38α, wild type, but not the HAT-defective, Tip60α induced acetylation of endogenous PRAK (Fig. 6E). Moreover, oncogenic ras induced acetylation of endogenous PRAK in BJ cells, which was abrogated by Tip60 shRNA (Fig. 6F). These findings reinforce the notion that Tip60 acetylates PRAK during ras-induced senescence.
Acetylation of K364 by Tip60 stimulates PRAK kinase activity and is essential for oncogenic ras-induced kinase activity and pro-senescent function of PRAK
Acetylation of PRAK by Tip60 prompted us to investigate the effect of Tip60 acetylation on PRAK activity and function. In an in vitro kinase assay, incubation with wild type Tip60α greatly increased the protein kinase activity of PRAK towards HSP27, as compared to when PRAK was not acetylated either in the absence of Tip60 or in the presence of HAT-defective Tip60α (HD) (Fig. S5A, compare lanes 1 and 2, and 3 and 4), or in the absence of acetyl-CoA (Fig. S5B). We cotransfected Tip60α and PRAK together with MKK3E and p38α into 293T cells, and measured the protein kinase activity of immunoprecipitated PRAK toward HSP27. Wild type (WT), but not HAT-defective (HD), Tip60α led to PRAK acetylation and strongly stimulated the HSP27 kinase activity of PRAK (Fig. S5C, compare lanes 2 and 4, and Fig. 7B). These indicate that PRAK kinase activity is induced upon acetylation by Tip60 in vitro and in cells. Furthermore, recombinant Tip60 failed to stimulate the protein kinase activity of PRAK carrying the K364R mutation that disrupted acetylation by Tip60 (Fig. 7A). The K51R mutation greatly reduced the basal kinase activity of PRAK, likely due to impairment of ATP binding since K51 is located in the ATP binding pocket. In the IP-kinase assay following 293T cotransfection, the K364R mutation also abrogated Tip60-induced PRAK kinase activity (Fig. 7B). Thus, acetylation of K364 is essential for the stimulation of PRAK kinase activity by Tip60 both in vitro and in cells.
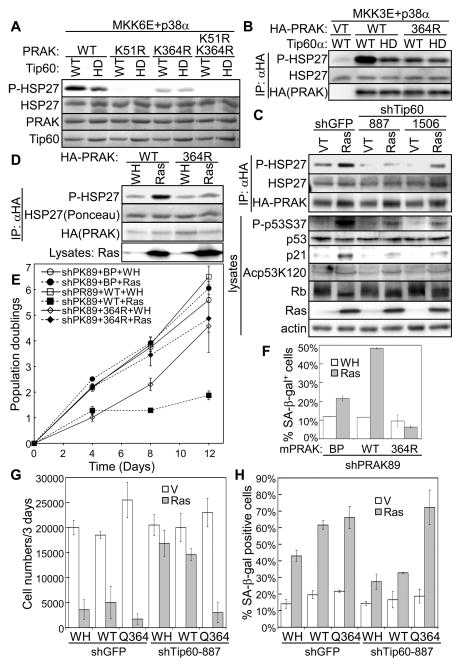
Fig. 7.
Acetylation of PRAK-K364 by Tip60 stimulates PRAK kinase activity in vitro and in cells and is required for the induction of the kinase activity and pro-senescent function of PRAK during ras-induced senescence.
(A) Wild type (WT) or indicated mutant of His- PRAK was incubated first with wild type (WT) or HAT-defective (HD) Tip60α, ATP, acetyl-CoA, p38α and MKK6E, and then with HSP27. Phosphorylated HSP27 (S82) was detected by Western blotting. HSP27 input and recombinant proteins were stained by Ponceau.
(B) HA-PRAK was immunoprecipitated from 293T cells transfected with wild type (WT) or K354R mutant of HA-PRAK or vector (VT), wild type (WT) or HAT-defective (HD) Tip60α, and MKK3E and p38α.
(C) HA-PRAK was immunoprecipitated from BJ cells transduced with HA-PRAK, shRNA for GFP or Tip60 (887, 1506), and HaRasV12 or vector (VT).
(D) HA-PRAK was immunoprecipitated from BJ cells transduced with wild type (WT) or K364R mutant of HA-PRAK, and HaRasV12 or vector (WH).
(B–D) Immunoprecipitated HA-PRAK was incubated with HSP27 and ATP. Phosphorylated HSP27 (S82) and total immunoprecipitated HA-PRAK were detected by Western blotting. HSP27 input was stained by Ponceau. Part of the lysates were also analyzed by Western blotting.
