Abstract
We have found that hydrogen (dihydrogen; H2) has beneficial lipid-lowering effects in high-fat diet-fed Syrian golden hamsters. The objective of this study was to characterize the effects of H2-rich water (0.9–1.0 l/day) on the content, composition, and biological activities of serum lipoproteins on 20 patients with potential metabolic syndrome. Serum analysis showed that consumption of H2-rich water for 10 weeks resulted in decreased serum total-cholesterol (TC) and LDL-cholesterol (LDL-C) levels. Western blot analysis revealed a marked decrease of apolipoprotein (apo)B100 and apoE in serum. In addition, we found H2 significantly improved HDL functionality assessed in four independent ways, namely, i) protection against LDL oxidation, ii) inhibition of tumor necrosis factor (TNF)-α-induced monocyte adhesion to endothelial cells, iii) stimulation of cholesterol efflux from macrophage foam cells, and iv) protection of endothelial cells from TNF-α-induced apoptosis. Further, we found consumption of H2-rich water resulted in an increase in antioxidant enzyme superoxide dismutase and a decrease in thiobarbituric acid-reactive substances in whole serum and LDL. In conclusion, supplementation with H2-rich water seems to decrease serum LDL-C and apoB levels, improve dyslipidemia-injured HDL functions, and reduce oxidative stress, and it may have a beneficial role in prevention of potential metabolic syndrome.
Keywords: apolipoprotein B, antioxidative property, low density lipoprotein, high density lipoprotein
Hydrogen (dihydrogen; H2), as the lightest and most abundant chemical element, is considered a novel antioxidant that can reduce oxidative stress (1). Hydrogen gas has come to the forefront of therapeutic medical gas research. Accumulated evidence in a variety of biomedical fields using clinical and experimental models for many diseases proves that H2, administered either through gas inhalation or consumption of an aqueous H2-containing solution, can act as a feasible therapeutic strategy in different disease models. For example, supplementation with H2-rich water was demonstrated to have a beneficial role in prevention of types 1 and 2 diabetes and insulin resistance (2, 3); chronic liver inflammation (4); acute oxidative stress; and focal brain ischemia/reperfusion injury (1). In addition, we have reported that consumption of H2-saturated saline for eight weeks prevent atherosclerosis in apolipoprotein E-knockout (apoE−/−) mice (5) and that administration of H2-saturated saline for four weeks decreases serum total cholesterol (TC) and LDL-cholesterol (LDL-C) levels in high-fat diet-fed hamsters (6). However, the lipid-regulating effect of H2 in humans has not yet been demonstrated. Therefore, the aim of this study was to characterize the effects of H2 on the content and composition of serum lipoproteins in patients with potential dyslipidemia.
Metabolic syndrome is characterized by a constellation of metabolic and anthropometric abnormalities, which include excess weight, insulin resistance, hyperglycemia, hypertension, low concentration of HDL-cholesterol (HDL-C), and dyslipidemia (7–9). Metabolic disease remains a serious concern in the world, and people with metabolic syndrome are at increased risk of developing cardiovascular disease, stroke, and type 2 diabetes (9, 10). It is known that the functions and concentrations of serum HDL have strong inverse correlations with risk of metabolic syndrome and atherosclerotic cardiovascular disease (11–13). HDL is known to undergo dramatic modification in structure and composition under the concerted actions of inflammation and oxidative stress (14, 15). As a result, HDL particles progressively lose normal biological activities and acquire altered properties. It is well known that H2 is an electron donor and therefore has a high reducing ability, and the beneficial effects of H2 on different disease models are mostly dependent on its antioxidative, anti-inflammatory, and antiapoptotic properties (16). Therefore, it is possible that dyslipidemia-injured HDL function might be improved by H2 treatment in animals or patients with hyperlipidemia or metabolic syndrome. We have previously demonstrated that administration of H2-saturated saline markedly improves the functional properties of the HDL particle in mice and golden hamsters (5, 6). However, whether the same effects of H2 could be observed in humans has not yet been determined. Here, we aimed to characterize the effects of H2 on functional properties of the HDL particle on 20 patients with potential metabolic syndrome.
METHODS
Subjects
The study protocol was approved by the Ethics Committee of Taishan Medical University. We recruited 20 subjects ≥ 43 years of age, males (n = 12) and females (n = 8), from existing patient databases or by advertisement. In this study, subjects were required to have one or more of the following conditions: prehypertension (diastolic blood pressure of 80–89 mmHg and systolic blood pressure of 139 mmHg or lower); prediabetes (fasting serum glucose from 5.2 to 6.9 mmol/l); TC > 5.18 mmol/l and/or LDL-C > 2.59 mmol/l; body mass index (BMI) between 25.0 and 34.9 kg/m2; or waist circumference≥ 100 cm for males and ≥ 88 cm for females. All participants provided written informed consent to participate before enrollment in the study.
Study design and preparation of H2 water
The patients consumed 0.9–1.0 l/day of H2-rich pure water for 10 weeks. A plastic shelled product (Premium FDR, Friendear, Tokyo, Japan) consisting of metallic magnesium (99.9% pure) and natural stones in 500 ml of polypropylene water bottles (NongFu Spring, HangZhou, China) was used to produce H2. The product was capable of generating H2 when placed in drinking water by the following chemical reaction: Mg + 2H2O → Mg (OH)2 + H2. The H2 water stick was placed into the sealed water bottle for 12 h before consumption by the patients. The sealed cap of the bottle protected the H2 from escaping, thus preserving the H2 ions and molecules in the water. The water was drunk by patients within 15 min after opening the sealed cap, and the H2 concentration was maintained between 0.2 and 0.25 mM and pH between 7.8 and 8.2 measured by a H2 sensor (Unisense, Denmark) for continuous 15 min after opening the sealed cap. Subjects were instructed to reuse the magnesium sticks by transferring the sticks to a new bottle of water after use. In summary, subjects were expected to consume 450–500 ml of H2-rich water two times/day for a total minimum consumption of 900 ml to a maximum consumption of 1,000 ml. The blood samples were collected at baseline (0 week) and after 10 weeks of drinking H2 water.
Serum analysis
Serum lipids.
Blood samples were obtained in the morning after an overnight fast. Serum glucose levels were measured by the glucose oxidase method. Serum TC, HDL-C, LDL-C, and triacylglycerols (TG) were measured by enzymatic methods on a chemical autoanalyzer (Hitachi Co, Tokyo, Japan). Lipoprotein profiles were obtained by fast-protein liquid chromatography (FPLC) using Superpose 6 10/300GL column (17). Briefly, 100 μl of fasting serum was applied to a Superose 6 column, and the samples were eluted in a mobile phase (0.15 M NaCl, 0.01% NaN3, and 2 mM EDTA, pH 7.5) at a rate of 0.3 ml/min in 60 fractions of 500 μl. Lipid composition of fractions corresponding to VLDL, LDL, and HDL were quantified by enzymatic assays using commercially available kits for TC (BioSino, Beijing, China).
