Abstract
Biological membrane functions are coupled to membrane curvature, the regulation of which often involves membrane-associated proteins. The membrane-binding N-terminal amphipathic helix-containing BIN/Amphiphysin/Rvs (N-BAR) domain of amphiphysin is implicated in curvature generation and maintenance. Improving the mechanistic understanding of membrane curvature regulation by N-BAR domains requires quantitative experimental characterization.
We have measured tube pulling force modulation by the N-BAR domain of Drosophila amphiphysin (DA-N-BAR) bound to tubular membranes pulled from micropipette-aspirated giant vesicles. We observed that fluorescently-labeled DA-N-BAR showed significantly higher protein density on tubules compared to the connected low-curvature vesicle membrane. Furthermore, we found the equilibrium tube pulling force to be systematically dependent on the aqueous solution concentration of DA-N-BAR, thereby providing the first quantitative assessment of spontaneous curvature generation. At sufficiently high protein concentrations, pulled tubes required no external force to maintain mechanical equilibrium, in agreement with the qualitative spontaneous tubulation previously reported for amphiphysin.

Keywords: curvature sorting, curvature sensing, pulling force, GUV, membrane mechanics
Aspects of lipid membrane curvature regulation have recently emerged as a forefront in physical chemistry1-3. The importance of membrane curvature is underscored by the fact that cellular organelles demonstrate a large variety of membrane shapes. Local curvatures range from essentially flat regions of the plasma membrane to highly bent spherical transport vesicles and tubular transport intermediates with cylindrical curvature. In Drosophila, the peripheral membrane-binding protein amphiphysin is known to localize on T-tubules4-6. Whereas non-vertebrates contain a single form of amphiphysin, two mammalian classes of amphiphysins are known: amphiphysin I, which aids in endocytosis7, and amphiphysin II, which also localizes on T-tubules8.
All identified isoforms of amphiphysin contain a highly-conserved BAR (Bin/Amphiphysin/Rvs) domain. BAR domains are known to form a banana shaped dimer along their charged membrane-binding surfaces9,10. This structure motivated use of the scaffolding mechanism – whereby a zero-spontaneous curvature membrane adopts the locally-bent shape of the curvature-generating protein11 – to explain the tubulation of liposomes incubated with amphiphysin12,13. Effective scaffolding likely requires cooperativity among adjacent proteins. A lattice network of domains was observed through cryo-EM reconstructions that indicated lateral and tip-to-tip interactions between neighboring dimers14. Various types of lattice arrangements for N-BAR domains have been explored through all-atom and coarse-grained simulations15. Drosophila amphiphysin’s BAR domain contains an N-terminal amphipathic helix (N-BAR). Such amphipathic helices are thought to aid in curvature generation via asymmetric insertion into the bilayer9,16,17.
We have incubated giant unilamellar vesicles (GUVs) with Drosophila amphiphysin N-BAR domains and combined pipette-aspiration18 with an optical trap19,20 to measure the force necessary to pull and then maintain a tube at equilibrium (Fig 1). For single-component lipid vesicles, it is well-established that the tube force is related to vesicle membrane tension and bending stiffness21. Molecules which act as curvature generators effectively lower the membrane bending energy22,23. An effective spontaneous curvature may then be observed through measurements of the static (equilibrium) tube force needed to maintain the protein-bound tube at fixed length.
Figure 1.

Schematic of experimental setup for membrane tube pulling force measurements showing: micropipette (inner radius Rp) used to fix vesicle (radius Rv) membrane tension via aspiration pressure difference Po-Pp; streptavidin-conjugated microsphere held by optical trap formed via a long-working distance (LWD) objective; and, tube resulting from retraction of bead after contact with vesicle.
