Abstract
Fetal asphyctic preconditioning, induced by a brief episode of experimental hypoxia-ischemia, offers neuroprotection to a subsequent more severe asphyctic insult at birth. Extensive cell stress and apoptosis are important contributing factors of damage in the asphyctic neonatal brain. Because ceramide acts as a second messenger for multiple apoptotic stimuli, including hypoxia/ischemia, we sought to investigate the possible involvement of the ceramide pathway in endogenous neuroprotection induced by fetal asphyctic preconditioning. Global fetal asphyxia was induced in rats by clamping both uterine and ovarian vasculature for 30 min. Fetal asphyxia resulted in acute changes in brain ceramide/sphingomyelin metabolic enzymes, ceramide synthase 1, 2, and 5, acid sphingomyelinase, sphingosine-1-phosphate phosphatase, and the ceramide transporter. This observation correlated with an increase in neuronal apoptosis and in astrocyte number. After birth, ceramide and sphingomyelin levels remained high in fetal asphyxia brains, suggesting that a long-term regulation of the ceramide pathway may be involved in the mechanism of tolerance to a subsequent, otherwise lethal, asphyctic event.
Keywords: apoptosis, inflammation, neonatal rat, neuroprotection, sphingomyelin
Perinatal asphyxia (PA) (hypoxia/ischemia) is one of the most common causes contributing to neonatal morbidity and mortality (1, 2). During periods of asphyxia, impaired gas exchange between mother and fetus occurs, leading to changes in blood gases and pH (3). As a consequence, asphyctic newborns develop multi-organ dysfunction (4); with the brain as one of the most affected organs reflecting severe and long-term cognitive and motor deficits (5, 6). Yet, no effective therapies are available, except for hypothermia in term neonates suffering from moderate encephalopathy (7). There is, however, evidence that molecular mechanisms of organ preconditioning result in tissue tolerance to a following more severe insult that would otherwise be lethal (8). Previously, we have shown in a rat model that fetal asphyxia, induced at embryonic day 17 (E17) by clamping the uterine vasculature for 30 min, induces brain tolerance to a stronger PA insult (9, 10). Moreover, less apoptotic cell death was seen in brains of Fetal asphyctic preconditioned animals compared with nonpreconditioned animals subjected to PA (10). Hence, the identification of the signaling pathways and effectors involved in brain tolerance is of primary importance for the development of new neuroprotective therapies.
Important mechanisms may involve changes in ceramide levels which have been reported in various models of hypoxia/ischemia (11, 12). Ceramide is the backbone of complex sphingolipids and is a very active metabolite (11). Increased ceramide synthesis in response to short periods of hypoxia induces DNA fragmentation and cell death (11, 13). Conversely, chronic exposure to hypoxia is characterized by the lack of ceramide accumulation (14) suggesting that a protective adaptive response to chronic hypoxia may exist. Because ceramide acts as a second messenger in apoptosis (15), its production is highly regulated and its levels inside the cell depend on the activity of several enzymes that participate in its synthesis and catabolism (11, 16, 17). In this study, we aim to investigate acute changes occurring in the sphingomyelin/ceramide pathway after a sublethal fetal asphyctic insult to identify molecules important in brain tolerance. Moreover, we study the crosstalk between ceramide metabolism and the brain apoptotic response. Better understanding of these mechanisms may allow the development of new neuroprotective therapies.
EXPERIMENTAL PROCEDURES
Animals
All experiments were approved by the Animal Ethics Board of Maastricht University on animal welfare according to Dutch governmental regulations. Timed-pregnant Sprague-Dawley rats (E14; Charles River, France) were kept under standard laboratory conditions (food and water given ad libitum, 21 ± 2°C environment temperature, and a 12 h light/dark schedule). Unsexed fetuses and male neonates were used within this study.
Experimental design
Fetal asphyxia was induced as previously described by Strackx et al. (9). Briefly, at E17 fetal asphyxia was induced by clamping both uterine and ovarian arteries with removable clamps for 30 min (Fig. 1A). All pups were born by caesarean section (CS). Animals were sacrificed directly after CS performed at 6, 12, and 72 h after the fetal asphyctic insult. Alternatively, CS was performed at birth (E21) and animals were sacrificed at 2 h, 6 h, and 7 days after CS (n = 6 for each group at every time point) (Fig. 1B). Control pups were born at the same time points by CS from untreated mothers. Pups belonging to the prenatal experimental groups were littermates from the same mother. The postnatal pups were born from at least two mothers. Total brain hemispheres of fetal and neonatal pups were collected and snap-frozen. All samples were stored at −80°C prior to further analysis.
Fig. 1.
Experimental design. Fetal asphyxia was induced at E17 by clamping the uterine vasculature for 30 min (A). At term birth, animals were delivered by caesarean section. Pups were euthanized at three different time points after fetal asphyxia prenatally and at three different time points postnatally (n = 6 per group per time point) (B). E, embryonic day; d, days.
RNA isolation
Total brain hemispheres were collected at 6 and 12 h after the fetal asphyctic insult and 2 h, 6 h and 7 days after birth. Total RNA was extracted from frozen tissue by homogenization of the samples with TRIzol reagent (Invitrogen, Breda, The Netherlands) according to the manufacturer's guidelines. Quality and quantity of the RNA were determined using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, MA). RNA integrity number values were determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, Amstelveen, The Netherlands) and RNA samples with RNA integrity number > 8 were included.
Reverse transcription
cDNA was synthesized by using the RevertAid First Strand cDNA synthesis kit (Fermentas, St. Leon Rot, Germany) according to the manufacturer's protocol. The samples were diluted 1 to 20 with DEPC-treated sterilized water and stored at −80°C prior to quantitative PCR analysis.