(E) Population doubling of BJ cells transduced with PRAK shRNA (shPK89); mPRAK (WT), mPRAK-K364R (364R) or vector (BP); and HaRasV12 (Ras) or vector (WH) over 12 days, starting on day-5 post ras transduction.
(F) % of SA-β-gal positive cells in the BJ cell populations described in (E) on day-12 post ras transduction.
(G) 1×104 of BJ cells transduced with shRNA for GFP (shGFP) or Tip60 (shTip60-887); human PRAK (WT), PRAK-K364Q (Q364) or vector (WH); and HaRasV12 (ras) or vector (V) were seeded in 12-well plates. Cell numbers were counted 3 days later.
(H) % of SA-β-gal positive cells in the BJ cell populations described in (G) on day-10 post ras transduction.
(E–H) Values are mean ± SD for triplicates.
See also Figure S5.
We further examined the effect of PRAK acetylation in ras-induced senescence. As described previously (Sun et al., 2007), PRAK immunoprecipitated from BJ cells undergoing ras-induced senescence displayed increased HSP27 kinase activity as compared to that from control cells (Fig. 7C). However, this induction of PRAK activity was greatly impaired in BJ cells stably expressing Tip60 shRNA (Fig.7C), suggesting that oncogenic ras stimulates PRAK kinase activity in a Tip60-dependent manner in senescent cells. Moreover, the K364R mutation that disrupts PRAK acetylation by Tip60 also abrogated ras-induced PRAK kinase activity (Fig. 7D). These results establish that during senescence induction, oncogenic ras stimulates the enzymatic activity of PRAK at least partly through Tip60-mediated acetylation of PRAK at K364.
We reported previously that shRNA targeting human PRAK disrupts ras-induced senescence, and that ectopic expression of murine PRAK, which cannot be silenced by the human PRAK shRNA, restores senescence in cells expressing the shRNA (Sun et al., 2007). Using this system, we tested the effect of Tip60 acetylation site on the ability of PRAK to mediate senescence. As expected, wild type murine PRAK restored ras-induced proliferative arrest (Fig. 7E) and expression of the SA-β-gal marker (Fig. 7F) in cells expressing the human PRAK shRNA (shPK89). In contrast, murine PRAK carrying the K364R mutation failed to restore senescence induction (Fig. 7E, F), suggesting that acetylation by Tip60 is critical for PRAK to mediate ras-induced senescence. Thus, acetylation of K364 by Tip60 is required for the induction of both kinase activity and pro-senescent function of PRAK during ras-induced senescence.
We showed previously that upon activation by oncogenic ras, PRAK activates p53 by phosphorylating p53 at Ser37 (Sun et al., 2007). Consistent with reduced PRAK protein kinase activity, we observed a reduction in ras-induced p53-S37 phosphorylation and ras-induced expression of p21WAF1, a transcriptional target of p53, in cells with Tip60 knockdown (Fig. 7C). Senescence is accompanied by accumulation of hypo-phosphorylated forms of Rb, which correlates with cell cycle arrest. We found that the ras-induced switch of Rb from hyper-phosphorylated forms to hypo-phosphorylated forms was attenuated in BJ cells with Tip60 knockdown (Fig. 7C), which is likely to be resulted from reduced expression of p21WAF1, an inhibitor of CDKs that phosphorylate Rb. Thus, our results indicate that the p38-Tip60-PRAK cascade impinges on p53-p21WAF1-Rb, a key effect pathway in senescence.
Tip60 regulates the pro-apoptotic activity of p53 by acetylating p53 at K120 (Charvet et al., 2011; Tang et al., 2006). Oncogenic ras induced acetylation of p53-K120; however ras-induced p53-K120 acetylation was not reduced by Tip60 shRNA (Fig. 7C), suggesting that p53-K120 acetylation in senescence may be mediated by a different acetyltransferase. Supporting this notion, MOZ, another member of the MYST family of histone acetyltransferases, acetylates p53-K120 and promotes senescence (Rokudai et al., 2013).