Curvature sorting of the N-BAR domain from Drosophila amphiphysin was first investigated with a fluorescently-labeled variant that was incubated with GUVs formed from the ternary lipid mixture DOPC/Chol/DOPS with the molar mixing fractions 77/3/20. Vesicles were symmetrically labeled with the curvature-insensitive lipid fluorophore Texas Red-DHPE19,24. In order to generate connected and equilibrated regions of high and low curvature lipid membrane, cylindrical tubes were pulled from pipette-aspirated vesicles18. Pipette aspiration achieves control of vesicle membrane tension, which along with the bending stiffness adjusts the radius of a pulled tube. Fig 2A shows the collected fluorescence from lipid and protein channels along the tube as well as part of the attached vesicle. Because the xyz-dimensions of the optical microscope’s focal volume are larger than the diameter of the tube (on the order of 10-100 nm), it is to be expected that the lipid signal on the tube is smaller than on the vesicle, assuming a constant fluorophore density24. However, it is apparent that this is not the case in the protein channel, where the relatively equal intensities of the tube and vesicle regions indicate a higher density of protein on the tube, compared to the attached vesicle.
Figure 2.
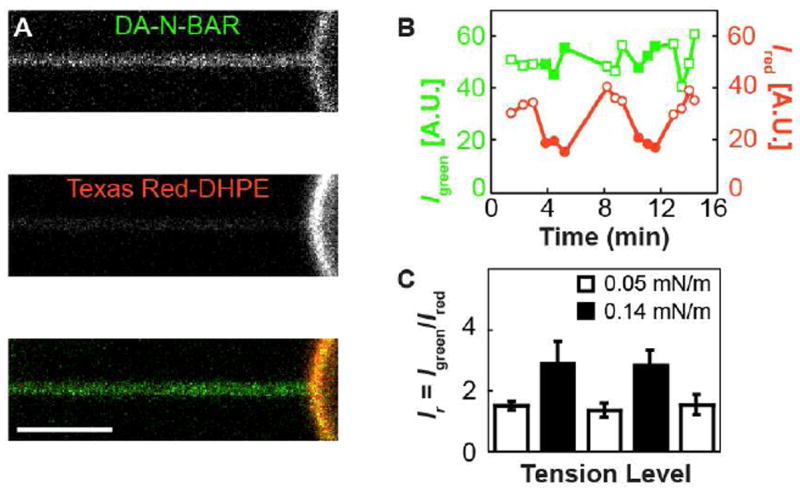
DA-N-BAR localization between connected membranes of different curvature. A). Top, green confocal channel detecting fluorescence from Alexa Fluor 488-labeled DA-N-BAR. Middle, red channel detecting Texas Red-DHPE fluorescence. Bottom, merged. Lateral tension σ= 0.1 mN/m. Vesicle composition is DOPC with 20% DOPS and 3% Chol, and protein solution concentration is 0.23 μM, with 15 mM NaCl. B) Tube fluorescence from components indicated in (A) (red and green points), for a vesicle with composition 86% DOPC, 11% DOPS and 3% Chol, is monitored as variations in the membrane tension [filled points indicate increased tension level; values in (C)] modulate the curvature of the tube (see Materials and Methods for details). Whereas the lipid fluorescence decreases as tube curvature is increased24,25, the DA-N-BAR fluorescence remains roughly constant over the tension levels considered. C) The ratio of fluorescence intensities from (B), Ir = Igreen/Ired, is averaged over 3-4 xz scans for each tension level. Error bars indicate SD. The p-value from Student’s t-test comparing all low (open bars) to all high (filled bars) tension Ir values is 1.1×10-3. The p-values corresponding to comparisons of Ir between consecutive segments of the cyclic tension change protocol are 0.077, 0.057, 0.022, and 0.025. Thus, Ir values respond with statistical significance to changes in membrane tension.
This high-curvature preference of DA-N-BAR is quantified in Fig 2C, where the intensities from each fluorophore on the tube were measured for a cycle of vesicle membrane tensions. The lipid signal responds as expected: increasing the membrane tension leads to a smaller tube radius, thereby reducing the number of imaged fluorophores. The curvature preference of the protein is demonstrated by the absence of a drop in intensity, which can be interpreted as a further increase in density concurrent to the decrease in tube area. This is quantified through the ratio of protein to lipid fluorescence intensities Ir, which follows the changes in applied vesicle tension (Fig 2C).