Real-time PCR
Primers were designed using Primer3plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi, Boston, MA) for the ceramide transporter protein (CERT), Goodpasture antigen-binding protein (GPBP), LAG1 homolog ceramide synthase (Lass)1 to Lass6, neutral sphingomyelinase 1 and 2 (nSMase1-2), acid sphingomyelinase (aSMase), sphingomyelin synthase 1 and 2 (SMS1-2), sphingosine kinase 1 (SphK1), sphingosine-1-phosphate phosphatase (Sph1PP), and oxysterol-binding protein 1 (OSBP1) (Table 1). All samples were analyzed in duplicate using the LightCycler 480 SYBR Green I Master kit (Roche, Almere, The Netherlands). Samples negative for RevertAid Reverse Transcriptase were used as control to ensure specific amplification. The real-time PCR was performed on a LightCycler 480 system (Roche) with 45 cycles: 20 s at 95°C, 15 s at 60°C, 15 s at 72°C. To standardize for the amount of cDNA, the geometric mean of three reference genes, β-actin, hypoxanthine-guanine phosphoribosyltransferase (HPRT), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1), was used. Quantification cycle values were analyzed with the Lightcycler 480 software (Conversion LC 480 and LingRegPCR version 9.19β) and calculated based on the cycle threshold (Ct) values.
TABLE 1.
Primer sequences for the selected genes of interest for quantitative PCR analysis
| Gene | Accession number | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
| β-actin | NM_031144.3 | TTGCTGACAGGATGCAGAAG | TGATCCACATCTGCTGGAAG |
| GAPDH | NM_017008.4 | CTCCCATTCTTCCACCTTTG | ATGTAGGCCATGAGGTCCAC |
| HPRT | NM_012583.2 | TTGCTGGTGAAAAGGACCTC | TCCACTTTCGCTGATGACAC |
| CERT | NM_005713.2 | ATAGAGGAACAGTCACAGAGTG | CTGTACCATCTCTTCAACCTTTTG |
| GPBP | NM_005713.2 | ATGTCCACAGATTCAGCTCCC | CTTCTTCTACAACCAGCTGCC |
| Lass1 | NM_001044230.2 | CTTCTTCAACACTCTGCTGCTG | TCTTCCAGTTCACGCATCTCG |
| Lass2 | NM_001033700 | GCCTTTGACTCCCTGACTTCA | GGGGTAGGGTGAGGGCATGTA |
| Lass3 | NM_001127561 | CTGGCTTCCTCCGACAATAAA | GGTGTGGCAACAAACTTTTCAAA |
| Lass4 | NM_001107117 | GTGGCTGTGGCAGGAGACATA | GCAAGGCCACGAATCTCTCAA |
| Lass5 | NM_001108993 | GGATGCTGTTCGAGCGATTTA | CATCCCAGTCCAGTTGCTTTGA |
| Lass6 | XM_345363 | CAGCGACACAGGAGTGGACAA | CGCACCATGAAGATGCAGAA |
| nSMase1 | NM_031360.2 | TCAGGAAGACCCTTGCTCTG | GACAGCCCCAAAATCATCAC |
| nSMase2 | NM_053605 | TGAAAACATTATTGAGCCTTGC | CTTTGCCACAGCCAATGTC |
| aSMase | NM_001006997.1 | TGACTCTAAGGGATGGAAGCC | AAAAGAGGGTGGAGAAGGGG |
| SMS1 | NM_181386.2 | GGTATCACACGATGGCCAAT | GATCGAGGTACAATCCCTTGG |
| SMS2 | DQ071571 | ACATCCAGATTTCCATGCCC | AAGAGAGCGTACACAAAGGC |
| SphK1 | NM_001270811.1 | AAACCCCTATGTAGCCTCCC | TAGTGACCTGCTCGTGCC |
| Sph1PP | NM_001191811.1 | CACGTTACCCTTAGCTATCCC | CAACATCCCTATGACGACCC |
Western blotting
Frozen brain hemispheres were collected at 6 and 12 h after the fetal asphyctic insult and 2 h, 6 h, and 7 days after birth and homogenized in CelLytic MT Mammalian Tissue Lysis/Extraction reagent (Sigma-Aldrich, St. Louis, MO). The samples were then centrifuged at 15,000 g for 10 min. The protein-containing supernatants were collected and the concentration of total protein was measured by Bradford, using BSA as a standard (18).
Equal amounts of total protein (30 μg) from each sample were separated using 10% SDS-PAGE gels and transferred onto a nitrocellulose membrane (Millipore, Amsterdam, The Netherlands). Blocking was performed with 5% BSA. Next, the membranes were incubated overnight at 4°C using rabbit polyclonal anti-GPBP/CERT (1:5,000 dilution, epitope 1-50 of human GPBP; Bethyl Laboratories, Montgomery, TX) (19) and with monoclonal mouse anti-rabbit GAPDH (1:2,000,000 dilution; Fitzgerald Industries, Concord, MA) as a loading control. After PBS washes, membranes were incubated with goat anti-rabbit-Alexa IRDye800CW (1:10,000 dilution; LI-COR Biosciences, Lincoln, NE) and donkey anti-mouse IRDye680DX conjugated (1:10,000 dilution; Rockland Immunochemicals, Gilbertsville, PA) for 1 h at room temperature. Targeted proteins were analyzed using the LICOR Odyssey scanner (Li-Cor Bioscience, Westburg, Leusden, The Netherlands) and ImageJ software (National Institutes of Health, Bethesda, MD).