Measurement of serum oxidative stress and oxidizability.
Serum levels of malondialdehyde (MDA), a marker for oxidative stress, were determined by a spectrophotometric measurement of thiobarbituric acid-reactive substances (TBARS) according to the manufacturer's instructions (Nanjing Jiancheng Biochemistry, China). The activity of superoxide dismutase (SOD), which acts as antioxidant and protects cellular components from being oxidized by reactive oxygen species, was measured by a commercial kit (Nanjing Jiancheng Biochemistry, China) according to the manufacturer's instructions. The activity of paraoxonase-1 (PON1), an antioxidant enzyme associated with HDL, was measured by adding serum to 1 ml of Tris-HCl buffer (100 mM, pH 8.0) containing 1 mM CaCl2 and 1 mM of phenylacetate (Sigma) as described previously (18). The rate of phenyl acetate hydrolysis was determined spectrophotometrically (Uvikon 930 spectrophotometer, Kontron) at 270 nm. PON1 activity was expressed in international units (U) per milliliter of serum. When measuring PON1 activity in lipoproteins, the activity was expressed in international units per gram of protein. The contents of the biologically active oxidized lipids in serum, including 12-hydroxy eicosatetraenoic acid (12-HETE), 13-hydroxy octadecadienoic acid (13-HODE), prostaglandin (PG) and 8-iso-prostaglandin F2α (8-iso-PGF2α), were determined by ELISA (Bluegene, Shanghai, China) according to the manufacturer's instructions.
Measurement of serum inflammatory factors.
Serum concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-6, were determined by ELISA kits (Bluegene, Shanghai, China) according to the manufacturer's instructions.
Western blots
For serum apolipoprotein measurement, 0.2 μl of serum was denatured at 90°C for 10 min and then subjected to western blot analysis using anti-apoB, anti-apoE, and anti-apoAI antibodies (Abcam). The proteins were visualized and quantified using a chemiluminescence method (Pierce) and Quantity One (Bio-Rad) software program.
Measurement of antioxidant properties of HDL
The serum of every 5–6 patients was pooled, and fasted serum lipoproteins were fractionated by ultracentrifugation at 40,000 rpm in a Beckman Optima LE-80K into VLDL (density less than 1.006 g/ml), LDL (density = 1.006–1.063 g/ml) and HDL3 (density = 1.125–1.21 g/ml) as described previously (19). Fractions were dialyzed in PBS at 4°C and LDL (100 μg protein/ml) was incubated with freshly prepared CuSO4 (10 μmol/l) in the presence or absence of the isolated HDL (200 μg protein/ml). After incubation at 37°C for 2 h, the extent of LDL oxidation was assessed by measuring TBARS formation (20) via a spectrophotometric method according to the manufacturer's instructions (Nanjing Jiancheng Biochemistry, China).
Endothelial cell monocyte adhesion assay
Monocyte adhesion assays were performed under static conditions as previously described (21) with minor modification. Human umbilical vein endothelial cells (HUVEC) were grown to confluence in 24-well plates and pretreated with or without HDL (100 μg protein/ml) for 18 h and stimulated with TNF-α (10 ng/ml) or LDL (100 μg protein/ml) for 6 h. THP-1 cells were labeled with a fluorescent dye, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM), by incubation with 10 µmol/l BCECF-AM at 37°C for 1 h in RPMI-1640 medium and were subsequently washed with EBM-2. Confluent HUVECs in 24-well plates were washed three times, and then labeled THP-1 cells (2 × 105 cells per 200 µl) were added to each well of HUVEC. THP-1 cells were allowed to adhere to HUVECs by incubation at 37°C for 60 min, and unbound THP-1 cells were removed by washing (three times, 5 min). THP-1 cells bound to HUVECs were counted under fluorescent microscope. The number of adherent leukocytes was determined by counting four fields per 100× high-power field well using fluorescent microscopy (Nikon, Japan) and photographed. Four randomly chosen high-power fields were counted per well. Experiments were performed in duplicate or triplicate and were repeated at least three times. The person counting the adherent monocytes was unaware of the treatment.
HDL-induced cholesterol efflux assay
Cholesterol efflux experiments were performed as described by Smith et al. (22). Acetyl LDL (AcLDL) was prepared according to the methods of Basu et al. (23). RAW264.7 macrophages at 50% confluence were cholesterol loaded and labeled in 1 ml of RGGB (RPMI 1640 supplemented with 50 mM glucose, 2 mM glutamine, and 0.1% BSA) containing [3H]cholesterol (1 μCi/ml) and AcLDL (100 μg protein/ml) for 30 min. Then macrophages were washed twice with 0.1% BSA-PBS and equilibrated with RGGB for 24 h. On the following day, the medium was then replaced with RGGB containing 200 μg protein/ml of HDL. After 12 h of incubation, the culture was centrifuged to remove cell debris, and 100 μl of the medium was removed for determination of radioactivity. At the end of the chase period, the macrophages were dissolved in 0.4 ml of 0.1 M sodium hydroxide, and the radioactivity per aliquot was measured. The percentage cholesterol efflux was calculated by dividing the media-derived radioactivity by the sum of the radioactivity in the media and the macrophages.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
HUVECs were grown to confluence in 96-well plates and pretreated with or without HDL (100 μg protein/ml) for 18 h and stimulated with TNF-α (15 ng/ml) for 6 h. Cells treated with medium only served as a negative control group. After removing the supernatant of each well and washing twice with PBS, 10 μl of MTT solution (5 mg/ml in PBS) and 100 μl of medium were then introduced. After incubation for another 4 h, the resultant formazan crystals were dissolved in dimethyl sulfoxide (150 μl), and the absorbance intensity was measured by a microplate reader (Tecan, Sweden) at 490 nm with a reference wavelength of 620 nm. All experiments were performed in quadruplicate, and the relative cell viability was expressed as a percentage relative to the untreated control cells.
LDL oxidation assay
LDL was isolated by ultracentrifugation from the serum. The MDA content of LDL was determined by the measurement of TBARS and expressed as nanomoles per milligram of protein. For determining LDL-mediated inflammation, 100 μg protein/ml of LDL was added to the cultured RAW264.7 macrophages and bone marrow-derived macrophages. After incubation for 24 h, quantitation of the secreted proinflammatory cytokines, including IL-6 and TNF-α, were performed from aliquots of conditioned medium by ELISA (Bluegene, Shanghai, China) according to the manufacturer's instructions. Bone marrow-derived macrophages in mice were obtained as described previously (24).