In addition to its curvature preference, the N-BAR domain can also act as a curvature generator9. The efficacy of membrane curvature generation and its dependence on protein solution concentration were investigated by measuring the force necessary to mechanically equilibrate tubes pulled from giant vesicles. Tubes pulled from single-phase vesicles with compositions far from a demixing boundary require a constant pulling force that is determined by the vesicle membrane tension (as set by the pipette aspiration) and the vesicle bending stiffness26. The vesicles here used, in absence of protein, follow this trend (data not shown). However, tubes pulled from vesicles that were incubated with DA-N-BAR were observed to require a maintenance force (tube force) that decreased over time immediately after pulling the tube. Fig 3A highlights three example force curves for vesicles incubated with three different solution concentrations of DA-N-BAR. Each equilibrium tube force, reached after tens of seconds, was measured to be lower than the initial tube force. This behavior deviates significantly from pulling forces measured with tubes incubated with the protein dynamin27. Dynamin has been observed to polymerize on tubular membranes in a curvature-dependent manner27. Polymerization has been shown to generate a polymerization force that is revealed as an abrupt decrease in tube pulling force once the tubular membrane becomes completely covered by dynamin27. Accordingly, the slow changes in pulling force after tube extension revealed in Fig 3A suggest a different mechanism for pulling force reduction by DA-N-BAR compared to dynamin. We interpret the time-dependent pulling force decay as reporting a concomitant increase in the protein density on the tube compared to the initial lower density of the vesicle it was pulled from, as evidenced by the tubular enrichment in Fig 2A.
Figure 3.

Tube pulling force is lowered by DA-N-BAR. For all results shown, vesicle membrane tension was fixed at 0.03±0.005 mN/m. A) Tube forces decreased over time for three vesicles incubated with DA-N-BAR at concentrations of 0.11 (light gray), 0.33 (gray), and 0.39 μM (black). Zero time is immediately after pulling a tube with a length of 20 μm from each vesicle. B) A tube is pulled from a vesicle in the absence of DA-N-BAR after which protein is injected into the measurement chamber. An initially constant force is observed before protein injection. After protein injection, tube force drops to near-zero. C) Equilibrium tube forces were observed following reversible tube length changes from the initial length of 20 μm at t = 0 s to 40 μm with a speed of 10 μm/s at t = 105 s and a subsequent return to 20 μm at t =205 s. Vesicle was incubated with 0.33 μM DA-N-BAR.
In Fig 3B, a tube pulled from a vesicle in the absence of DA-N-BAR required constant force until the protein was injected into the chamber at a nominal solution concentration of 1.4 μM. Following injection, tube force was observed to markedly decrease to a near-zero equilibrium value; this is in qualitative agreement with the spontaneous tubulation of liposomes13.
At lower protein concentrations, tube length could be changed reversibly. In Fig 3C, the tube force of a vesicle incubated with DA-N-BAR was measured to decrease following initial formation, as in Fig 3A. After ~100 s of equilibration, the tube was extended from 20 to 40 μm in length in 2 s. The tube force correspondingly increased sharply to near the initial value, then decreased again in similar manner to the first decay but at a somewhat higher equilibrium force28. A subsequent decrease in tube length (from 40 to 20 μm) led to a rapid drop in the tube force; the ensuing reestablishment of the minimum force value first measured demonstrates mechanical and compositional equilibrium.
As was indicated by Fig 3A, the equilibrium tube force was observed to depend on the protein solution concentration that vesicles were incubated in. Systematic measurements of resulting, protein-equilibrated tube forces are shown in Fig 4A. Equilibrium tube forces consistently decreased with increases in DA-N-BAR solution concentration. Sufficiently high concentrations of protein led to near complete loss of tube force, as expected for a protein with tubulation activity.
Figure 4.

Reductions in tube force depend on DA-N-BAR concentration. A) Average equilibrium tube force decreases non-linearly with DA-N-BAR incubation concentration. Averages are taken of 8-10 vesicle/tube pairs at each concentration; SEMs are shown in black, SDs are shown in gray. Each vesicle membrane tension was kept constant at 0.03±0.005 mN/m. Tubes were pulled to 20 μm at 10 μm/s and equilibrium forces were measured after 200 s. B) Effective spontaneous curvatures calculated using equilibrium tube forces in A) and Eq 3. Error bars represent propagated SEMs from force measurements in A) and uncertainties in bending stiffness and lateral tension.