Measurement of sphingolipids
Sphingolipid levels were determined at 6 and 12 h after the fetal asphyctic insult and 2 h, 6 h, and 7 days after birth. Brain pieces were homogenized using a Zymo Research bead beater for 2 × 20 s at 6 m/s in water yielding a 250 mg/ml tissue homogenate. Glycosphingolipid content was determined as previously described (20) with slight modifications. Briefly, 50 μl of brain homogenate was extracted with 600 μl of CHCl3/methanol 1/2 (v/v). The extract was centrifuged for 10 min at 13,200 rpm and the pellet discarded. Five hundred and ten microliters of CHCl3/MQ-H2O 1/1.3 (v/v) was added, mixed, and centrifuged for 3 min at 13,200 rpm to separate the phases. The lower phase was collected and the upper phase reextracted with 400 μl of CHCl3. The combined lower phases were dried under N2 flow, taken up in 500 μl of freshly prepared 0.1 M NaOH in methanol, and deacylated in a microwave (SAM-155, CEM Corp.) for 60 min. Fifty microliters of this solution was derivatized with 25 μl ortho-phthalaldehyde (OPA) reagent. The OPA-derivatized lipids were separated by HPLC and quantified as previously described, using C17 sphinganine as an internal standard (Avanti Polar Lipids, Alabaster, AL) (20). Sphingomyelin content was determined by incubating the samples with 125 mU of sphingomyelinase from Bacillus cereus (Sigma-Aldrich, St. Louis, MO) for 1 h at 37°C. The samples were then extracted as previously described (20), glycosphingolipids and sphingomyelin levels where calculated by subtracting the ceramide levels from treated and nontreated samples.
Flow cytometric analysis
At 72 h after fetal asphyxia, brains were collected for fluorescence-activated cell sorting (FACS) analysis. Total brains were placed in ice-cold plating medium consisting of DMEM supplemented with 10% fetal bovine serum (FBS), penicilline/streptavidine, and glutamate. The tissue was mechanically disrupted using a glass homogenizer. The cell suspension was centrifuged and the pellet was resuspended in 5 ml of plating medium. The crude cell suspension was then passed through a 100 μm nylon cell strainer to remove large cell clumps. Next, cells numbers were counted using a Bürker's chamber and divided into different Eppendorf tubes (106 cells/0.5 ml per tube). The viability of the cells was monitored using trypan blue (21).
Apoptotic and necrotic cells were detected with AnnexinV conjugated to FITC and propidium iodide. The staining was executed according to the manufacturer's instructions (AnnexinV-FITC Apoptosis Detection Kit; BD Biosciences Pharmingen, Breda, The Netherlands).
To identify different cell types, extracellular staining for OX42 (microglia) and intracellular staining for GFAP (astroglia), CNPase (oligodendrocytes), and NF-200 (neurons) was performed. Shortly, the cells were washed with staining buffer (PBS + 2% FBS). After washing, cells were incubated for 30 min at room temperature with the primary antibody: mouse anti-rat CD11b/c (clone OX42, BD Pharmingen, 1:100 dilution). The cells were washed twice followed by incubation with the secondary antibody (donkey anti-mouse alexa 488, 1:100 dilution). For intracellular staining, cells were fixed with 4% paraformaldehyde in staining buffer for 20 min at room temperature. Permeabilization was done after two more washing steps with permeabilization buffer (0.05% saponin + 2% FBS + PBS) for 10 min at room temperature. The cell suspension was then incubated with the primary antibody for 30 or 45 min at room temperature: mouse anti-ovine CNPase (Clone 11-5B, 1:200 dilution; Sigma-Aldrich), rabbit anti-ovine GFAP (1:200 dilution; DAKO, Heverlee Belgium), and mouse anti-NF-200 (1:100 dilution; Abcam, Cambridge, UK). After two washing steps, the cell suspension was incubated with the secondary antibody (donkey anti-mouse or donkey anti-rabbit alexa 488, 1:100 dilution) for 30 min at room temperature. Finally, all samples were washed and kept at 4°C in the dark until analysis.
All samples were measured on a FACScalibur flow cytometry system (BD Biosciences, Breda, The Netherlands) equipped with an argon ion laser (488 nm). Analysis was done using the Cell Quest Pro software (BD Biosciences). Forward and sideward light angle scatters were collected from all samples. Samples were gated (R1) to exclude cell debris and cellular aggregates for further analysis. For each marker, the mean fluorescence intensity and the percentage of positive cells stained above background were measured for a total of 10,000 cells per sample within the gate (R1). The cut off was defined using control tissue negative for the different markers processed and stained alongside the experimental samples. The mean fluorescence intensity was corrected for autofluorescence using the values from cells only incubated with secondary antibody.
Statistical analysis
All results were expressed as mean + SEM. Statistical analyses were conducted using GraphPad Prism software. Normality was tested using the Kolmogorov-Smirnov test. All results were normally distributed. Comparisons between control and fetal asphyctic animals were analyzed using unpaired t-test, one-way ANOVA, or two-way ANOVA with Bonferroni post hoc test. P < 0.05 was considered statistically significant.
RESULTS
A global fetal asphyctic preconditioning stimulus induces neuroprotection by lowering the amount of apoptotic cell death in PA animals (10) preserving both locomotor activity and cognitive function (9). Because ceramide is an important regulator of cell growth and apoptosis (22), we studied the acute effect of fetal asphyxia in the ceramide signaling pathway (Fig. 2) at several prenatal and postnatal time points (Fig. 1B).
Fig. 2.
Ceramide metabolism pathway. Ceramide can be generated by the activity of three general pathways. i) The de novo pathway that occurs at the cytosolic surface of the ER from condensation of L-serine with palmitoyl CoA. Once formed, de novo-generated ceramide is transported by CERT/GPBP to the Golgi apparatus and ii) converted to sphingomyelin by sphingomyelin synthase (SMS) (sphingomyelinase pathway) or to glucosylceramide (GalCer + GlcCer). iii) The salvage pathway, that takes place at the membrane of different organelles in which ceramide is formed by degradation of sphingomyelin by different sphingomyelinases (SMases). Ceramide generated in lysosomes can be used as a substrate for acid ceramidase for the generation of sphingosine and, from this, the generation of sphingosine-1-phosphate in other compartments. SphK, sphingosine kinase; Sph1PP, sphingosine-1-phosphate phosphatase.