Statistical analysis
Statistical analysis was performed by one-way ANOVA (ANOVA) test with the GraphPad Prism v.4.0. Results are expressed as means ± SD. P < 0.05 was considered significant.
RESULTS
Subject characteristics
The baseline demographics of subjects are presented in Table 1. Subjects enrolled in the study included those who had TC > 5.18 mmol/l (n = 17), LDL-C >2.59 mmol/l (n = 18), BMI 25–34.9 (n = 17), and/or smokers (n = 10). All subjects showed mean normal clinical levels of baseline biometric parameters, clinical chemistry, and hematology. All smokers were occasional smokers.
TABLE 1.
Patient characteristics and effects of drinking H2-rich water
| Characteristic | Before Drinking H2-rich Water | After Drinking H2-rich Water | |
| Number of patients | 20 | ||
| Male | 12 | ||
| Female | 8 | ||
| Age (years) | 55.8 ± 10.6 | ||
| Mean systolic BP (mmHg) | 134 ± 16.6 | 128.8 ± 16.8 | |
| Mean diastolic BP (mmHg) | 82.6 ± 11.5 | 80.1 ± 11.5 | |
| Height (cm) | 164.8 ± 8.5 | 164.8 ± 8.5 | |
| Weight (kg) | 74.1 ± 13.4 | 72.0 ± 10.5 | |
| Waist circumference (cm) | 99.7 ± 8.5 | 94.6 ± 6.6 | |
| Body mass index (kg/m2) | 27.3 ± 2.6 | 26.5 ± 2.1 | |
| Fasting glucose (mmol/l) | 5.7 ± 0.6 | 5.8 ± 1.0 | |
| Alcohol use | |||
| Daily | 3 | ||
| Weekly | 3 | ||
| Occasional | 14 | ||
| Tobacco use | |||
| Yes | 10 | ||
| No | 10 |
Effect of H2 on serum lipoprotein profiles and serum levels of antioxidative and inflammatory biomarkers
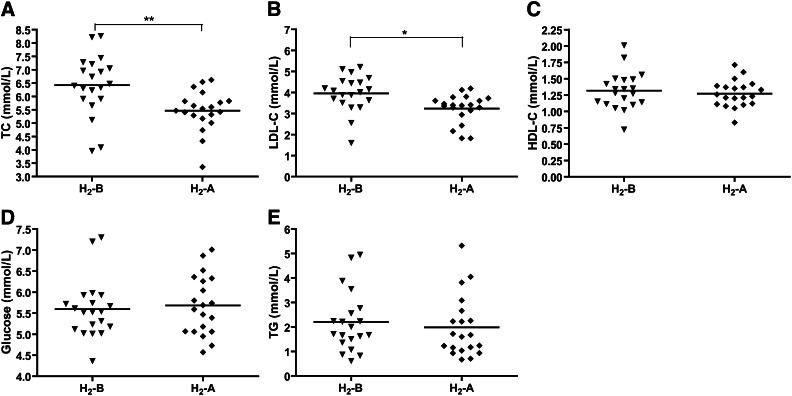
The serum lipid levels of each individual are presented in Table 2, and the distribution of lipid levels is shown in Fig. 1. We can see that serum TC and LDL-C levels were significantly decreased after 10 weeks of H2 treatment in all of the 20 patients with potential metabolic syndrome. Among 20 patients, 18 had decreased and 1 (patient 14) had increased TC and LDL-C levels, and 1 (patient 8) was not altered after H2 consumption.
TABLE 2.
Effect of H2 on levels of serum lipids and glucose
| Before Drinking H2-rich Water | After Drinking H2-rich Water | |||||||||||
| Patient | Gender | Smoker? | TC (mM) | LDL-C (mM) | HDL-C (mM) | TG (mM) | Glucose (mM) | TC (mM) | LDL-C (mM) | HDL-C (mM) | TG (mM) | Glucose (mM) |
| 1 | M | N | 6.96 | 4.2 | 1.34 | 2.24 | 5.93 | 6.15 | 3.14 | 1.2 | 2.26 | 6.33 |
| 2 | F | N | 5.91 | 3.88 | 1.35 | 1.71 | 5.67 | 5.77 | 3.73 | 1.37 | 1.04 | 6.52 |
| 3 | F | N | 6.4 | 3.88 | 1.82 | 1.07 | 5.72 | 5.83 | 3.29 | 1.6 | 0.94 | 6.36 |
| 4 | F | N | 6.23 | 4.01 | 1.15 | 2.01 | 5.52 | 5.32 | 2.94 | 0.83 | 4.05 | 5.39 |
| 5 | F | N | 6.92 | 4.44 | 1.5 | 1.68 | 5.18 | 5.42 | 3.46 | 1.39 | 1.72 | 5.18 |
| 6 | F | N | 6.47 | 4.47 | 1.42 | 0.87 | 5.52 | 5.6 | 3.78 | 1.33 | 1.6 | 5.69 |
| 7 | F | N | 8.26 | 4.69 | 2.01 | 1.66 | 5.54 | 6.62 | 3.8 | 1.71 | 0.93 | 5.59 |
| 8 | M | N | 5.12 | 3.29 | 1.11 | 0.83 | 5.02 | 5.17 | 3.38 | 1.23 | 0.71 | 5.06 |
| 9 | F | N | 5.66 | 3.7 | 1.34 | 0.61 | 5.12 | 5.29 | 3.28 | 1.35 | 1.24 | 4.57 |
| 10 | M | N | 7.21 | 5.1 | 1.48 | 2.21 | 5.01 | 6.54 | 4.12 | 1.5 | 2.23 | 5.06 |
| 11 | F | Y | 6.33 | 4.08 | 1.1 | 2.23 | 5.74 | 5.47 | 3.36 | 1.11 | 1.68 | 6.04 |
| 12 | M | Y | 6.74 | 4.15 | 1.02 | 3.55 | 5.02 | 5.65 | 3.4 | 1.21 | 1.23 | 5.07 |
| 13 | M | Y | 8.22 | 5.21 | 1.56 | 2.56 | 5.98 | 6.36 | 4.19 | 1.42 | 3.09 | 5.47 |
| 14 | M | Y | 3.96 | 1.6 | 1.2 | 4.95 | 5.61 | 4.33 | 1.82 | 1.08 | 2.66 | 5.74 |
| 15 | M | Y | 7.44 | 3.63 | 1.05 | 4.83 | 4.36 | 5.01 | 2.43 | 1.05 | 5.32 | 4.73 |
| 16 | M | Y | 5.91 | 3.51 | 1.14 | 2.77 | 7.2 | 5.82 | 3.41 | 1.12 | 2.22 | 5.8 |
| 17 | M | Y | 6.3 | 3.28 | 1.49 | 3.88 | 7.3 | 4.74 | 2.16 | 1.37 | 3.82 | 7.01 |
| 18 | M | Y | 7.05 | 4.97 | 1.27 | 1.36 | 5.93 | 5.54 | 3.6 | 1.1 | 1.18 | 6.26 |
| 19 | M | Y | 4.09 | 2.55 | 0.72 | 1.62 | 5.23 | 3.36 | 1.82 | 1.26 | 0.67 | 4.95 |
| 20 | M | Y | 7.28 | 4.54 | 1.28 | 1.51 | 5.31 | 5.41 | 3.61 | 1.21 | 1.16 | 6.87 |
| Mean of nonsmokers | 6.51 ± 0.88 | 4.17 ± 0.53 | 1.45 ± 0.28 | 1.49 ± 0.60 | 5.42 ± 0.32 | 5.77 ± 0.52* | 3.49 ± 0.36# | 1.35 ± 0.24 | 1.67 ± 1.00 | 5.58 ± 0.65 | ||
| Mean of smokers | 6.33 ± 1.38 | 3.75 ± 1.10 | 1.18 ± 0.24 | 2.93 ± 1.33 | 5.77 ± 0.92 | 5.17 ± 0.85* | 2.98 ± 0.84# | 1.19 ± 0.13 | 2.30 ± 1.45 | 5.79 ± 0.77 | ||
| Mean of all patients | 6.42 ± 1.13 | 3.96 ± 0.86 | 1.32 ± 0.29 | 2.21 ± 1.24 | 5.60 ± 0.69 | 5.47 ± 0.75** | 3.24 ± 0.68# | 1.27 ± 0.20 | 1.99 ± 1.25 | 5.68 ± 0.71 | ||
*P < 0.05 compared with TC levels before drinking H2-rich water; **P < 0.01 compared with TC levels before drinking H2-rich water; #P < 0.05 compared with LDL-C levels before drinking H2-rich water. M, male; F, female.