For the measurements shown in Fig 4, salt concentrations were kept low in order to promote binding of DA-N-BAR to the negatively-charged vesicles, ranging between 21 and 27 mM. Over this range and a fixed DA-N-BAR solution concentration, no trend in the resulting equilibrium tube forces could be found. However, even higher salt levels led to higher tube forces, likely indicating reduced binding of protein to the tubular membrane due to electrostatic screening. Increasing the salt concentration to 150 mM in the 0.28 μM DA-N-BAR chamber increased the equilibrium tube force to 11.4±0.7 pN, while an even larger increase to 432 mM salt nearly abolished the force reduction, with equilibrium tube forces approaching the bare tube force. We will systematically investigate salt effects on pulling force in future studies.
As a starting point in understanding the lowered equilibrium tube force dependence on solution concentration of DA-N-BAR, we model our membrane as a homogenous tube partially covered with protein such that the tube adopts an effective spontaneous curvature Cs, the value of which depends on the curvature and membrane binding density of the protein. The free energy F is then given as:
| (1) |
where R and L are the tube radius and length, respectively, κ is the membrane bending stiffness, σ is the lateral tension, and f is the tube force. Minimizations of F with respect to the radius and length lead to a Cs-dependent tube force:
| (2) |
or, equivalently,
| (3) |
From Eq. 3 we calculate an effective spontaneous curvature as a function of protein solution concentrations (Fig 4B). Membrane tension and bare tube bending stiffness were measured as described previously19,20 and were assumed to remain constant at 0.03±0.005 mN/m and 108±7 pN·nm (measured on 5 vesicles in absence of DA-N-BAR), respectively, for all DA-N-BAR solution concentrations. The assumption of tube bending stiffness being unaffected by protein binding has previously been made27. In light of theoretical predictions for membrane inserting peptides1, and experimental measurements involving the peripheral protein Sar129, this assumption needs to be carefully tested in future measurements. From Fig 4B we conclude that for the highest solution concentrations, spontaneous curvatures on the order of 10 nm are found, consistent with the molecular curvature of the DA-N-BAR domain9.
We note that the curvature sorting of DA-N-BAR observed in Fig 2 leads to a transition region between high protein density on the tube and low density on the vesicle. In the presence of a phase boundary, we have recently shown that its exact position within the high curvature neck region influences the measured pulling forces19. Future research will assess the possible contribution of this effect on the measurements shown here.
To summarize, we have found systematic solution concentration dependence of the spontaneous curvature conferred by DA-N-BAR. We note that evidence for intramolecular inhibition of N-BAR domain membrane binding by the SH3 domain of full length amphiphysin exists30,31. Future experiments therefore should address the role of the SH3 domain on membrane curvature sensing and generation by amphiphysin and related BAR domain proteins.
We also note that discrepancies have arisen in recent theoretical work pertaining to the role of the N-terminal amphipathic helix H0 found in N-BAR domains (but not in all BAR domains) in achieving the membrane bending observed for proteins such as amphiphysin32,33. We expect that quantitative measurements of membrane curvature generation, such as shown here, comparing different types of BAR domains and their mutants, will help elucidate mechanisms underlying BAR domain function.
MATERIALS & EXPERIMENTAL METHODS
Materials
1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (DOPC), 1,2-Dioleoyl-sn-Glycero-3-Phospho-L-Serine (DOPS), and 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Biotinyl(Polyethylene Glycol)2000] (DSPE-Bio-PEG2000) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Casein, Tris, and ethylenediaminetetraacetic acid (EDTA) were obtained from Fisher Scientific (Fairlawn, NJ). Cholesterol (Chol) and fatty-acid free bovine serum albumin (BSA) was purchased from Sigma Chemical Co. (St. Louis, MO). Texas Red-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine triethylammonium salt (Texas Red-DHPE) was from Invitrogen (Carlsbad, CA). Streptavidin-conjugated microspheres with mean diameter of ~6 μm were from Polysciences, Inc. (Warrington, PA).
Preparation of Giant Unilamellar Vesicles
Unless noted otherwise, lipid solutions were prepared to contain 68 mol% DOPC, 12 mol% Chol, and 20 mol% DOPS dissolved in chloroform for a total lipid concentration of 0.6 mM, and additionally contained 0.3 mol% Texas Red-DHPE, and 0.3-0.5 mol% DSPE-Bio-PEG2000. GUVs were prepared by electroformation in 1 M sucrose solution as described34.
