Abstract
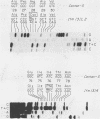
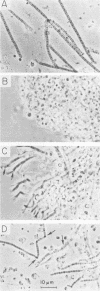
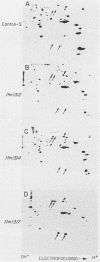
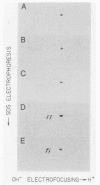
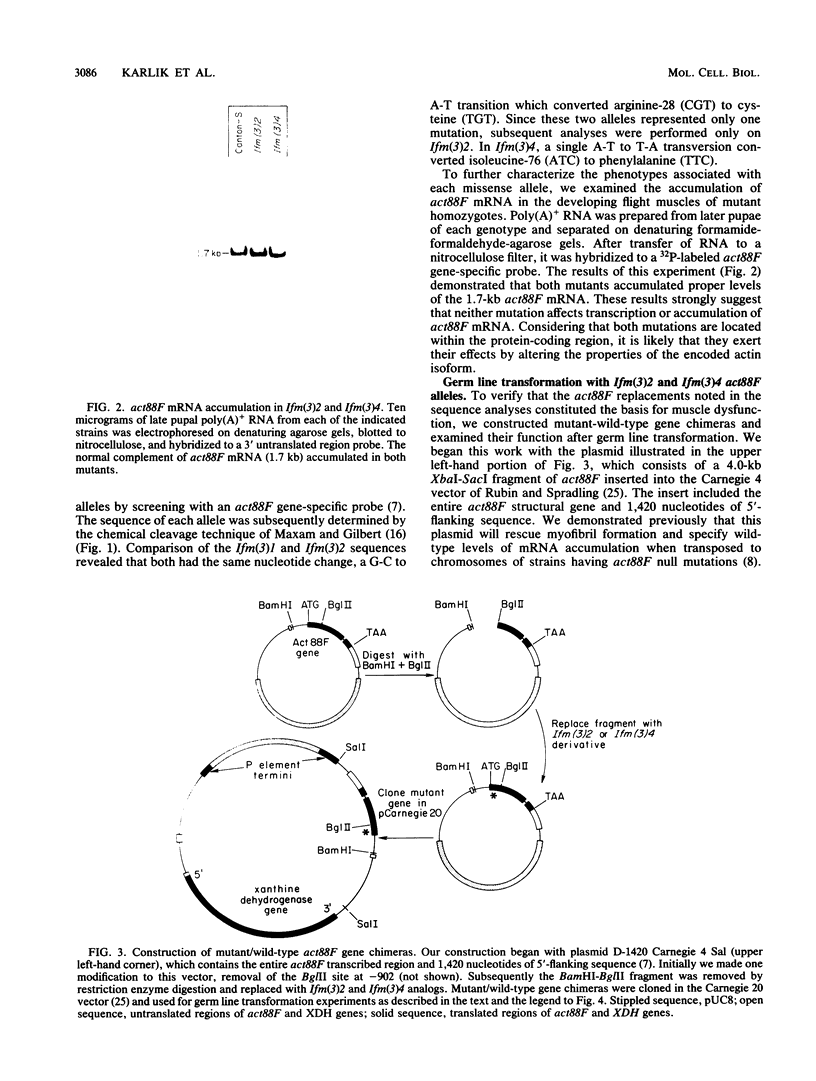
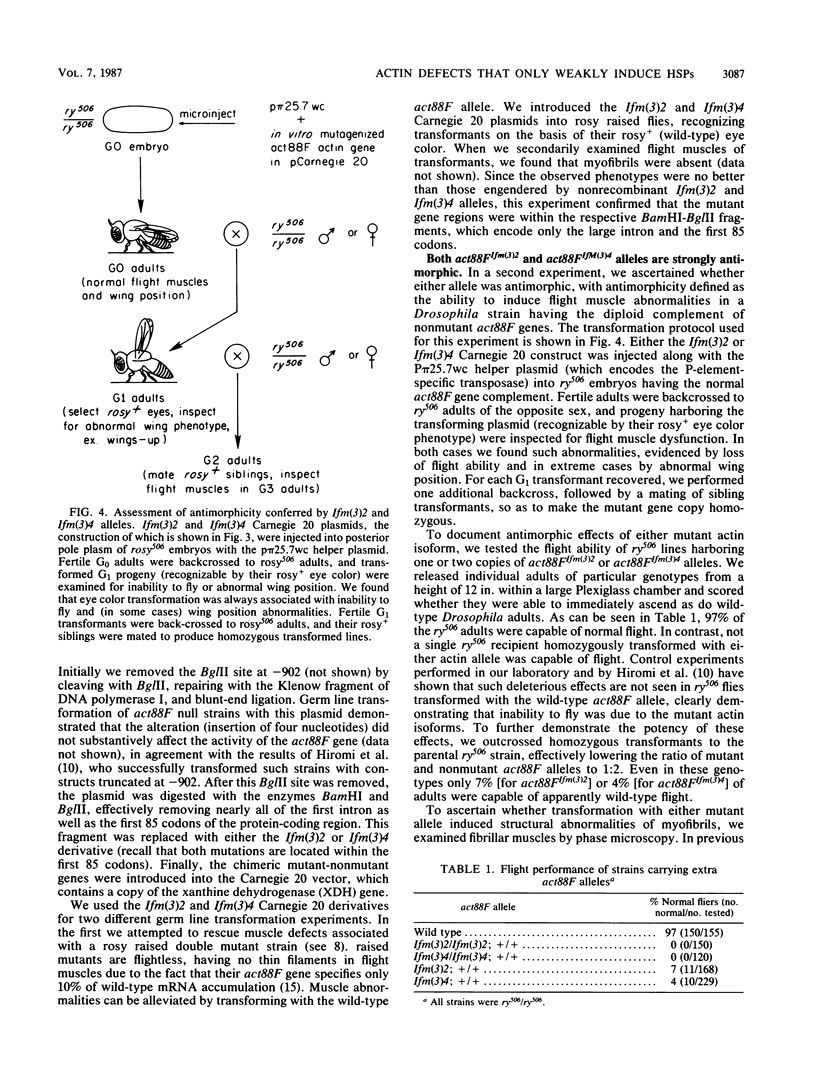
We have characterized two extant mutations of the flight muscle-specific act88F actin gene of Drosophila melanogaster. Both defective alleles were recovered from flightless mutants isolated previously (K. Mogami and Y. Hotta, Mol. Gen. Genet. 183:409-417, 1981). By directly sequencing the mutant alleles, we demonstrated that in act88FIfm(3)2 a single G-C to A-T transition converted arginine-28 to cysteine and that in act88FIfm(3)4 a single A-T to T-A transversion changed isoleucine-76 to phenylalanine. We showed that the actins encoded by either allele were strongly antimorphic. Mutant alleles effectively disrupted myofibril structure and function in the flight muscles of strains having the diploid complement of wild-type act88F genes. However, unlike antimorphic actins encoded by three previously characterized act88F alleles, neither that encoded by act88FIfm(3)2 nor that encoded by act88FIfm(3)4 was a strong inducer of heat shock protein synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Curmi P. M., Barden J. A., dos Remedios C. G. Conformational studies of G-actin containing bound lanthanide. Eur J Biochem. 1982 Feb;122(2):239–244. doi: 10.1111/j.1432-1033.1982.tb05872.x. [DOI] [PubMed] [Google Scholar]
- Deak I. I. Mutations of Drosophila melanogaster that affect muscles. J Embryol Exp Morphol. 1977 Aug;40:35–63. [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Fyrberg E. A. 5'-flanking sequence required for regulated expression of a muscle-specific Drosophila melanogaster actin gene. Mol Cell Biol. 1986 Oct;6(10):3388–3396. doi: 10.1128/mcb.6.10.