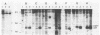Abstract
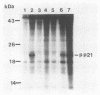
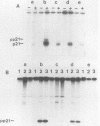
Point mutations of p21 proteins were constructed by oligonucleotide-directed mutagenesis of the v-rasH oncogene, which substituted amino acid residues within the nucleotide-binding consensus sequence, GXG GXGK. When the glycine residue at position 10, 13, or 15 was substituted with valine, the viral rasH product p21 lost its GTP-binding and autokinase activities. Other substitutions at position 33, 51, or 59 did not impair its binding activity. G418-resistant NIH 3T3 cell lines were derived by transfection with constructs obtained by inserting the mutant proviral DNA into the pSV2neo plasmid. Clones with a valine mutation at position 13 or 15 were incapable of transforming cells, while all other mutants with GTP-binding activity were competent. A mutant with a substitution of valine for glycine at position 10 which had lost its ability to bind GTP and its autokinase activity was fully capable of transforming NIH 3T3 cells. These cells grew in soft agar and rapidly formed tumors in nude mice. The p21 of cell lines derived from tumor explants still lacked the autokinase activity. These findings suggest that the glycine-rich consensus sequence is important in controlling p21 activities and that certain mutations may confer to p21 its active conformation without participation of ligand binding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. GTP-binding proteins. One molecular machine can transduce diverse signals. 1986 Jun 26-Jul 2Nature. 321(6073):814–816. doi: 10.1038/321814a0. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Maryak J. M., Wei C. M., Shih T. Y., Shober R., Cheung H. L., Ellis R. W., Hager G. L., Scolnick E. M., Lowy D. R. Functional organization of the Harvey murine sarcoma virus genome. J Virol. 1980 Jul;35(1):76–92. doi: 10.1128/jvi.35.1.76-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield R. G., Jones S. S., Lo K. M., Weinberg R. A. Activation of Ha-ras p21 by substitution, deletion, and insertion mutations. Mol Cell Biol. 1985 Aug;5(8):1809–1813. doi: 10.1128/mcb.5.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton D. J., Hattori S., Shih T. Y. Mutations of the ras gene product p21 that abolish guanine nucleotide binding. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5076–5080. doi: 10.1073/pnas.83.14.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Pan B. T., Roberts T. M., Cooper G. M. Isolation of ras GTP-binding mutants using an in situ colony-binding assay. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4607–4611. doi: 10.1073/pnas.83.13.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Ellis R. W., Scolnick E. M. Autophosphorylation of v-Ha-ras p21 is modulated by amino acid residue 12. Proc Natl Acad Sci U S A. 1984 May;81(9):2674–2678. doi: 10.1073/pnas.81.9.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Hattori S., Clanton D. J., Satoh T., Nakamura S., Kaziro Y., Kawakita M., Shih T. Y. Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol Cell Biol. 1987 May;7(5):1999–2002. doi: 10.1128/mcb.7.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Yamashita T., Copeland T. D., Oroszlan S., Shih T. Y. Reactivity of a sulfhydryl group of the ras oncogene product p21 modulated by GTP binding. J Biol Chem. 1986 Nov 5;261(31):14582–14586. [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Aaronson S. A. Activation of ras p21 transforming properties associated with an increase in the release rate of bound guanine nucleotide. Mol Cell Biol. 1986 Dec;6(12):4214–4220. doi: 10.1128/mcb.6.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenberger J. A., Ulsh L., Shih T. Y., Papas T. S. High-level expression in Escherichia coli of enzymatically active Harvey murine sarcoma virus p21ras protein. Science. 1983 Aug 26;221(4613):858–860. doi: 10.1126/science.6308763. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Clark B. F., la Cour T. F., Kjeldgaard M., Norskov-Lauritsen L., Nyborg J. A model for the tertiary structure of p21, the product of the ras oncogene. Science. 1985 Oct 4;230(4721):78–82. doi: 10.1126/science.3898366. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Stokes P. E., Smythers G. W., Dhar R., Oroszlan S. Characterization of the phosphorylation sites and the surrounding amino acid sequences of the p21 transforming proteins coded for by the Harvey and Kirsten strains of murine sarcoma viruses. J Biol Chem. 1982 Oct 10;257(19):11767–11773. [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O. Oncogenes and cancer: the p21 ras genes. Cancer Invest. 1984;2(2):109–123. doi: 10.3109/07357908409020294. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Trahey M., Milley R. J., Cole G. E., Innis M., Paterson H., Marshall C. J., Hall A., McCormick F. Biochemical and biological properties of the human N-ras p21 protein. Mol Cell Biol. 1987 Jan;7(1):541–544. doi: 10.1128/mcb.7.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Clark S. G., Levinson A. D. The oncogenic activation of human p21ras by a novel mechanism. Science. 1986 Aug 8;233(4764):649–652. doi: 10.1126/science.3487832. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Kung H. F., Bekesi E., Robins T., Johnsen M., Vass W. C., Lowy D. R. Mutational analysis of a ras catalytic domain. Mol Cell Biol. 1986 Jul;6(7):2646–2654. doi: 10.1128/mcb.6.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]