Preparation of Drosophila amphiphysin N-BAR (DA-N-BAR)
Within the N-BAR domain (DA-N-BAR, residues 1-244), native cysteines (residues 66 and 82) were mutated to alanine, and a cysteine for specific labeling was introduced by mutagenesis at residue 130 (E130C). Protein purification was as described35. For fluorescence measurements, DA-N-BAR was coupled with Alexa Fluor 488-C5-maleimide. The protein was stored in 500 mM NaCl, 20 mM HEPES, pH 7.4.
Preparation of micropipettes
Micropipettes were fashioned from glass capillaries (World Precision Instruments Inc., Sarasota, FL) that were stretched using a pipette puller. Pipette tips were cut using a microforge at desired inner diameters of ~3 μm. Irreversible adhesion of membrane to the pipette was prevented by incubation of pipette tips with 0.5 mg/mL BSA dissolved in 1X PBS. Pipettes were filled with 1 M sucrose solution using a MicroFil needle (WPI, Sarasota, FL).
Tube force reduction induced by DA-N-BAR
A sample chamber was formed from two coverslips overhanging both sides of a glass microscope slide, creating a 1 mm thick cell that was open on three sides to allow the insertion of a micropipette (see Fig 1). The coverslips were pretreated by immersion in a solution of 2.5 mg/mL casein, 20 mM Tris, and 2 mM EDTA and subsequent rinsing with deionized water. The chamber was filled with 100 μL of 1 M sucrose, 1.5 μL of 10X PBS, 0.2 μL of microsphere dispersion, and 10 μL of vesicle dispersion. DA-N-BAR was added to reach the desired nominal concentration. The chamber was mounted on an inverted microscope (IX81; Olympus, Center Valley, PA) equipped with a home-built optical trap19 which uses a second, independently positioned objective (60X, 1.1NA, water immersion, long working distance; Olympus) oriented opposite the imaging objective to introduce a 1064 nm laser into the chamber. Micropipettes were moved via a three-dimensional motorized micromanipulator system (Luigs & Neumann, Ratingen, Germany). Aspiration pressure was controlled through adjustments in the height of an attached water reservoir, and it was measured with a pressure transducer (Validyne Engineering, Los Angeles, CA). An individual vesicle typically between 5 and 10 μm in radius was aspirated under constant pressure to fix the membrane (reservoir) tension at 0.03±0.005 mN/m. A subsequently trapped bead was brought into contact with the vesicle and then retracted at 10 μm/s, generating a tube 20 μm in length. Forces exerted on the bead were measured in real-time. Each vesicle and tube pair was maintained for ~ 200 seconds to allow for both mechanical and compositional equilibration, here defined as a constant, time-independent tube force.
Optical trap design and calibration
Our home-built optical trapping setup has been described previously19. A trapped bead could be translated, via a movable objective lens, with sub-micron precision along the axis of tubes pulled from vesicles without measurable change in trap stiffness. The stiffness of the trap was measured for each bead using the drag-force method36 and was typically 0.05 pN/nm. Tube forces were measured from the displacement of the bead from the trap center.
Confocal fluorescence microscopy
Membrane tubes and GUVs bound by DA-N-BAR were imaged by confocal fluorescence microscopy as described19,24-26. Sample chamber preparation, membrane tube formation, and membrane tension modulation, were as described in the preceding section, except that the polystyrene bead was held in a second micropipette and the coverslips did not require pre-treatment.
Image analysis
Quantitative image analysis was achieved using IMAGEJ (National Institutes of Health, Bethesda, MD). For analysis of the curvature dependence of membrane component localization (Fig 2), fluorescence intensities of green (labeled protein) and red (labeled lipid) channels of the confocal microscope, respectively, were measured from line-sequential scan confocal micrographs of tube cross sections (defined as xz images) as described24,25. Background was determined from the mean fluorescence intensity within a region surrounding the tube, in individual xz images, and subtracted from the mean of the region containing the tube yielding the fluorescence intensity values Igreen and Ired.
Acknowledgments
This project was funded by NSF (MCB-0718569, TB), the Alfred P. Sloan foundation (TB), and NIH (GM063915, RL). We acknowledge helpful discussions with S. Das.