Changes in mRNA levels of enzymes involved in ceramide metabolism at 6 and 12 h after fetal asphyxia
To investigate whether ceramide metabolism genes change at 6 and 12 h after fetal asphyctic preconditioning, we examined by RT-PCR the expression of several enzymes involved in ceramide metabolism: Lass1 to Lass6, SMS1-2, aSMase, nSMase1-2, SphK1, and Sph1PP. We observed significant mRNA changes in ceramide synthases [the enzymes which convert sphinganine to ceramide (23)], Lass1 (P = 0.021), Lass2 (P = 0.006), and Lass5 (P = 0.016) after fetal asphyxia (Fig. 3). No significant changes were seen in mRNA levels of SMS1 and SMS2, which catalyze the formation of sphingomyelin from ceramide (11), nSMase1 and -2, which break down sphingomyelin to generate ceramide (24), and SphK1, which catalyzes the phosphorylation of sphingosine to form sphingosine-1-phosphate (Fig. 3). On the other hand, aSMase which is localized in lysosomes and regulates cellular membrane turnover (17), and Sph1PP involved in regulating levels of sphingosine-1-phosphate (25) were increased after fetal asphyxia compared with untreated controls (aSMase, P = 0.002; Sph1PP, P = 0.039) (Fig. 3). Once generated in the endoplasmatic reticulum (ER), ceramide must be transported by CERT/GPBP to the Golgi apparatus in order to be converted to more complex sphingolipids (17). Fetal asphyxia significantly increased CERT/GPBP mRNA synthesis (P = 0.045), however, we found CERT/GPBP protein levels reduced after fetal asphyxia (P = 0.033) (Fig. 3, bottom right). This suggests that the increased mRNA synthesis of CERT/GPBP is a compensatory response caused by a reduction in CERT/GPBP protein levels, probably to increase ceramide transport for the production of sphingomyelin.
Fig. 3.
Changes in mRNA and protein levels of enzymes involved in ceramide metabolism at 6 and 12 h after fetal asphyxia. A: Prenatal relative mRNA levels of Lass1 to Lass6, nSMase1-2, aSMase, SMS1-2, SPK1, Sph1PP, and CERT/GPBP in control and fetal asphyctic animals. mRNA levels are relative to the geomean of measured housekeeping genes and normalized to control levels. B: Relative protein levels of CERT/GPBP normalized to GAPDH as loading control 6 and 12 h after fetal asphyxia with corresponding control levels. Comparisons between control and fetal asphyctic animals were analyzed using one-way ANOVA; P < 0.05 is indicated for each particular gene/protein.
Using HPLC, we investigated whether the changes in ceramide enzymes lead to changes in ceramide and ceramide metabolite levels. Ceramide and sphingomyelin levels were unaffected shortly after fetal asphyxia (data not shown), suggesting that this phase is mainly characterized by alterations at transcriptional level.
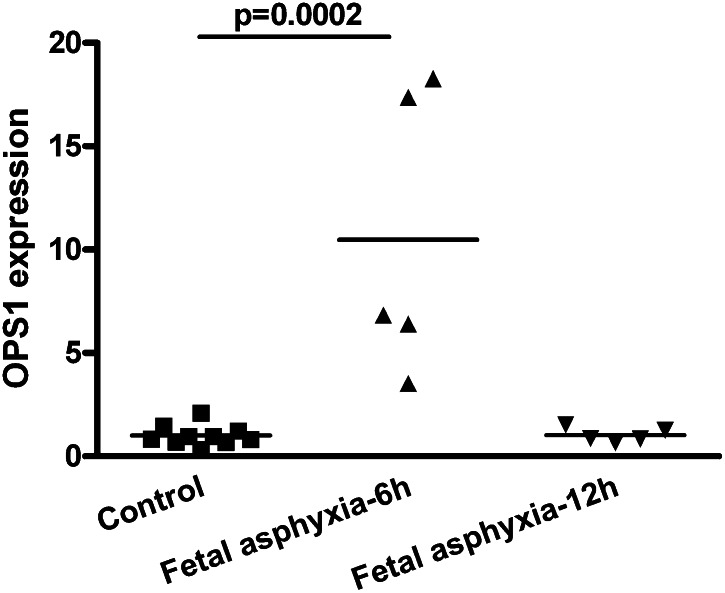
OSBP1 levels are increased 6 h after fetal asphyxia
We investigated if fetal asphyxia could also acutely affect other genes involved in lipid metabolism. We studied OSBP1, a member of a family of sterol-binding proteins, with structural homology to GPBP/CERT (17). We found a significant increase in OSBP1 mRNA levels 6 h after fetal asphyxia compared with controls (P < 0.001) (Fig. 4). However, there was no significant increase in OSBP1 mRNA levels at 12 h after fetal asphyxia compared with controls (P > 0.05). The on-off effect of fetal asphyxia on OSBP1 mRNA levels is in marked contrast to the long-lasting effects of fetal asphyxia on some ceramide metabolism genes which remained affected 12 h after fetal asphyxia (Fig. 3).
Fig. 4.
OSBP1 levels are increased 6 h after fetal asphyxia. Prenatal relative mRNA levels of OSBP1 in control and fetal asphyctic animals. mRNA levels are relative to the geomean of measured housekeeping genes and normalized to control levels. Comparisons between control and fetal asphyctic animals were analyzed using one-way ANOVA and with Bonferroni post hoc test, P < 0.033.