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Hotta Y. Actin gene mutations in Drosophila; heat shock activation in the indirect flight muscles. EMBO J. 1985 Jul;4(7):1681–1687. doi: 10.1002/j.1460-2075.1985.tb03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Okamoto H., Gehring W. J., Hotta Y. Germline transformation with Drosophila mutant actin genes induces constitutive expression of heat shock genes. Cell. 1986 Jan 31;44(2):293–301. doi: 10.1016/0092-8674(86)90763-4. [DOI] [PubMed] [Google Scholar]
- Johnson P., Blazyk J. M. Involvement of an arginine residue of actin in tropomyosin binding. Biochem Biophys Res Commun. 1978 Jun 14;82(3):1013–1018. doi: 10.1016/0006-291x(78)90884-7. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Coutu M. D., Fyrberg E. A. A nonsense mutation within the act88F actin gene disrupts myofibril formation in Drosophila indirect flight muscles. Cell. 1984 Oct;38(3):711–719. doi: 10.1016/0092-8674(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Mahaffey J. W., Coutu M. D., Fyrberg E. A., Inwood W. The flightless Drosophila mutant raised has two distinct genetic lesions affecting accumulation of myofibrillar proteins in flight muscles. Cell. 1985 Jan;40(1):101–110. doi: 10.1016/0092-8674(85)90313-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mogami K., Hotta Y. Isolation of Drosophila flightless mutants which affect myofibrillar proteins of indirect flight muscle. Mol Gen Genet. 1981;183(3):409–417. doi: 10.1007/BF00268758. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. What turns on heat shock genes? Nature. 1985 Oct 10;317(6037):477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Hiromi Y., Ishikawa E., Yamada T., Isoda K., Maekawa H., Hotta Y. Molecular characterization of mutant actin genes which induce heat-shock proteins in Drosophila flight muscles. EMBO J. 1986 Mar;5(3):589–596. doi: 10.1002/j.1460-2075.1986.tb04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palter K. B., Watanabe M., Stinson L., Mahowald A. P., Craig E. A. Expression and localization of Drosophila melanogaster hsp70 cognate proteins. Mol Cell Biol. 1986 Apr;6(4):1187–1203. doi: 10.1128/mcb.6.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]