Tip60 mediates DNA damage responses induced by genotoxic agents or oncogenes (Gorrini et al., 2007; Sun et al., 2005), and DNA damage is an intermediate step in ras-induced senescence (Bartkova et al., 2006; Di et al., 2006). We found that Tip60 shRNA reduced ras-induced formation of γH2AX-positive DNA damage foci (Fig. S5D, E), suggesting that Tip60 also contributes to DNA damage responses in senescence. Thus, Tip60 likely regulates senescence through multiple pathways. To investigate the relative contribution of the PRAK-dependent pathway, we tested the effect of the PRAK-K364Q mutation that mimics Tip60-mediated acetylation on senescence induction in Tip60 knockdown cells. The PRAK-K364Q mutant restored ras-induced senescence in BJ cells expressing Tip60 shRNA (Fig. 7G, 7H), indicating that PRAK acetylation is at least one of the major signaling components that Tip60 relies on to mediate senescence.
DISCUSSION
The current study identifies a novel cascade of posttranslational modifications underlies the essential roles of p38, Tip60, and PRAK in oncogene-induced senescence. Despite the importance of Tip60 in multiple cellular processes, little is known about the mechanism underlying the regulation of Tip60 activity by upstream signaling. An early report suggested that Tip60 activity is modulated by cyclin B/Cdc2-mediated phosphorylation of Ser90 (Lemercier et al., 2003). More recent studies demonstrate that GSK3 phosphorylates Tip60 on Ser86, which allows Tip60 to participate in growth factor deprivation-induced autophage or to promote apoptosis (Charvet et al., 2011; Lin et al., 2012). We demonstrate that Tip60 is phosphorylated by p38 on Thr158, which represents a novel mechanism that regulates the enzymatic activity as well as the biological function of Tip60. Thr158 is located in the hinge region between the chromodomain and the catalytic MYST domain. Phosphorylation of Thr158 may induce a conformational change in Tip60, leading to increased acetyltransferase activity.
We reported that PRAK plays an essential role in oncogene-induced senescence in cell culture and mouse cancer models (Sun et al., 2007). The only previously known mode of regulation of PRAK activity is through phosphorylation. In this study, we demonstrate for the first time that PRAK kinase activity and pro-senescent function can be stimulated via acetylation.
Our finding on the requirement of Tip60 for oncogene-induced senescence is in line with the tumor suppressing activity of Tip60 (Gorrini et al., 2007). At least part of the tumor suppressing activity of Tip60 has been previously attributed to its ability to mediate apoptosis through direct acetylation of p53-K120, which does not contribute to proliferative arrest (Charvet et al., 2011; Tang et al., 2006). The ability of Tip60 to mediate oncogene-induced senescence through acetylation of PRAK, as reported in the current study, offers a novel mechanism underlying the tumor suppressing activity of Tip60.
We show that Tip60 plays an essential role in oncogenic ras-induced senescence by stimulating the activity and pro-senescent function of PRAK. Since Tip60 is a multi-functional acetyltransferase, we cannot rule out the possibility that Tip60 may participate in oncogene-induced senescence through additional, PRAK-independent routes. For example, Tip60 may directly activate p53, a key senescence effector, as a transcriptional coactivator of p53 (Berns et al., 2004; Doyon and Cote, 2004). In addition, Tip60 can mediate DNA damage responses by activating ATM through direct acetylation (Sun et al., 2005). Thus, Tip60 may contribute to senescence by regulating ATM-mediated DNA damage response, which is an intermediate step in senescence induction. Indeed, Tip60 shRNA reduced ras-induced formation of DNA damage foci (Fig. S5D, E). Nevertheless, data presented in the current study demonstrate that PRAK acetylation and activation is at least one of the major signaling step acting downstream of Tip60 to mediate senescence.
Although the currently study focuses on oncogenic ras-induced senescence, the function of the p38-Tip60-PRAK pathway may also be operational in other cellular processes, especially those induced by stresses that activate p38. One recent paper reported that Tip60 functions to maintain adult cardiomyocytes in growth arrested state and promote apoptosis under stress-induced conditions (Fisher et al., 2012). It will be intriguing to determine whether the posttranslational modification cascade involving p38, Tip60 and PRAK also plays a role in this biological setting.
EXPERIMENTAL PROCEDURES
Cell culture
BJ fibroblasts were maintained in Minimum Essential medium supplemented with 10% fetal calf serum, non-essential amino acids, glutamine and antibiotics. WI38 and IMR90 fibroblasts, 293T, H1299, and LinX-A retroviral packaging cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum, glutamine and antibiotics. If necessary, 10 μM of SB203580 were added to medium to inhibit p38.
Yeast 2-hybrid screen
Yeast 2-hybrid screen was performing with the Matchmaker system (Clontech) using PRAK as bait, as described in details in Supplemental Experimental Procedures.