Increased neuronal apoptosis and increased astroglia cell numbers in fetal asphyctic brain
Growing evidence indicates a signaling role for ceramide in asphyxia-induced apoptosis (26, 27). To determine the effects of ceramide changes on apoptotic levels, we quantified the amount of live apoptotic and necrotic cells in total brain of control versus fetal asphyctic animals by FACS analysis at 72 h post-fetal asphyxia. Figure 5A shows that although approximately 35% of the cells undergo apoptosis (due to normal development and the used methodology), the amount of live cells decreased after fetal asphyxia (unpaired t-test, P < 0.001) due to increased apoptosis (unpaired t-test, P = 0.002) but not necrosis, and that neurons contribute most to this increase in apoptosis (unpaired t-test, P = 0.04) (Fig. 5B). Moreover, the asphyctic brain has larger numbers of astrocytes than the control brain (unpaired t-test, P = 0.005) (Fig. 5B). In conclusion, fetal asphyxia induces increased numbers of apoptotic cells, less neurons, and more astrocytes.
Fig. 5.
Increased neuronal apoptosis and increased astroglia cell numbers in fetal asphyxia brain. Amount of live, apoptotic, and necrotic cells (A) and amount of specific cell types (B) 72 h after fetal asphyxia compared with respective control levels. Data shown as mean + SEM. Comparisons between control and fetal asphyctic animals were analyzed using unpaired t-test, P < 0.05 is indicated. White bars represent control and black bars, fetal asphyxia brain.
Increased ceramide levels are sustained until 7 days after birth
To investigate whether the changes in ceramide metabolism after sublethal fetal asphyxia would persist after birth, we assessed ceramide synthesis 2 h, 6 h, and 7 days after birth (Fig. 1B) in fetal asphyxia-subjected animals and controls. At all postnatal time points, ceramide levels were increased after fetal asphyxia (P < 0.0005) (Fig. 6A). Sphingomyelin levels were also increased after fetal asphyxia (P < 0.02) (Fig. 6B). Because the recovered ceramide and sphingomyelin amounts in our rat brain samples were relatively low compared with similar sphingolipids recovered from adult mouse brain (28), we tested whether the amount of SMase used in our assay to determine sphingomyelin concentration in the brain samples was sufficient. We incubated the brain samples with five times more SMase and this did not result in detection of more ceramide (supplementary Table I). Additionally, we investigated a potential negative matrix effect by brain lipids on ceramide detection. Therefore we spiked brain samples with a known amount of ceramide and looked for the recovery of this spike. These results showed that ceramide measurements were not altered in our samples (supplementary Table I).
Fig. 6.
Increased ceramide levels are sustained until 7 days after birth. Ceramide (A) and sphingomyelin (B) concentrations expressed in nmol/mg protein in control (white bars) and fetal asphyxia (black bars) animals. mRNA levels of aSMase (C) are presented relative to the geomean of measured housekeeping genes. Relative protein levels of CERT/GPBP normalized to GAPDH as loading control in control (white bars) and fetal asphyxia (black bars) animals (D). Data shown as mean + SEM. Comparisons between control and fetal asphyctic animals were analyzed using two-way ANOVA; P < 0.05 is indicated for each particular gene/protein. White bars represent control and black bars fetal asphyxia brain.
To correlate the observed changes in ceramide/sphingomyelin levels to expression of genes of the ceramide pathway, we tested by real-time PCR the same set of genes studied prenatally. We found a significant increase in mRNA levels of aSMase after fetal asphyxia (P < 0.0004) (Fig. 6C). Additionally, we could detect a significant upregulation of GPBP/CERT protein levels at 7 days after birth (P < 0.05) in fetal asphyxia subjected animals (Fig. 6D). This increase in sphingolipids in fetal asphyctic animals after birth suggests the persistent activation of the catabolic as well as the de novo ceramide metabolic pathways.
DISCUSSION
In the present study, we found evidence for acute and persisting changes in the ceramide metabolism in the brain due to fetal asphyxia. Before birth, Fetal asphyxia induced a rapid upregulation in expression levels of Lass1, Lass2, aSMase, CERT/GPBP, and Sph1PP and downregulation of Lass5, higher apoptotic cell rate, and increased glia cell numbers. After birth, ceramide and sphingomyelin were found elevated in brains of fetal asphyxia subjected animals. Together, these data demonstrate that fetal asphyxia induces an increase in ceramide levels in the brain and suggest that ceramide may have a role for the observed neuroprotection against a later more severe insult.
Many studies investigated the induction of tolerance to hypoxic-ischemic insults. Nevertheless, the mechanism by which a period of sublethal fetal asphyxia can provide neuroprotection against a subsequent more severe insult is not completely understood. Changes in ceramide levels have been reported in various models of hypoxia. Previously, we reported that an asphyctic episode before birth can induce chronic changes in expression levels of ceramide metabolism genes in the brain (29). Our work suggested that the combination of two subsequent asphyctic insults provides long-lasting brain neuroprotection probably by maintaining normal apoptosis and promoting cell proliferation. Here, we investigated if ceramide metabolism changes can also be observed acutely after fetal asphyxia. We studied early time points (6, 12, and 72 h before birth and 2 and 6 h after birth) and a late time point (7 days after birth). We analyzed the ceramide pathway at early time points because it has been described that hypoxia can induce early inflammatory changes in the brain (30, 31) and ceramides have been associated to inflammation (32). Additionally, we included 7 days after birth because at this time point we have previously demonstrated in our model that apoptosis is increased after severe perinatal asphyxia compared with preconditioned animals (fetal asphyxia-PA) (9).