Fig. 1.
Effect of H2 on the levels of serum lipids and glucose. (A) Serum TC, (B) LDL-C, (C) HDL-C, (D) glucose, and (E) TG in patients with potential metabolic syndrome before and after 10 weeks of H2 consumption were determined by enzymatic method. Data (means ± SD, n = 20) were expressed as mmol per liter. H2-B, before drinking H2-rich water; H2-A, after drinking H2-rich water. *P < 0.05, **P < 0.01.
In addition, among the 20 patients, 10 were smokers and 10 were nonsmokers. As shown in Table 2, H2 treatment decreased serum TC and LDL-C levels not only in smokers but also in nonsmokers. And it seems that the lipid-lowering effects of H2 on smokers were better than those on nonsmokers, although there is no significant difference. Serum levels of HDL-C and glucose were not altered by consumption of H2 (Table 2 and Fig. 1C, D). Interestingly, serum TG levels were decreased by H2 treatment in 10 smokers, although there is no significant difference (Table 2), which caused a slight decrease in TG levels in all of the 20 patients (Table 2 and Fig. 1E).
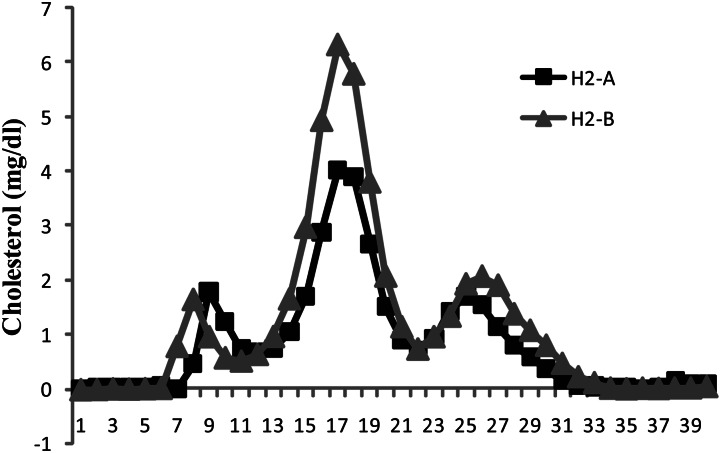
Moreover, serum lipoprotein profile by FPLC further confirmed the decrease in serum LDL-C levels in H2-treated subjects and revealed that serum VLDL-C and HDL-C remained unchanged after intake of H2 water (Fig. 2).
Fig. 2.
FPLC cholesterol profiles using pooled serum samples of n = 5 patients per group showing cholesterol content (mg/dl) of serum lipoprotein fractions.
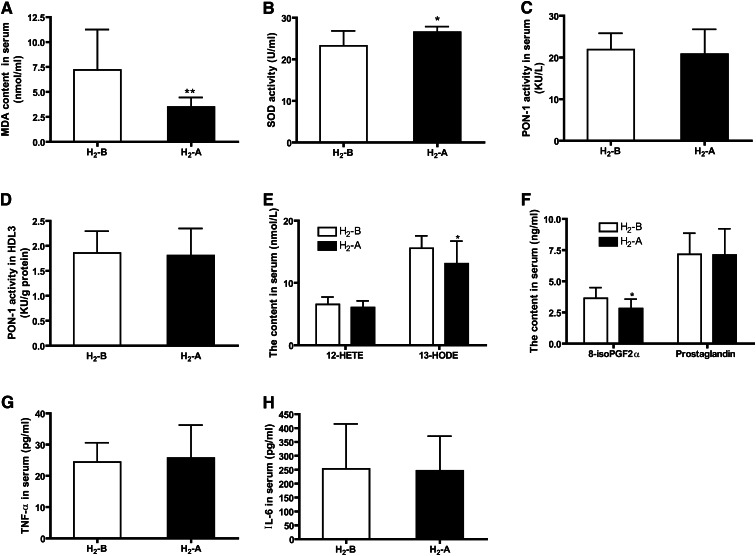
Changes in biomarkers of oxidative and inflammatory status after 10 weeks of consumption of H2 water are shown in Fig. 3. Serum levels of MDA, one of the most frequently used indicators of lipid peroxidation, were decreased significantly (Fig. 3A), and the activity of SOD, which acts as an antioxidant and protects cellular components from being oxidized by reactive oxygen species, was increased after H2 water consumption (Fig. 3B). However, the activity of PON1, an antioxidant enzyme associated with HDL, was not altered in either serum or HDL3 fractions by H2 water (Fig. 3C, D). In addition, we detected serum concentrations of several biologically active oxidized lipids, including 12-HETE, 13-HODE, PG, and 8-iso-PGF2α. The data in Fig. 3E, F shows that 13-HODE and 8-iso-PGF2α levels were improved following the H2 treatment; however, 12-HETE and PG levels were not altered by H2. There was no significant effect of intake of H2 water on serum levels of inflammatory biomarkers, including TNF-α and IL-6 (Fig. 3G, H).
Fig. 3.