Plasmids
Plasmids used in the study are described in Supplemental Experimental Procedures.
Retrovirus-based gene transduction
Retroviral-based transduction was carried out as previously described (Sun et al., 1998). Transduced cells were purified with 120 μg/ml hygromycin B, 400 μg/ml G418, 5 μg/ml blasticidin and/or 1.2 μg/ml puromycin.
Analysis of senescence
Analysis of senescence was performed using growth curve assay starting on day-4-5 and SA-β-gal staining on day-6-12 post ras transduction as described previously (Wang et al., 2002). To quantify SA-β-gal positives, at least 200 cells were counted in random fields in each of the triplicated wells.
Western blot analysis
Western blotting was performed with lysates prepared 6–8 days after transduction of Ras or MKK3/6E from sub-confluent cells as described (Wang et al., 2002). Primary antibodies are described in Supplemental Experimental Procedures.
Reverse transcription-coupled PCR
RNA was isolated using TRIzol reagent (Life Technologies). RT-PCR was performed using One-Step RT-PCR kit (Qiagen). Details are provided in Supplemental Experimental Procedures.
Recombinant proteins
Recombinant Hsp27, GST-ATF2, His-PRAK, GST-MKK6E, and His-p38α, β, γ and δ isoforms were prepared as described previously (Kwong et al., 2009; New et al., 1998; New et al., 2003). Histones were purchased from Millipore and Sigma. His-Tip60 and GST-PRAK were prepared as described in Supplemental Experimental Procedures.
GST pull down
1 μg of GST-PRAK or GST protein were incubated with 1 μg of wild type or deletion mutant of Tip60α in PBST (phosphate-buffered saline with 0.5% Triton X-100) at 4° C for 1 hour. 10 μl of Glutathione-agarose beads were added. After 1 hour of additional incubation, beads were spun down at 2,000g, 4° C for 1 minute, washed 3 times with PBST, and resuspended in 2X Laemmli buffer. All samples were heated at 95° C and resolved by SDS-PAGE. GST-PRAK and GST proteins were visualized by Coomassie Brilliant Blue R staining, and Tip60 proteins were detected by Western blotting.
Co-Transfection in 293T and H1299 cells
293T and H1299 cells were in general transfected by Lipofectamine 2000 (Life Technologies) according to manufacturer’s protocol. In some cases, 297T cells were transfected with calcium phosphate, as described in Supplemental Experimental Procedures.
Immunoprecipitation
Immunoprecipitation was performed in 293T or BJ cells as described in details in Supplemental Experimental Procedures.
PRAK kinase assay
Acetylation-coupled PRAK kinase assays were performed as described in details in Supplemental Experimental Procedures.
Tip60 acetyltransferase assay
Phosphorylation-coupled Tip60 acetyltransferase assays were performed as described in details in Supplemental Experimental Procedures.
Mass spectrometry
p38 phosphorylation site on Tip60 and Tip60 acetylation sites on PRAK were identified by mass spectrometry. Details are provided in Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
p38, Tip60 and PRAK functionally interact to mediate oncogene-induced senescence
p38 induces activity and prosenescent function of Tip60 by phosphorylating Thr158
Tip60 induces the activity and prosenescent function of PRAK by acetylating K364
Acknowledgments
We thank Drs. Didier Trouche, John Lough, David Engelberg and Oded Livnah for providing reagents. This work was supported by NIH (CA106768 and CA131231). The Scripps manuscript number is 22090.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askari N, Diskin R, Avitzour M, Capone R, Livnah O, Engelberg D. Hyperactive variants of p38alpha induce, whereas hyperactive variants of p38gamma suppress, activating protein 1-mediated transcription. J Biol Chem. 2007;282:91–99. doi: 10.1074/jbc.M608012200. [DOI] [PubMed] [Google Scholar]
- Avitzour M, Diskin R, Raboy B, Askari N, Engelberg D, Livnah O. Intrinsically active variants of all human p38 isoforms. FEBS J. 2007;274:963–975. doi: 10.1111/j.1742-4658.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M, Nijkamp W, Weigelt B, Agami R, Ge W, Cavet G, Linsley PS, Beijersbergen RL, Bernards R. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Charvet C, Wissler M, Brauns-Schubert P, Wang SJ, Tang Y, Sigloch FC, Mellert H, Brandenburg M, Lindner SE, Breit B, Green DR, McMahon SB, Borner C, Gu W, Maurer U. Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol Cell. 2011;42:584–596. doi: 10.1016/j.molcel.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- Di MR, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre’ M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, dda di FF. