Abstract
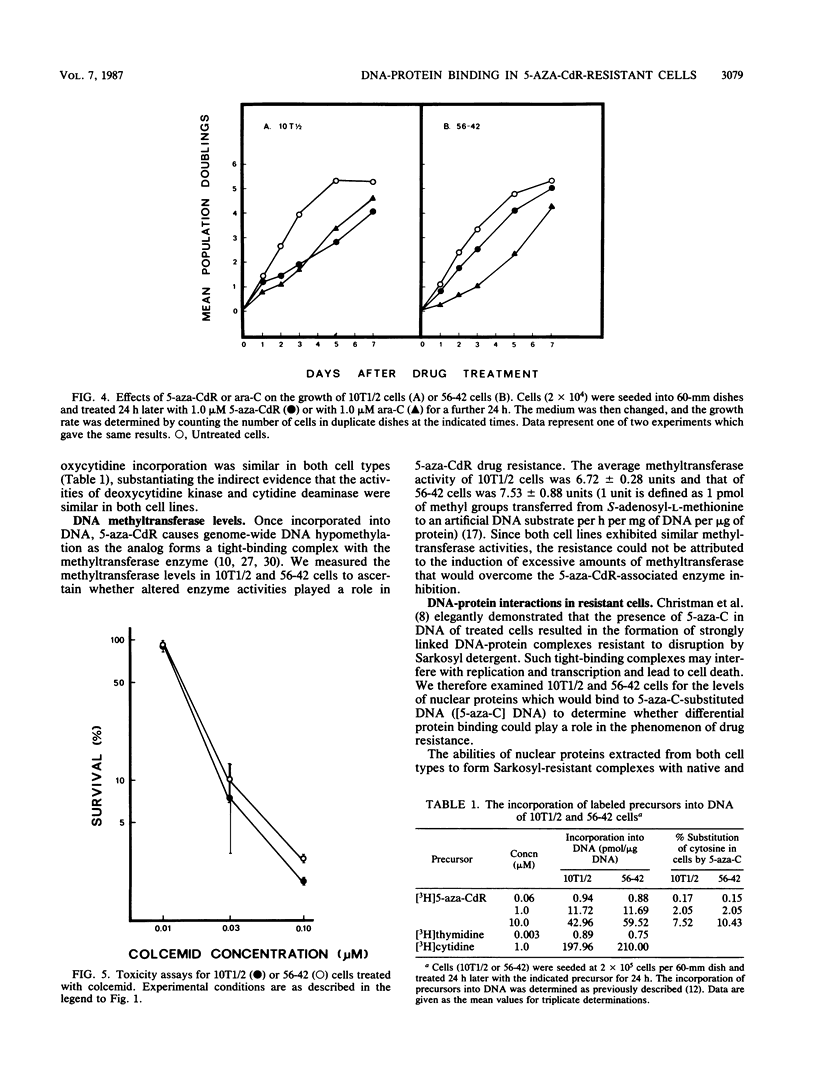
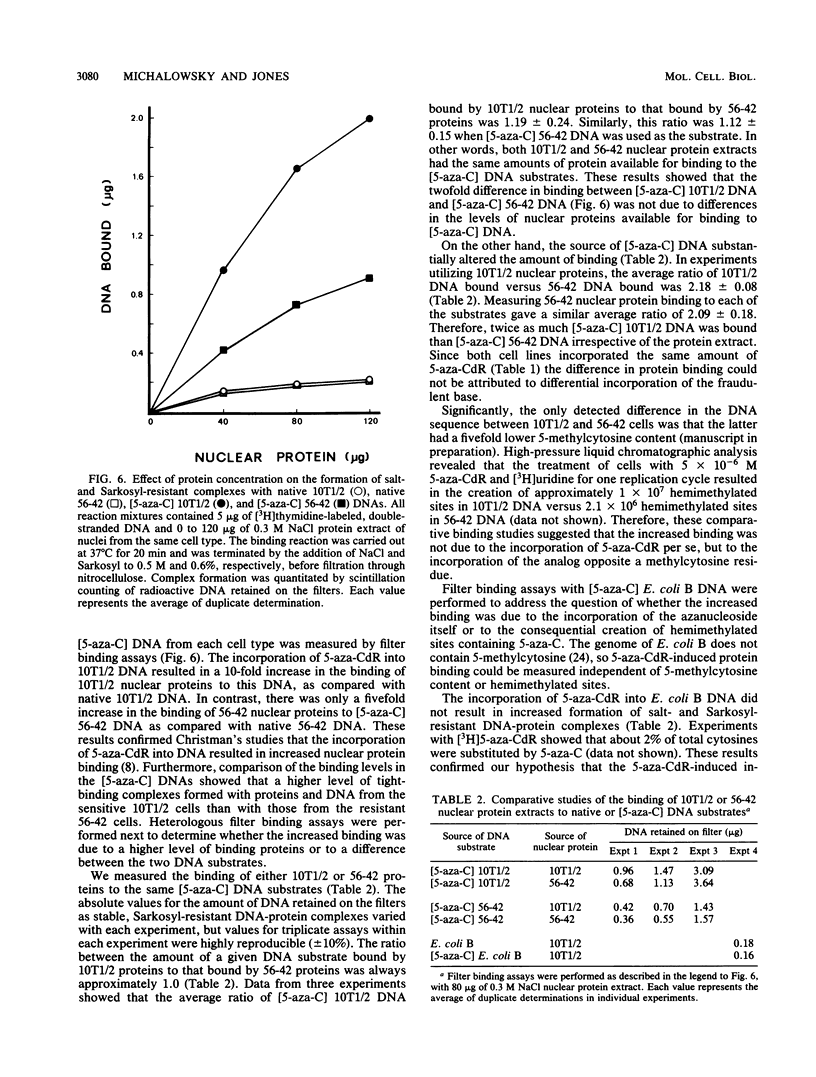
A clonal cell line (56-42) that was stably and exclusively resistant to the toxic effects of the antileukemic agent 5-aza-2'-deoxycytidine (5-aza-CdR) was derived from C3H 10T1/2 C18 cells after multiple treatments with 5-aza-CdR. The 50% lethal dose of 5-aza-CdR for these cells was 1.3 microM, which was 15-fold greater than that for the parental cells. Cell line 56-42 was slightly cross-resistant to the ribo-analog 5-azacytidine, but was sensitive to the nucleoside analog 1-beta-D-arabinofuranosylcytosine and to colcemid. Both parental and resistant cell lines incorporated equimolar amounts of 5-aza-CdR into DNA. Resistance was therefore not due to decreased activation, increased detoxification, or reduced incorporation of the drug. The overall level of cytosine methylation in the resistant clone was 80% lower than the level in the sensitive cells. Therefore, the potential number of hemimethylated sites created by the incorporation of equivalent amounts of 5-aza-CdR into the DNA of the two cell types was much greater in the sensitive cells. Furthermore, 5-azacytosine-substituted DNA from the sensitive cells bound 100% more nuclear protein in the form of highly stable complexes. The incorporation of 5-aza-CdR opposite methylated cytosine residues in DNA of the sensitive cells thus resulted in increased nuclear protein binding at hemimethylated sites. This relative increase in tight-binding protein complexes was shown to occur in living cells and may well disrupt replication and transcription and instigate cell death. The differential binding of proteins to hypomethylated, azacytosine-containing DNA may thus mediate a novel mechanism of drug resistance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Berkowitz D. M., Kakefuda T., Sporn M. A simple and rapid method for the isolation of enzymatically active HeLa cell nuclei. J Cell Biol. 1969 Sep;42(3):851–854. doi: 10.1083/jcb.42.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation--how important in gene control? Nature. 1984 Feb 9;307(5951):503–504. doi: 10.1038/307503a0. [DOI] [PubMed] [Google Scholar]