Effect of H2 on serum levels of antioxidative and inflammatory biomarkers. (A) Serum concentrations of MDA. (B) SOD activity in serum. (C and D) PON1 activity in serum and HDL3 fraction. (E) Serum concentrations of 12-HETE and 13-HODE. (F) Serum concentrations of 8-iso-PGF2α and PG. (G) Serum concentrations of TNF-α. (H) Serum concentrations of IL-6. n = 20, *P < 0.05, **P < 0.01.
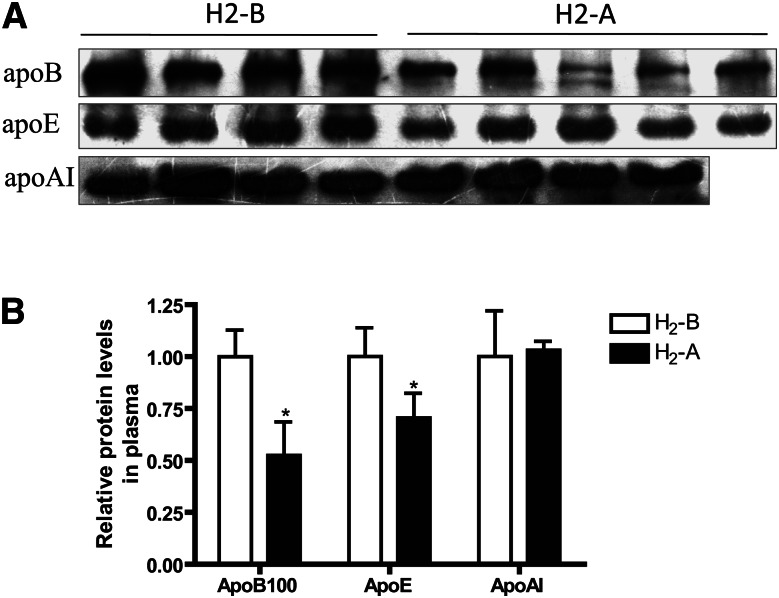
H2 treatment decreases apoB and apoE protein levels in serum
Serum LDL and HDL are particles composed of a variety of lipids and protein components; thus, it is necessary to clarify which content of the lipoprotein could be affected by H2 treatment. Consistent with the difference observed for LDL-C, apoB100, the major protein on LDL, was significantly decreased by consumption of H2 water in patients with potential metabolic syndrome (Fig. 4). The apoE protein, which is mainly present in VLDL and LDL particles, was also lowered with the treatment of H2 (Fig. 4). ApoAI, the major protein on HDL, was not altered after intake of H2 water (Fig. 4), which is consistent with the changes observed for HDL-C. These data suggested that H2 could decrease the expression of the major protein constituents of LDL and VLDL.
Fig. 4.
Effect of H2 on serum levels of apoB, apoE, and apoAI proteins. (A) Effect of H2 on serum apoB, apoE, and apoAI protein levels by western blots. (B) Densitometric quantitation of western blot data (n = 4–5) by Quantity One software. *P < 0.05.
H2 improves the oxidation and the functional properties of the HDL particle
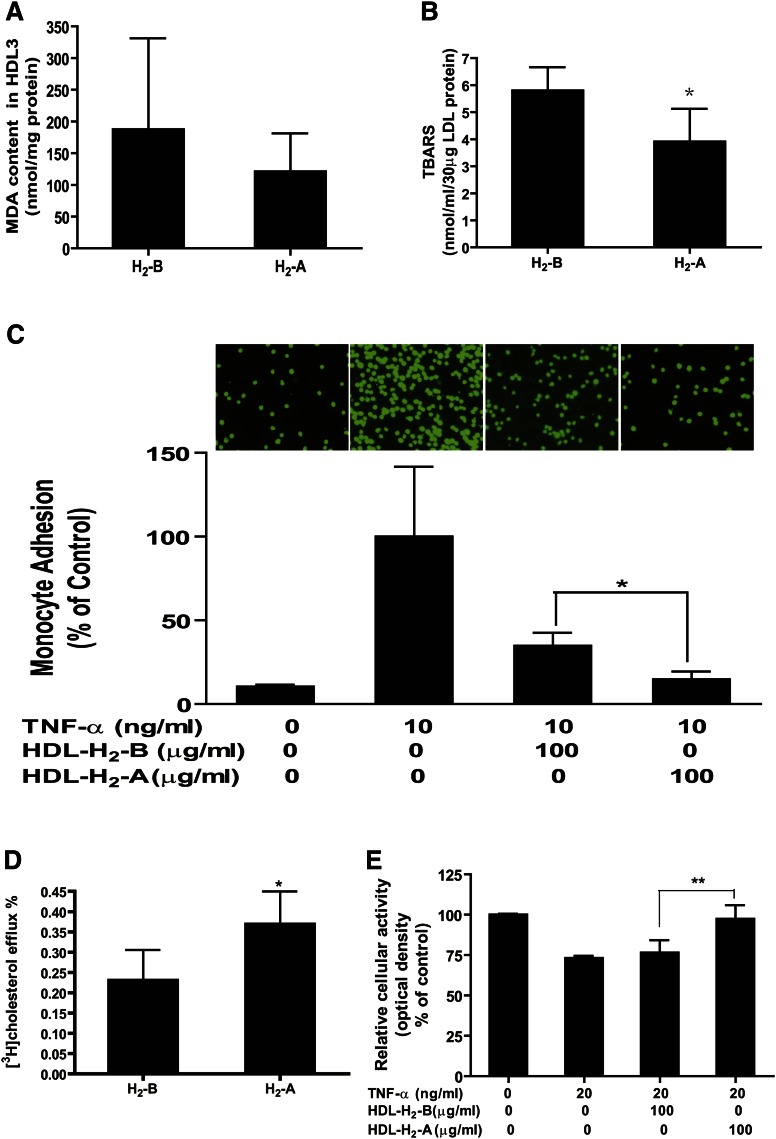
HDL is known to undergo dramatic modification in structure and composition under the concerted actions of inflammation and oxidative stress (14, 15). Recent evidence indicates that H2 acts as a therapeutic medical gas in a variety of disease models by exerting antioxidant and anti-inflammatory effects (3, 25, 26). Therefore, it is possible that administration of H2 might improve the functional quality of HDL particle. First, H2 water supplementation tended to decrease the MDA content in HDL3 (Fig. 5A), suggesting that H2 improves the oxidation of HDL particle.
Fig. 5.
H2 seems to improve the oxidation and functional properties of the HDL particle. Serum of every 5–6 patients was pooled, and HDL3 was isolated by ultracentrifugation from the serum. (A) MDA content in HDL3 particles. HDL function was determined as (B) protection of LDL against oxidation, (C) inhibition of TNF-α-induced THP-1 monocyte adhesion to endothelial cells, (D) stimulation of cholesterol efflux from macrophage foam cells, and (E) protection of endothelial cells from TNF-α-induced apoptosis. Assays were performed as detailed in Methods. n = 3–4 pooled serum samples. *P < 0.05, **P < 0.01.