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Doyon Y, Cote J. The highly conserved and multifunctional NuA4 HAT complex. Curr Opin Genet Dev. 2004;14:147–154. doi: 10.1016/j.gde.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G, de Stanchina E, Lin AW, Querido E, McCurrach ME, Hannon GJ, Lowe SW. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol Cell Biol. 2002;22:3497–3508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JB, Kim MS, Blinka S, Ge ZD, Wan T, Duris C, Christian D, Twaroski K, North P, Auchampach J, Lough J. Stress-induced cell-cycle activation in tip60 haploinsufficient adult cardiomyocytes. PLoS One. 2012;7:e31569. doi: 10.1371/journal.pone.0031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrini C, Squatrito M, Luise C, Syed N, Perna D, Wark L, Martinato F, Sardella D, Verrecchia A, Bennett S, Confalonieri S, Cesaroni M, Marchesi F, Gasco M, Scanziani E, Capra M, Mai S, Nuciforo P, Crook T, Lough J, Amati B. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature. 2007;448:1063–1067. doi: 10.1038/nature06055. [DOI] [PubMed] [Google Scholar]
- Haq R, Brenton JD, Takahashi M, Finan D, Finkielsztein A, Damaraju S, Rottapel R, Zanke B. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 2002;62:5076–5082. [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Kwong J, Hong L, Liao R, Deng Q, Han J, Sun P. p38alpha and p38gamma mediate oncogenic ras-induced senescence through differential mechanisms. J Biol Chem. 2009;284:11237–11246. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemercier C, Legube G, Caron C, Louwagie M, Garin J, Trouche D, Khochbin S. Tip60 acetyltransferase activity is controlled by phosphorylation. J Biol Chem. 2003;278:4713–4718. doi: 10.1074/jbc.M211811200. [DOI] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, Van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Li TY, Liu Q, Zhang C, Li X, Chen Y, Zhang SM, Lian G, Liu Q, Ruan K, Wang Z, Zhang CS, Chien KY, Wu J, Li Q, Han J, Lin SC. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science. 2012;336:477–481. doi: 10.1126/science.1217032. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Narita M, Lowe SW. Senescence comes of age. Nat Med. 2005;11:920–922. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- New L, Jiang Y, Han J. Regulation of PRAK subcellular location by p38 MAP kinases. Mol Biol Cell. 2003;14:2603–2616. doi: 10.1091/mbc.E02-08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood LJ, Kato Y, Parry GC, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke B, Bastien J, Khanna SJ, Warne PH, Cowling V, Cook SJ, Peters G, Delpuech O, Schulze A, Berns K, Mullenders J, Beijersbergen RL, Bernards R, Ganesan TS, Downward J, Hancock DC. Involvement of MINK, a Ste20 family kinase, in Ras oncogene-induced growth arrest in human ovarian surface epithelial cells. Mol Cell. 2005;20:673–685. doi: 10.1016/j.molcel.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci U S A. 2013;110:3895–3900. doi: 10.1073/pnas.1300490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzi V, Logan IR, Robson CN. Cellular functions of TIP60. Int J Biochem Cell Biol. 2006;38:1496–1509. doi: 10.1016/j.biocel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shi Y, Gaestel M. In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol Chem. 2002;383:1519–1536. doi: 10.1515/BC.2002.173. [DOI] [PubMed] [Google Scholar]
- Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 2006;16:433–442. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Sun P, Dong P, Dai K, Hannon GJ, Beach D. p53-independent role of MDM2 in TGF-beta1 resistance. Science. 1998;282:2270–2272. doi: 10.1126/science.282.5397.2270. [DOI] [PubMed] [Google Scholar]
- Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, Xie C, Chen J, Deng Q, Yamout M, Dong MQ, Frangou CG, Yates JR, III, Wright PE, Han J. PRAK Is Essential for ras-Induced Senescence and Tumor Suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, McMahon SB. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo J, Zhang W, Gu W. Tip60-Dependent Acetylation of p53 Modulates the Decision between Cell-Cycle Arrest and Apoptosis. Mol Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Kitabayashi I. Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX. Biochem Biophys Res Commun. 2009;390:1355–1360. doi: 10.1016/j.bbrc.2009.10.156. [DOI] [PubMed] [Google Scholar]
- Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.