References
- 1.Zemel A, Ben-Shaul A, May S. Modulation of the Spontaneous Curvature and Bending Rigidity of Lipid Membranes by Interfacially Adsorbed Amphipathic Peptides. J Phys Chem B. 2008;112:6988. doi: 10.1021/jp711107y. [DOI] [PubMed] [Google Scholar]
- 2.Groves JT. Bending Mechanics and Molecular Organization in Biological Membranes. Annu Rev Phys Chem. 2007;58:697. doi: 10.1146/annurev.physchem.56.092503.141216. [DOI] [PubMed] [Google Scholar]
- 3.Noid WG, Chu JW, Ayton GS, Krishna V, Izvekov S, Voth GA, Das A, Andersen HC. The multiscale coarse-graining method. I. A Rigorous Bridge Between Atomistic and Coarse-Grained Models. J Chem Phys. 2008;128 doi: 10.1063/1.2938860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leventis PA, Chow BM, Stewart BA, Iyengar B, Campos AR, Boulianne GL. Drosophila Amphiphysin is a Post-Synaptic Protein Required for Normal Locomotion but Not Endocytosis. Traffic. 2001;2:839. doi: 10.1034/j.1600-0854.2001.21113.x. [DOI] [PubMed] [Google Scholar]
- 5.Razzaq A, Robinson IM, McMahon HT, Skepper JN, Su Y, Zelhof AC, Jackson AP, Gay NJ, O’Kane CJ. Amphiphysin is Necessary for Organization of the Excitation-Contraction Coupling Machinery of Muscles, but Not for Synaptic Vesicle Endocytosis in Drosophila. Genes Dev. 2001;15:2967. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelhof AC, Bao H, Hardy RW, Razzaq A, Zhang B, Doe CQ. Drosophila Amphiphysin is Implicated in Protein Localization and Membrane Morphogenesis but Not in Synaptic Vesicle Endocytosis. Development. 2001;128:5005. doi: 10.1242/dev.128.24.5005. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Zelhof AC. Amphiphysins: Raising the BAR for Synaptic Vesicle Recycling and Membrane Dynamics. Traffic. 2002;3:452. doi: 10.1034/j.1600-0854.2002.30702.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule Biogenesis in Muscle. Science. 2002;297:1193. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 9.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT. BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure. Science. 2004;303:495. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 10.Casal E, Federici L, Zhang W, Fernandez-Recio J, Priego EM, Miguel RN, DuHadaway JB, Prendergast GC, Luisi BF, Laue ED. The Crystal Structure of the BAR Domain from Human Bin1/Amphiphysin II and its Implications for Molecular Recognition. Biochemistry. 2006;45:12917. doi: 10.1021/bi060717k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon HT, Gallop JL. Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature. 2005;438:590. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 12.Blood PD, Voth GA. Direct observation of Bin/amphiphysin/Rvs (BAR) Domain-Induced Membrane Curvature by Means of Molecular Dynamics Simulations. Proc Natl Acad Sci U S A. 2006;103:15068. doi: 10.1073/pnas.0603917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takei K, Slepnev VI, Haucke V, De Camilli P. Functional Partnership Between Amphiphysin and Dynamin in Clathrin-Mediated Endocytosis. Nat Cell Biol. 1999;1:33. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 14.Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural Basis of Membrane Invagination by F-BAR Domains. Cell. 2008;132:807. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin Y, Arkhipov A, Schulten K. Simulations of Membrane Tubulation by Lattices of Amphiphysin N-BAR Domains. Structure. 2009;17:882. doi: 10.1016/j.str.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallop JL, Jao CC, Kent HM, Butler PJG, Evans PR, Langen R, McMahon HT. Mechanism of Endophilin N-BAR Domain-Mediated Membrane Curvature. EMBO J. 2006;25:2898. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N. Endophilin BAR Domain Drives Membrane Curvature by Two Newly Identified Structure-Based Mechanisms. EMBO J. 2006;25:2889. doi: 10.1038/sj.emboj.7601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochmuth RM, Wiles HC, Evans EA, Mccown JT. Extensional Flow of Erythrocyte-Membrane from Cell Body to Elastic Tether .2. Experiment. Biophys J. 1982;39:83. doi: 10.1016/S0006-3495(82)84493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinrich M, Tian T, Esposito C, Baumgart T. Dynamic Sorting of Lipids and Proteins in Curvature Gradients: A Moving Phase Boundary Problem. Proc Natl Acad Sci U S A. 2010;107:7208. doi: 10.1073/pnas.0913997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuvelier D, Derenyi I, Bassereau P, Nassoy P. Coalescence of Membrane Tethers: Experiments, Theory, and Applications. Biophys J. 2005;88:2714. doi: 10.1529/biophysj.104.