Second, the biological effect of H2 on the antioxidative functionality of HDL was tested, namely, the protection of LDL particles from oxidation. As shown in Fig. 5B, H2 treatment significantly inhibited the formation of TBARS.
Third, the effect of H2 on the anti-inflammatory properties of HDL was tested, including protection of cytokine-induced monocyte adhesion to endothelial cells and stimulation of endothelial nitric oxide production, which has been suggested as an important endothelial-atheroprotective effect of HDL. As shown in Fig. 5C, after incubation of HUVECs for 6 h with TNF-α, adhesion of monocytes to HUVECs was significantly increased, and preincubation of HUVECs with HDL3 isolated from patients after 10 weeks of drinking H2 water (HDL-H2-A) markedly reduced TNF-α-induced adhesion of monocyte to HUVECs compared with those of HDL3 isolated at baseline (0 week) (HDL-H2-B). These data suggest that the anti-inflammatory function of HDL was improved by H2 water. We also determined the effects of HDL-H2-A compared with HDL-H2-B on endothelial nitric oxide production. Unfortunately, the nitric oxide production was not altered significantly after intake of H2 water (data not shown), which might be attributable to the sensitivity of Griess method.
Fourth, the ability of the isolated HDL particles to elicit efflux from cholesterol-loaded macrophages was tested. As shown in Fig. 5D, HDL particles isolated from the serum after H2 treatment exhibited dramatically higher efflux properties compared with the HDL particles isolated from the serum before H2 treatment, indicating that the cholesterol efflux ability mediated by HDL particles was increased by H2.
Finally, the biological effect of H2 on the antiapoptotic functionality of HDL was determined. As shown in Fig. 5E, H2 treatment significantly inhibited TNF-α induced endothelial cell apoptosis. These data indicate that dyslipidemia-injured HDL functions, including the ability to protect against LDL oxidation, the ability to inhibit cytokine-induced monocyte adhesion to endothelial cells, the ability to stimulate cholesterol efflux from macrophage foam cells, and the ability to protect endothelial cell apoptosis, were markedly improved by administration of H2 water in patients with potential metabolic syndrome.
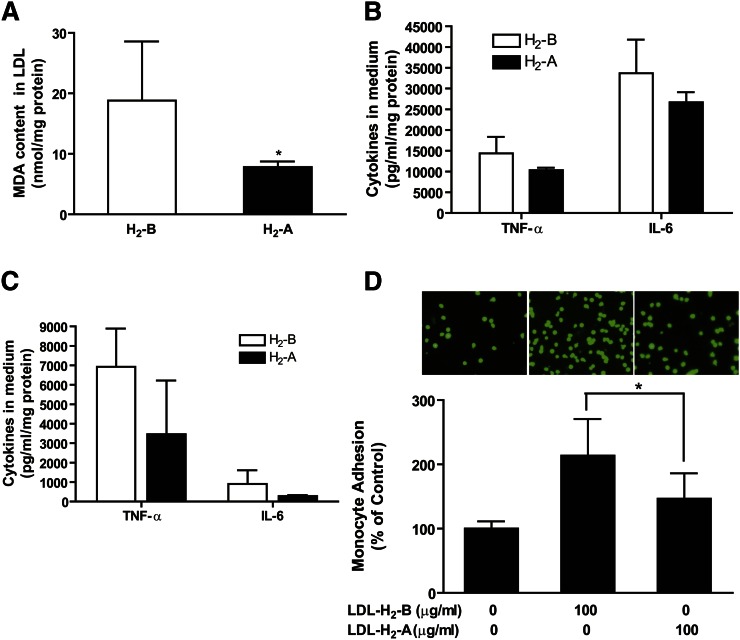
H2 reduces the oxidation of LDL and LDL-mediated inflammation
The oxidation of LDL plays an important role in atherogenesis and may influence lipid metabolism. In this study, we determined the effects of H2 water on the oxidation of LDL and LDL-mediated inflammation. As shown in Fig. 6A, the MDA content of the isolated LDL was reduced by H2 treatment, suggesting that H2 reduces the oxidation of LDL. To determine LDL-mediated inflammation, 100 μg protein/ml of the isolated LDL was added to the cultured RAW264.7 macrophages and bone marrow-derived macrophages for 24 h. As shown in Fig. 6B, C, the secretion of TNF-α and IL-6 by macrophages was reduced by H2 treatment. Furthermore, we tested the effects of H2 on LDL-induced monocyte adhesion to endothelial cells. As shown in Fig. 6D, consumption of H2 water significantly decreased LDL-induced monocyte adhesion to endothelial cells. These data reveal that H2 reduces LDL-mediated inflammatory properties in culture.
Fig. 6.
H2 seems to reduce oxidation of LDL in patients and LDL-mediated inflammation in macrophages. Serum of every 5–6 patients was pooled, and LDL was isolated by ultracentrifugation from the serum. (A) MDA content of the isolated LDL. (B) One hundred micrograms of protein per milliliter of isolated LDL was added to cultured RAW264.7 macrophages. Supernatants were harvested at 24 h for measurement of TNF-α and IL-6 by ELISA assay. (C) One hundred micrograms of protein per milliliter of isolated LDL was added to the cultured bone marrow-derived macrophages, and cytokines in medium were measured after 24 h incubation. (D) HUVECs were stimulated with LDL (100 μg protein/ml) for 6 h and THP-1 cells, labeled with a fluorescent dye BCECF-AM, were added to HUVECs. After adhering, THP-1 cells bound to HUVECs were counted under fluorescent microscope as described in Methods. Results are expressed as means ± SD (n = 3–4 pooled serum samples). *P < 0.05.
DISCUSSION
In a previous study, we found that H2 has beneficial lipid-lowering effects in high-fat diet-fed Syrian golden hamsters (6). However, it remains unknown whether H2 has effects on lipid and lipoprotein metabolism in humans. The key finding of our present study is that the novel antioxidant chemical element H2 seems to alleviate lipid metabolism disorder, including hyperlipidemia and defective HDL function, in patients with potential metabolic syndrome. Our results were not consistent with that of human studies in type 2 diabetes mellitus, as they showed significant decreases in modified LDL-C levels and no effect on total and LDL-C (3). The inconsistency might be attributable to the difference in pathological conditions, the dosage of administration, or the intervention period. Despite this possibility, the discrepancy might be explained by the fact that modified LDL, like oxidized LDL, largely existed in the hyperlipidemia model, which was used in our study.