We found acute prenatal and postnatal changes in ceramide metabolism genes. In particular Lass1 and Lass2 genes were upregulated and Lass5 downregulated prenatally after fetal asphyxia compared with controls. Six isoforms of Lass enzyme have been identified, each responsible for the synthesis of ceramides of distinct fatty acid length (33). Increased ceramide synthase activity has been shown following a brief exposure to hypoxia and oxygen/glucose deprivation (34, 35) to promote the synthesis of ceramide, sphingomyelin, and glycosphingolipids (23). It is not clear which Lass isoform is specifically responsible for the asphyxia-induced ceramide synthesis because data suggest that changes in ceramide synthase expression can be counter-regulated by changes in the other Lass isoforms (36). However results from our rat asphyxia model show that Lass1 and Lass2 are acutely sensitive to oxygen limitation and suggest that they may be partially responsible for the postnatal increase in ceramide levels. Other ceramide metabolism genes that are sensitive to asphyxia are aSMase, Sph1PP (salvage pathway), and CERT/GPBP (de novo pathway). These genes are all acutely upregulated after fetal asphyxia. In the salvage pathway aSMase forms ceramide via lysosomal catabolism of sphingomyelin and Sph1PP catalyzes the degradation of S1P and recycling of sphingosine into long-chain ceramides (37). Both aSMase and Sph1PP sensitivity to acute cellular oxygen changes has already been reported (15, 23, 38, 39). Additionally, we found that mRNA levels of the ceramide transporter CERT/GPBP were elevated before birth after fetal asphyxia. In contrast, protein levels of CERT/GPBP were found to be reduced. As CERT/GPBP is responsible for the transport of ceramide to the Golgi, it is possible that changes in CERT/GPBP expression levels represent a regulatory mechanism to process excessively produced ceramide in response to fetal asphyxia; by reducing/enhancing ceramide transport and modulating the conversion of ceramide to sphingomyelin at the Golgi. Although we identified acute prenatal changes in ceramide metabolism genes and proteins, no changes were seen in overall ceramide or sphingomyelin levels at these time points.
After birth we observed an increase in ceramide and sphingomyelin levels. The acute prenatal stimulation of ceramide synthesis in the de novo and salvage pathways could account for the postnatal increased levels of ceramide and sphingomyelin after fetal asphyxia. The amounts of ceramide and sphingomyelin measured in our samples were markedly lower than those measured in adult mouse brain (28). We attribute the relatively low sphingolipid levels detected to the young age of the fetal and neonatal pups. The postnatal elevation in ceramide and sphingomyelin contents was correlated with increased levels of aSMase, highlighting the persistent activation of this gene, and the importance of the salvage pathway in the response to fetal asphyxia. In contrast, postnatal Lass1 mRNA levels were not changed in fetal asphyxia animals. Because we did not study Lass2 to Lass6 genes at these time points, we cannot estimate the total contribution of the de novo pathway to the generation of postnatal ceramide. Seven days after birth we found more CERT/GPBP protein in fetal asphyctic animals; this may be a compensatory mechanism to respond to a higher production of ceramide in the ER. Additionally, the increase in CERT/GPBP levels can also be a result of the acute inflammatory response post fetal asphyxia (40) as inflammation, and in particular cytokines, are associated with increased CERT/GPBP-mediated transport (17, 41).
Initial studies investigated the chronic effect of FA in our rat asphyxia model, where we observed that ceramide metabolism is chronically changed after fetal asphyxia and PA (28). In contrast when fetal asphyxia and PA were combined, expression levels of genes of the ceramide pathway were normal (29). We showed that apoptotic cell numbers were not different from those of controls when animals were subjected to fetal asphyxia in combination with a later severe asphyctic insult (10).
Here we studied the short-term effect of fetal asphyxia in the same model and found higher ceramide synthesis and higher numbers of apoptotic neurons in fetal asphyctic brains relative to controls. This is apparently contradictory with the idea that fetal asphyxia is neuroprotective. Besides the general knowledge that higher ceramide levels will induce apoptosis and this will have a negative impact in the organism, there are several facts to consider: i) The dual role of ceramide ranging from protective to pro-apoptotic pointed out by several studies. Research has indicated a detrimental role for ceramide and shown that reducing ceramide production might protect against hypoxic-ischemic neuronal injury (15, 27, 38). However, this effect of ceramide was observed in a chronic model of ischemia and the induction of ischemia was in older animals. On the other hand, in hippocampal and cortical neurons it has been suggested that ceramide induces protection against injury, apoptosis, and free radical forms (26) and can mediate hypoxia tolerance induced by hypoxic preconditioning (42). ii) Neuronal cell death occurs during normal brain development and may serve a variety of biological functions including the elimination of cells carrying abnormalities (43), and its dysregulation results in developmental brain pathology (26, 44). iii) Apoptotic cells can protect the tissue-reducing levels of circulating cytokines (45). In our model we observed postnatally that several cytokines are upregulated at the transcriptional level in fetal asphyxia-treated animals compared with controls (40). Increased apoptosis might be a mechanism to reduce brain injury after fetal asphyxia including inflammatory damage.
Finally we also observed larger numbers of astrocytes in fetal asphyctic brains. Other studies have shown increased cell numbers after hypoxic-ischemic brain injury. One of the explanations for this may be that the neonatal animal responds to brain injury with cell proliferation to stimulate recovery (46, 47).
In summary, fetal asphyxia induced a rapid upregulation of enzymes involved in ceramide metabolism leading to the upregulation of ceramide and increased apoptosis. Follow-up studies should focus on targeting enzymes which are found differentially regulated after fetal asphyxia and the combination of fetal asphyxia with a subsequent severe asphyctic insult to further study their potentially protective effect against asphyxia.
Supplementary Material
Acknowledgments
The authors thank Fabian Loidl (MD, PhD) for his help in Figure 1A.
Footnotes
Abbreviations:
- Abbreviations: aSMase
- acid sphingomyelinase
- CERT
- ceramide transporter protein
- CS
- caesarean section
- E17
- embryonic day 17
- ER
- endoplasmic reticulum
- FACS
- fluorescence-activated cell sorting
- GPBP
- Goodpasture antigen-binding protein
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- Lass
- LAG1 homolog ceramide synthase
- nSMase1-2
- neutral sphingomyelinase 1 and 2
- OPA
- o-phthalaldehyde
- OSBP1
- oxysterol-binding protein 1
- PA
- perinatal asphyxia
- SMS1-2
- sphingomyelin synthase 1 and 2
- SphK1
- sphingosine kinase 1
- Sph1PP
- sphingosine-1-phosphate phosphatase
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table.