- Bouchard J., Momparler R. L. Incorporation of 5-Aza-2'-deoxycytidine-5'-triphosphate into DNA. Interactions with mammalian DNA polymerase alpha and DNA methylase. Mol Pharmacol. 1983 Jul;24(1):109–114. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chandler L. A., DeClerck Y. A., Bogenmann E., Jones P. A. Patterns of DNA methylation and gene expression in human tumor cell lines. Cancer Res. 1986 Jun;46(6):2944–2949. [PubMed] [Google Scholar]
- Christman J. K., Schneiderman N., Acs G. Formation of highly stable complexes between 5-azacytosine-substituted DNA and specific non-histone nuclear proteins. Implications for 5-azacytidine-mediated effects on DNA methylation and gene expression. J Biol Chem. 1985 Apr 10;260(7):4059–4068. [PubMed] [Google Scholar]
- Christman J. K., Schneiderman N., Acs G. Interaction of DNA methyltransferase and other non-histone proteins isolated from Friend erythroleukemia cell nuclei with 5-azacytosine residues in DNA. Prog Clin Biol Res. 1985;198:105–118. [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Flatau E., Gonzales F. A., Michalowsky L. A., Jones P. A. DNA methylation in 5-aza-2'-deoxycytidine-resistant variants of C3H 10T1/2 C18 cells. Mol Cell Biol. 1984 Oct;4(10):2098–2102. doi: 10.1128/mcb.4.10.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A. DNA methylation and cancer. Cancer Res. 1986 Feb;46(2):461–466. [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Hemimethylated duplex DNAs prepared from 5-azacytidine-treated cells. Nucleic Acids Res. 1981 Jun 25;9(12):2933–2947. doi: 10.1093/nar/9.12.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowsky L. A., Jones P. A. DNA methylation and drug resistance in variants of C3H10T1/2 C1 8 cells. Prog Clin Biol Res. 1986;226:391–399. [PubMed] [Google Scholar]
- Momparier R. L., Gonzales F. A. Effect of intravenous infusion of 5-aza-2'-deoxycytidine on survival time of mice with L1210 leukemia. Cancer Res. 1978 Sep;38(9):2673–2678. [PubMed] [Google Scholar]
- Nikolskaya I. I., Tkatcheva Z. G., Vanyushin B. F., Tikchonenko T. I. On the presence of minor bases in DNA of phages DD7 and Sd and their hosts. Biochim Biophys Acta. 1968 Feb 26;155(2):626–629. doi: 10.1016/0005-2787(68)90211-6. [DOI] [PubMed] [Google Scholar]