Subanalysis was conducted on 20 subjects, and we found the serum TC levels of 17 subjects with hyperlipidemia (TC > 5.18 mmol/l) was decreased by intake of H2 water for 10 weeks, and the serum TC levels of the remaining 3 subjects without hyperlipidemia (TC < 5.18 mmol/l) was not significantly altered by H2 treatment, although the TC level of 1 subject was slightly increased after treatment (Table 2, patient 14). The data gave us a clue that H2 might exert a lipid-lowering effect on the patients with hyperlipidemia but have little effect on the population without hyperlipidemia.
It is well known that high cholesterol level is one of the important risk factors for atherosclerosis. Therefore, the elucidation of the mechanism by which H2 decreases serum cholesterol will provide solid evidence for the application of H2 in cardiovascular disease therapy. Serum LDL and VLDL are particles composed of a variety of lipids and protein components; thus, it is necessary to clarify which content of the lipoprotein could be affected by H2 treatment. First, LDL is a contributing factor to the development of atherosclerosis. ApoB100 is the major protein present in LDL particles, and like LDL-C, the serum apoB level has been positively correlated with risk for atherosclerotic disease. In the present study, we found H2 treatment not only decreased serum LDL-C levels, but also remarkably lowered apoB100 and apoE protein levels in serum. The data gave us a clue that H2 might regulate the metabolism of LDL-C by decreasing the synthesis of apoB or increasing the catabolism of LDL-C. Second, like LDL-C, VLDL-C is considered a type of ‘‘bad’’ cholesterol because elevated levels are associated with an increased risk of coronary artery disease (27). We found that apoB and apoE, the major proteins in VLDL, were lowered by H2, although cholesterol analysis by FPLC revealed no changes of VLDL-C after H2 treatment. The inconsistencies in protein and cholesterol levels might be explained by the suspicion that the functional target of H2 might be apolipoprotein expression, not cholesterol. Taken together, the data indicate that molecular H2 dissolved in water might have a beneficial regulating effect on lipid abnormality in patients with metabolic syndrome, especially in patients with hyperlipidemia. This effect is partially related to its regulation of lipid and protein contents of LDL and VLDL. Further experiments are needed to identify the mechanisms by which H2 regulates the lipid and protein contents of lipoprotein particles and improves the serum lipoprotein profile. Previous studies have demonstrated that administration of H2-rich saline improves insulin sensitivity (3), which, in our view, could partly contribute to the improved lipid metabolism in our study. Furthermore, liver cells sense the reduced levels of hepatic intracellular cholesterol and seek to compensate by synthesizing LDL receptors to draw cholesterol out of the circulation (28). Future studies on hepatic HMG-CoA reductase and LDL receptors may elucidate the mechanism by which H2 acts on lipoprotein regulation.
HDL is known to protect against the development of atherosclerosis and is widely documented as a “negative risk factor” for coronary heart diseases (29). The antiatherogenic activity of HDL is principally attributable to a variety of antioxidative, anti-inflammatory, and antiapoptotic properties and the reverse transport of cholesterol (30). In the present study, we that found H2 treatment seems to improve the functionality of HDL3 without altering HDL-C serum levels. It is known that the beneficial effects of H2 on different disease models are mostly dependent on its antioxidative, anti-inflammatory, and antiapoptotic properties (16). Therefore, it is possible that the protective effect of H2 on HDL function in our study is attributable, at least in part, to the antioxidative and anti-inflammatory properties of H2. A number of therapeutic strategies are being developed to target HDL-C in an attempt to inhibit the progression or induce regression of atherosclerosis and reduce cardiovascular events. Therefore, the protective effect of H2 on HDL function in the our study might provide a further evidence for H2 application in vascular disease therapy. We did not observe any unwanted side effects of H2, including headache, diarrhea, and vomiting (data not shown), suggesting fewer toxic and adverse effects of H2.
The oxidation of LDL plays an important role in atherogenesis and may influence lipid metabolism. Our data reveals that H2 seems to reduce the oxidation of LDL and LDL-mediated inflammation, and they further support our prediction that H2 might improve lipid metabolism in patients with hyperlipidemia by inhibiting LDL-mediated inflammation. Previous studies have demonstrated that administration of H2 reduces atherogenesis in apoE−/− mice (5); in our view, this could partly contribute to the observed protective effects of H2 on the oxidation of LDL and LDL-mediated inflammation in our study.
Indeed, our data give us a clue that H2 might have the potential to be used as a novel lipid-regulating agent with the advantage of no toxicity compared with other commonly used lipid-regulating drugs that have side-effects on the liver and kidney. Further understanding of the mechanisms underlying the signaling pathways involved in the ability of H2 to influence lipid and cellular metabolism is required to fully exploit intake of H2 gas as a therapeutic strategy.
There are several limitations to the present study. First, the number of subjects recruited is small and it is hard to draw a solid conclusion, particularly having smokers among the subjects. It should, however, be emphasized that we analyzed the data in smokers and nonsmokers separately, and the response to H2 in smokers seems to be stronger than that in nonsmokers, but the difference did not reach statistical significance. It would be useful to enlarge the sample size and examine the reaction to H2 in smokers due to the antioxidative property of H2. Another limitation of the study was that it was not a double-blind, randomized, controlled trial comparing H2-rich water to placebo and comparing experimental endpoints from H2-treated metabolic syndrome subjects with those measured in untreated healthy individuals. It is possible that during the 10 weeks of the study, the subjects altered their lifestyles, which could have affected the parameters studied. Therefore, it is difficult for us to draw a solid conclusion, and it limits the conclusions to stating that H2 treatment appear to regulate lipid metabolism in patients.
In summary, our data show that in vivo administration of H2 water seems to decrease serum TC and LDL-C levels and improve HDL functions in patients with potential metabolic syndrome, suggesting that H2 may be used as a newer pharmacological agent to treat or control lipid metabolism disorder.
Footnotes
Abbreviations:
- BMI
- body mass index
- FPLC
- fast-protein liquid chromatography
- HDL-C
- HDL-cholesterol
- 12-HETE
- 12-hydroxy eicosatetraenoic acid
- 13-HODE
- 13-hydroxy octadecadienoic acid
- HUVEC
- human umbilical vein endothelial cell
- IL
- interleukin
- 8-iso-PGF2α
- 8-iso-prostaglandin F2α
- LDL-C
- LDL cholesterol
- MDA
- malondialdehyde
- PG
- prostaglandin
- PON1
- paraoxonase-1
- SOD
- superoxide dismutase
- TBARS
- thiobarbituric acid-reactive substance
- TC
- total cholesterol
- TG
- triacylglycerol
- TNF
- tumor necrosis factor
- VLDL-C
- VLDL cholesterol
This work was supported by the Taishan Scholars Foundation of Shandong Province (zd056, zd057); special research funding of Taishan Medical University (2008); National Natural Science Foundation of China (81200216), and Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (BS2012YY034).