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derenyi I, Julicher F, Prost J. Formation and Interaction of Membrane Tubes. Phys Rev Lett. 2002;88:2381011. doi: 10.1103/PhysRevLett.88.238101. [DOI] [PubMed] [Google Scholar]
- 22.Leibler S. Curvature Instability in Membranes. J Phys (Paris) 1986;47:507. [Google Scholar]
- 23.Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost J, Goud B, Bassereau P. Curvature-Driven Lipid Sorting Needs Proximity to a Demixing Point and is Aided by Proteins. Proc Natl Acad Sci U S A. 2009;106:5622. doi: 10.1073/pnas.0811243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian A, Baumgart T. Sorting of Lipids and Proteins in Membrane Curvature Gradients. Biophys J. 2009;96:2676. doi: 10.1016/j.bpj.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capraro BR, Yoon Y, Cho W, Baumgart T. Curvature Sensing by the Epsin N-Terminal Homology (ENTH) Domain Measured on Cylindrical Lipid Membrane Tethers. J Am Chem Soc. 2010;132:1200. doi: 10.1021/ja907936c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian AW, Capraro BR, Esposito C, Baumgart T. Bending Stiffness Depends on Curvature of Ternary Lipid Mixture Tubular Membranes. Biophys J. 2009;97:1636. doi: 10.1016/j.bpj.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux A, Koster G, Lenz M, Sorre B, Manneville JB, Nassoy P, Bassereau P. Membrane Curvature Controls Dynamin Polymerization. Proc Natl Acad Sci U S A. 2010;107:4141. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waugh RE, Song J, Svetina S, Zeks B. Local and Nonlocal Curvature Elasticity in Bilayer-Membranes by Tether Formation from Lecithin Vesicles. Biophys J. 1992;61:974. doi: 10.1016/S0006-3495(92)81904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Settles EI, Loftus AF, McKeown AN, Parthasarathy R. The Vesicle Trafficking Protein Sar1 Lowers Lipid Membrane Rigidity. Biophys J. 2010;99:1539. doi: 10.1016/j.bpj.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao YJ, Ma QJ, Vahedi-Faridi A, Sundborger A, Pechstein A, Puchkov D, Luo L, Shupliakov O, Saenger W, Haucke V. Molecular Basis for SH3 Domain Regulation of F-BAR-Mediated Membrane Deformation. Proc Natl Acad Sci U S A. 2010;107:8213. doi: 10.1073/pnas.1003478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farsad K, Slepnev V, Ochoa G, Daniell L, Hauke V, De Camilli P. A Putative Role for Intramolecular Regulatory Mechanisms in the Adaptor Function of Amphiphysin in Endocytosis. Neuropharmacology. 2003;45:787. doi: 10.1016/s0028-3908(03)00306-x. [DOI] [PubMed] [Google Scholar]
- 32.Arkhipov A, Yin Y, Schulten K. Membrane-Bending Mechanism of Amphiphysin N-BAR Domains. Biophys J. 2009;97:2727. doi: 10.1016/j.bpj.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campelo F, McMahon HT, Kozlov MM. The Hydrophobic Insertion Mechanism of Membrane Curvature Generation by Proteins. Biophys J. 2008;95:2325. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathivet L, Cribier S, Devaux PF. Shape Change and Physical Properties of Giant Phospholipid Vesicles Prepared in the Presence of an AC Electric Field. Biophys J. 1996;70:1112. doi: 10.1016/S0006-3495(96)79693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varkey J, Isas JM, Mizuno M, Jensen MB, Bhatia VK, Jao CC, Petriova J, Voss JC, Stamou DG, Steven AC, et al. Membrane Curvature Induction and Tubulation are Common Features of Synucleins and Apolipoproteins. J Biol Chem. 2010;285:32486. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svoboda K, Block SM. Biological Applications of Optical Forces. Annu Rev Biophys Biomol Struct. 1994;23:247. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]