- Razin A., Szyf M. DNA methylation patterns. Formation and function. Biochim Biophys Acta. 1984 Sep 10;782(4):331–342. doi: 10.1016/0167-4781(84)90043-5. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Santi D. V., Garrett C. E., Barr P. J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983 May;33(1):9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- Sorm F., Veselý J. Effect of 5-aza-2'-deoxycytidine against leukemic and hemopoietic tissues in AKR mice. Neoplasma. 1968;15(4):339–343. [PubMed] [Google Scholar]
- Sugiyama M., Patierno S. R., Cantoni O., Costa M. Characterization of DNA lesions induced by CaCrO4 in synchronous and asynchronous cultured mammalian cells. Mol Pharmacol. 1986 Jun;29(6):606–613. [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Mechanism of action of eukaryotic DNA methyltransferase. Use of 5-azacytosine-containing DNA. J Mol Biol. 1982 Dec 15;162(3):679–692. doi: 10.1016/0022-2836(82)90395-3. [DOI] [PubMed] [Google Scholar]
- Veselý J., Cihák A. Incorporation of a potent antileukemic agent, 5-aza-2'-deoxycytidine, into DNA of cells from leukemic mice. Cancer Res. 1977 Oct;37(10):3684–3689. [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Association of decreased uridine and deoxycytidine kinase with enhanced RNA and DNA polymerase in mouse leukemic cells resistant to 5-azacytidine and 5-aza-2'-deoxycytidine. Cancer Res. 1970 Aug;30(8):2180–2186. [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Biochemical mechanisms of drug resistance. IV. Development of resistance to 5-azacytidine and simultaneous depression of pyrimidine metabolism in leukemic mice. Int J Cancer. 1967 Nov 15;2(6):639–646. doi: 10.1002/ijc.2910020625. [DOI] [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Characteristics of mouse leukemic cells resistant to 5-azacytidine and 5-aza-2'-deoxycytidine. Cancer Res. 1968 Oct;28(10):1995–2000. [PubMed] [Google Scholar]
- Von Hoff D. D., Slavik M., Muggia F. M. 5-Azacytidine. A new anticancer drug with effectiveness in acute myelogenous leukemia. Ann Intern Med. 1976 Aug;85(2):237–245. doi: 10.7326/0003-4819-85-2-237. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Jones P. A., Momparler R. L. Inhibition of DNA methylation in L1210 leukemic cells by 5-aza-2'-deoxycytidine as a possible mechanism of chemotherapeutic action. Cancer Res. 1983 Aug;43(8):3493–3496. [PubMed] [Google Scholar]