REFERENCES
- 1.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13: 688–694 [DOI] [PubMed] [Google Scholar]
- 2.Amitani H., Asakawa A., Cheng K., Amitani M., Kaimoto K., Nakano M., Ushikai M., Li Y., Tsai M., Li J. B., et al. 2013. Hydrogen improves glycemic control in type1 diabetic animal model by promoting glucose uptake into skeletal muscle. PLoS ONE. 8: e53913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajiyama S., Hasegawa G., Asano M., Hosoda H., Fukui M., Nakamura N., Kitawaki J., Imai S., Nakano K., Ohta M., et al. 2008. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 28: 137–143 [DOI] [PubMed] [Google Scholar]
- 4.Gharib B., Hanna S., Abdallahi O. M., Lepidi H., Gardette B., De Reggi M. 2001. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C. R. Acad. Sci. III. 324: 719–724 [DOI] [PubMed] [Google Scholar]
- 5.Song G., Tian H., Qin S., Sun X., Yao S., Zong C., Luo Y., Liu J., Yu Y., Sang H., et al. 2012. Hydrogen decreases athero-susceptibility in apolipoprotein B-containing lipoproteins and aorta of apolipoprotein E knockout mice. Atherosclerosis. 221: 55–65 [DOI] [PubMed] [Google Scholar]
- 6.Zong C., Song G., Yao S., Li L., Yu Y., Feng L., Guo S., Luo T., Qin S. 2012. Administration of hydrogen-saturated saline decreases plasma low-density lipoprotein cholesterol levels and improves high-density lipoprotein function in high-fat diet-fed hamsters. Metabolism. 61: 794–800 [DOI] [PubMed] [Google Scholar]
- 7.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114: 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holvoet P., Lee D. H., Steffes M., Gross M., Jacobs D. R., Jr 2008. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 299: 2287–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford E. S., Giles W. H., Dietz W. H. 2002. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 287: 356–359 [DOI] [PubMed] [Google Scholar]
- 10.Grundy S. M., Brewer H. B., Jr, Cleeman J. I., Smith S. C., Jr, Lenfant C. 2004. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 109: 433–438 [DOI] [PubMed] [Google Scholar]
- 11.Cifuentes-Goches J. C., Gomez-Lopez Jde D., Hernandez-Ancheyta L., Flores-Fuentes S. E., Inchaustegui-Arias J. L., Canas-Urbina A. O. 2012. Hypertriglyceridemia and low HDL cholesterol as high impact factors for metabolic syndrome diagnosis in apparently healthy adults [in Spanish]. Rev. Med. Inst. Mex. Seguro Soc. 50: 301–306 [PubMed] [Google Scholar]
- 12.Van Lenten B. J., Navab M., Shih D., Fogelman A. M., Lusis A. J. 2001. The role of high-density lipoproteins in oxidation and inflammation. Trends Cardiovasc. Med. 11: 155–161 [DOI] [PubMed] [Google Scholar]
- 13.Tamada M., Makita S., Abiko A., Naganuma Y., Nagai M., Nakamura M. 2010. Low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a useful marker for early-stage carotid atherosclerosis. Metabolism. 59: 653–657 [DOI] [PubMed] [Google Scholar]
- 14.Khovidhunkit W., Kim M. S., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45: 1169–1196 [DOI] [PubMed] [Google Scholar]
- 15.Esteve E., Ricart W., Fernandez-Real J. M. 2005. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin. Nutr. 24: 16–31 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y., Sano M., Hayashida K., Ohsawa I., Ohta S., Fukuda K. 2009. Are the effects of alpha-glucosidase inhibitors on cardiovascular events related to elevated levels of hydrogen gas in the gastrointestinal tract? FEBS Lett. 583: 2157–2159 [DOI] [PubMed] [Google Scholar]
- 17.Jiang X. C., Masucci-Magoulas L., Mar J., Lin M., Walsh A., Breslow J. L., Tall A. 1993. Down-regulation of mRNA for the low density lipoprotein receptor in transgenic mice containing the gene for human cholesteryl ester transfer protein. Mechanism to explain accumulation of lipoprotein B particles. J. Biol. Chem. 268: 27406–27412 [PubMed] [Google Scholar]
- 18.Itahara T., Suehiro T., Ikeda Y., Inoue M., Nakamura T., Kumon Y., Kawada M., Hashimoto K. 2000. Serum paraoxonase and arylesterase activities in hemodialysis patients. J. Atheroscler. Thromb. 7: 152–158 [DOI] [PubMed] [Google Scholar]
- 19.Jiang X. C., Bruce C., Mar J., Lin M., Ji Y., Francone O. L., Tall A. R. 1999. Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J. Clin. Invest. 103: 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rikitake Y., Hirata K., Kawashima S., Akita H., Yokoyama M. 1998. Inhibitory effect of inducible type nitric oxide synthase on oxidative modification of low density lipoprotein by vascular smooth muscle cells. Atherosclerosis. 136: 51–57 [DOI] [PubMed] [Google Scholar]
- 21.McCrohon J. A., Jessup W., Handelsman D. J., Celermajer D. S. 1999. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation. 99: 2317–2322 [DOI] [PubMed] [Google Scholar]
- 22.Smith J. D., Miyata M., Ginsberg M., Grigaux C., Shmookler E., Plump A. S. 1996. Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J. Biol. Chem. 271: 30647–30655 [DOI] [PubMed] [Google Scholar]
- 23.Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. 1981. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc. Natl. Acad. Sci. USA. 78: 7545–7549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori M., Sadahira Y., Kawasaki S., Hayashi T., Awai M. 1990. Macrophage heterogeneity in bone marrow culture in vitro. J. Cell Sci. 95: 481–485 [DOI] [PubMed] [Google Scholar]
- 25.Ohsawa I., Nishimaki K., Yamagata K., Ishikawa M., Ohta S. 2008. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun. 377: 1195–1198 [DOI] [PubMed] [Google Scholar]
- 26.Kolovou G., Anagnostopoulou K., Mikhailidis D. P., Cokkinos D. V. 2008. Apolipoprotein E knockout models. Curr. Pharm. Des. 14: 338–351 [DOI] [PubMed] [Google Scholar]
- 27.Geurian K., Pinson J. B., Weart C. W. 1992. The triglyceride connection in atherosclerosis. Ann. Pharmacother. 26: 1109–1117 [DOI] [PubMed] [Google Scholar]
- 28.Ma P. T., Gil G., Sudhof T. C., Bilheimer D. W., Goldstein J. L., Brown M. S. 1986. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc. Natl. Acad. Sci. USA. 83: 8370–8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714 [DOI] [PubMed] [Google Scholar]
- 30.Kontush A., Chapman M. J. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374 [DOI] [PubMed] [Google Scholar]