REFERENCES
- 1.Lawn J. E., Kerber K., Enweronu-Laryea C., Cousens S. 2010. 3.6 million neonatal deaths–what is progressing and what is not? Semin. Perinatol. 34: 371–386 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization 2005. The World Health Report: Make every mother and child count. World Health Organization, [Google Scholar]
- 3.Low J. A. 1997. Intrapartum fetal asphyxia: definition, diagnosis, and classification. Am. J. Obstet. Gynecol. 176: 957–959 [DOI] [PubMed] [Google Scholar]
- 4.Tarcan A., Tiker F., Guvenir H., Gurakan B. 2007. Hepatic involvement in perinatal asphyxia. J. Matern. Fetal Neonatal Med. 20: 407–410 [DOI] [PubMed] [Google Scholar]
- 5.Jensen A., Garnier Y., Middelanis J., Berger R. 2003. Perinatal brain damage–from pathophysiology to prevention. Eur. J. Obstet. Gynecol. Reprod. Biol. 110(Suppl. 1): S70–S79 [DOI] [PubMed] [Google Scholar]
- 6.Low J. A. 2004. Determining the contribution of asphyxia to brain damage in the neonate. J. Obstet. Gynaecol. Res. 30: 276–286 [DOI] [PubMed] [Google Scholar]
- 7.Perlman J. M. 2006. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin. Ther. 28: 1353–1365 [DOI] [PubMed] [Google Scholar]
- 8.Hagberg H., Dammann O., Mallard C., Leviton A. 2004. Preconditioning and the developing brain. Semin. Perinatol. 28: 389–395 [DOI] [PubMed] [Google Scholar]
- 9.Strackx E., Van den Hove D. L., Prickaerts J., Zimmermann L., Steinbusch H. W., Blanco C. E., Gavilanes A. W., Vles J. S. 2010. Fetal asphyctic preconditioning protects against perinatal asphyxia-induced behavioral consequences in adulthood. Behav. Brain Res. 208: 343–351 [DOI] [PubMed] [Google Scholar]
- 10.Strackx E., Zoer B., Van den Hove D., Steinbusch H., Blanco C., Vles J. S., Villamor E., Gavilanes A. W. 2010. Brain apoptosis and carotid artery reactivity in fetal asphyctic preconditioning. Front. Biosci. (Schol. Ed.). 2: 781–790 [DOI] [PubMed] [Google Scholar]
- 11.Novgorodov S. A., Gudz T. I. 2009. Ceramide and mitochondria in ischemia/reperfusion. J. Cardiovasc. Pharmacol. 53: 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuiyan M. I., Islam M. N., Jung S. Y., Yoo H. H., Lee Y. S., Jin C. 2010. Involvement of ceramide in ischemic tolerance induced by preconditioning with sublethal oxygen-glucose deprivation in primary cultured cortical neurons of rats. Biol. Pharm. Bull. 33: 11–17 [DOI] [PubMed] [Google Scholar]
- 13.Ueda N., Kaushal G. P., Hong X., Shah S. V. 1998. Role of enhanced ceramide generation in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Kidney Int. 54: 399–406 [DOI] [PubMed] [Google Scholar]
- 14.Bitar F. F., Mroueh S., El Khatib M., Bitar H., Tarrabain M., El Sabban M., Obeid M., Nasser M., Dbaibo G. S. 2003. Tissue-specific ceramide response in the chronically hypoxic rat model mimicking cyanotic heart disease. Prostaglandins Other Lipid Mediat. 72: 155–163 [DOI] [PubMed] [Google Scholar]
- 15.Ohtani R., Tomimoto H., Kondo T., Wakita H., Akiguchi I., Shibasaki H., Okazaki T. 2004. Upregulation of ceramide and its regulating mechanism in a rat model of chronic cerebral ischemia. Brain Res. 1023: 31–40 [DOI] [PubMed] [Google Scholar]
- 16.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426: 803–809 [DOI] [PubMed] [Google Scholar]
- 17.Mencarelli C., Losen M., Hammels C., De Vry J., Hesselink M. K., Steinbusch H. W., De Baets M. H., Martinez-Martinez P. 2010. The ceramide transporter and the Goodpasture antigen binding protein: one protein–one function? J. Neurochem. 113: 1369–1386 [DOI] [PubMed] [Google Scholar]
- 18.Xu S. S., Yan C. L., Liu L. M., Zeng Q. L. 2008. Effects of different cell lysis buffers on protein quantification. Zhejiang Da Xue Xue Bao Yi Xue Ban. 37: 45–50 [DOI] [PubMed] [Google Scholar]
- 19.Mencarelli C., Hammels C., Van Den Broeck J., Losen M., Steinbusch H., Revert F., Saus J., Hopkins D. A., De Baets M. H., Steinbusch H. W., et al. 2009. The expression of the Goodpasture antigen-binding protein (ceramide transporter) in adult rat brain. J. Chem. Neuroanat. 38: 97–105 [DOI] [PubMed] [Google Scholar]
- 20.Groener J. E., Poorthuis B. J., Kuiper S., Helmond M. T., Hollak C. E., Aerts J. M. 2007. HPLC for simultaneous quantification of total ceramide, glucosylceramide, and ceramide trihexoside concentrations in plasma. Clin. Chem. 53: 742–747 [DOI] [PubMed] [Google Scholar]
- 21.Gavilanes A. W., Strackx E., Kramer B. W., Gantert M., Van den Hove D., Steinbusch H., Garnier Y., Cornips E., Steinbusch H., Zimmermann L., et al. 2009. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am. J. Obstet. Gynecol. 200: 437. e1–8 [DOI] [PubMed] [Google Scholar]
- 22.Mencarelli C., Martinez-Martinez P. 2013. Ceramide function in the brain: when a slight tilt is enough. Cell. Mol. Life Sci. 70: 181–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry R. J., Ridgway N. D. 2005. Molecular mechanisms and regulation of ceramide transport. Biochim. Biophys. Acta. 1734: 220–234 [DOI] [PubMed] [Google Scholar]
- 24.Jana A., Hogan E. L., Pahan K. 2009. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 278: 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson K. R., Johnson K. Y., Becker K. P., Bielawski J., Mao C., Obeid L. M. 2003. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J. Biol. Chem. 278: 34541–34547 [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Ginis I., Hallenbeck J. M. 2001. The protective effect of ceramide in immature rat brain hypoxia-ischemia involves up-regulation of bcl-2 and reduction of TUNEL-positive cells. J. Cereb. Blood Flow Metab. 21: 34–40 [DOI] [PubMed] [Google Scholar]
- 27.Feng Y., LeBlanc M. H. 2006. N-tosyl-L-phenylalanyl-chloromethyl ketone reduces ceramide during hypoxic-ischemic brain injury in newborn rat. Eur. J. Pharmacol. 551: 34–40 [DOI] [PubMed] [Google Scholar]
- 28.Lee S., Lee Y. S., Choi K. M., Yoo K. S., Sin D. M., Kim W., Lee Y. M., Hong J. T., Yun Y. P., Yoo H. S. 2012. Quantitative analysis of sphingomyelin by high-performance liquid chromatography after enzymatic hydrolysis. Evid. Based Complement. Alternat. Med. 2012: 396218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlassaks E., Gavilanes A. W. D., Vles J. S. H., Deville S., Kramer B. W., Strackx E., Martinez-Martinez P. 2013. The effects of fetal and perinatal asphyxia on neuronal cytokine levels and ceramide metabolism in adulthood. J. Neuroimmunol. 255: 97–101. [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Alconada D., Hilario E., Alvarez F. J., Alvarez A. 2012. Apoptotic cell death correlates with ROS overproduction and early cytokine expression after hypoxia-ischemia in fetal lambs. Reprod. Sci. 19: 754–763 [DOI] [PubMed] [Google Scholar]
- 31.Ashdown H., Joita S., Luheshi G. N., Boksa P. 2008. Acute brain cytokine responses after global birth hypoxia in the rat. J. Neurosci. Res. 86: 3401–3409 [DOI] [PubMed] [Google Scholar]
- 32.Grösch S., Schiffmann S., Geisslinger G. 2012. Chain length-specific properties of ceramides. Prog. Lipid Res. 51: 50–62 [DOI] [PubMed] [Google Scholar]
- 33.Bartke N., Hannun Y. A. 2009. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 50(Suppl): S91–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menuz V., Howell K. S., Gentina S., Epstein S., Riezman I., Fornallaz-Mulhauser M., Hengartner M. O., Gomez M., Riezman H., Martinou J. C. 2009. Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science. 324: 381–384 [DOI] [PubMed] [Google Scholar]
- 35.Basnakian A. G., Ueda N., Hong X., Galitovsky V. E., Yin X., Shah S. V. 2005. Ceramide synthase is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. Am. J. Physiol. Renal Physiol. 288: F308–F314 [DOI] [PubMed] [Google Scholar]
- 36.Mullen T. D., Spassieva S., Jenkins R. W., Kitatani K., Bielawski J., Hannun Y. A., Obeid L. M. 2011. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J. Lipid Res. 52: 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandala S. M., Thornton R., Galve-Roperh I., Poulton S., Peterson C., Olivera A., Bergstrom J., Kurtz M. B., Spiegel S. 2000. Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1- phosphate and induces cell death. Proc. Natl. Acad. Sci. USA. 97: 7859–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Z. F., Nikolova-Karakashian M., Zhou D., Cheng G., Schuchman E. H., Mattson M. P. 2000. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J. Mol. Neurosci. 15: 85–97 [DOI] [PubMed] [Google Scholar]
- 39.Llacuna L., Mari M., Garcia-Ruiz C., Fernandez-Checa J. C., Morales A. 2006. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 44: 561–572 [DOI] [PubMed] [Google Scholar]
- 40.Vlassaks E., Strackx E., Vles J., Nikiforou M., Martinez-Martinez P., Kramer B. W., Gavilanes A. W. 2013. Fetal asphyctic preconditioning modulates the acute cytokine response thereby protecting against perinatal asphyxia in neonatal rats. J. Neuroinflammation. 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granero F., Revert F., Revert-Ros F., Lainez S., Martinez-Martinez P., Saus J. 2005. A human-specific TNF-responsive promoter for Goodpasture antigen-binding protein. FEBS J. 272: 5291–5305 [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Ginis I., Spatz M., Hallenbeck J. M. 2000. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am. J. Physiol. Cell Physiol. 278: C144–C153 [DOI] [PubMed] [Google Scholar]
- 43.Chun J., Schatz D. G. 1999. Rearranging views on neurogenesis: neuronal death in the absence of DNA end-joining proteins. Neuron. 22: 7–10 [DOI] [PubMed] [Google Scholar]
- 44.Roth K. A., D'Sa C. 2001. Apoptosis and brain development. Ment. Retard. Dev. Disabil. Res. Rev. 7: 261–266 [DOI] [PubMed] [Google Scholar]
- 45.Ren G, S. J., Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. 2008. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J. Immunol. (5): 3277–3284 [DOI] [PubMed] [Google Scholar]
- 46.Bartley J., Soltau T., Wimborne H., Kim S., Martin-Studdard A., Hess D., Hill W., Waller J., Carroll J. 2005. BrdU-positive cells in the neonatal mouse hippocampus following hypoxic-ischemic brain injury. BMC Neurosci. 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong J., Plane J. M., Parent J. M., Silverstein F. S. 2005. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr. Res. 58: 600–606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.