Abstract
Walter (Jahrb Wiss Bot 87:750–860, 1939) proposed a two-layer hypothesis, an equilibrium explanation for coexistence of savanna trees and grasses. This hypothesis relies on vertical niche partitioning and assumed that grasses are more water-use efficient than trees and use subsurface water while trees also have access to deeper water sources. Thus, in open savannas, grasses were predicted to predominate because of their water use efficiency and access to subsurface water. This hypothesis has been a prominent part of the savanna literature since first proposed. We review the literature on Walter’s hypothesis and reconsider his original intentions. Walter intended this hypothesis to be restricted to dry savannas. In his opinion, mesic and humid savannas were controlled by biotic factors and disturbances. We surveyed the global savanna literature for records of vertical niche partitioning by grasses and trees. We find that, within the scope of Walter’s original intentions, this hypothesis works remarkably well, and in some cases is appropriate for deserts as well as for dry temperate systems and even some mesic savannas.
Electronic supplementary material
The online version of this article (doi:10.1007/s00442-012-2538-y) contains supplementary material, which is available to authorized users.
Keywords: Savanna, Tree–grass coexistence, Codominance, Equilibrium theory, Patch dynamics, Spatial heterogeneity, Resource partitioning, Root distributions, Water
Introduction
Savannas cover about 20 % of the earth’s land surface (Sankaran et al. 2005). They are generally described as biomes with continuous grass strata and discontinuous tree or shrub strata (Walker 1985; Belsky 1990). The identification of the mechanisms leading to this tree–grass codominance in savannas is a widely and often controversially discussed topic (e.g. Sarmiento 1984; Belsky 1990; Scholes and Archer 1997; Sankaran et al. 2004; Scheiter and Higgins 2007). Among a variety of concepts seeking to explain tree–grass codominance, spatial resource partitioning is one of the most favoured but also most disputed (Walter 1939; Walker and Noy-Meir 1982; Jeltsch et al. 2000; Ward 2005; Lehmann et al. 2009). According to this concept, tree and grass roots occupy different vertical niches, providing each vegetation stratum with almost exclusive access to essential resources such as water and nutrients (Breshears and Barnes 1999; Kambatuku et al. 2012). In its purest form, when woody and herbaceous niches do not overlap, no competition for resources between the plants in these strata would exist and, therefore, tree–grass codominance would be in a stable equilibrium.
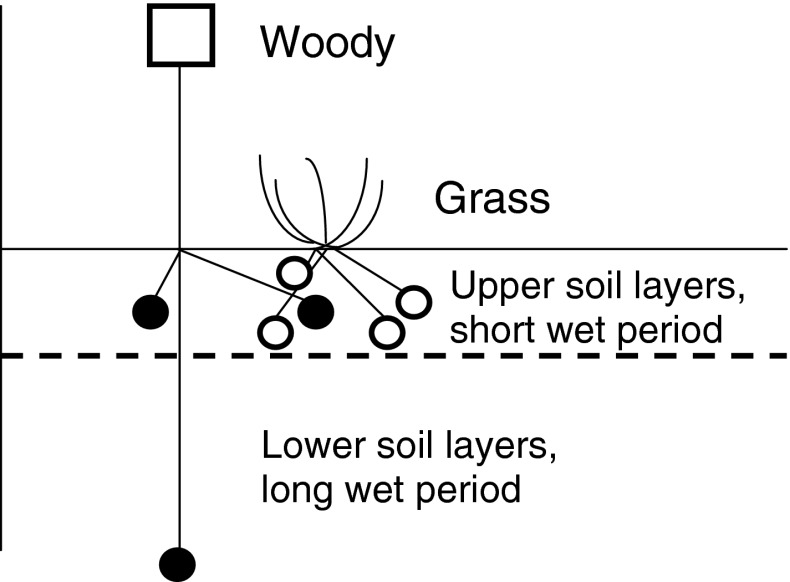
Exemplary among such spatial concepts is Walter’s (1939) two-layer hypothesis which has often been used to explain niche separation between trees and grasses (Walker et al. 1981; Walker and Noy-Meir 1982). In this two-layer hypothesis, it has been proposed that grasses with their intense and shallow root system would use water only from the subsurface layers (hereafter termed “topsoil”). In contrast, woody savanna trees would only use little of the topsoil water but would have exclusive access to and primarily rely on subsoil water below the grass roots (Fig. 1). Spatial niche separation in the soil would account for the equilibrium-like codominance of trees and grasses. However, this explanation, which may be found in most publications citing the two-layer hypothesis, was initially proposed by Walker and Noy-Meir (1982). It is a simplification of the original hypothesis of Walter (1939, 1971) and it has often been disputed or even rejected (e.g. Higgins et al. 2000; Jeltsch et al. 2000; Scheiter and Higgins 2007; Kulmatiski et al. 2010). The goals of this article are to explain the origin of the two-layer hypothesis and to review empirical evidence for it (Walter 1939, 1971).
Fig. 1.
A minimal model of vertical resource partitioning (from Walker and Noy-Meir 1982). Filled circles tree roots, open circles grass roots. Note that competition only occurs in the upper soil layer, where water is available for a short period only prior to evaporation
The foundation for the two-layer hypothesis was laid by the German botanist Andreas F.W. Schimper (1898) who emphasized different rooting depths of woody and herbaceous plants in his book Pflanzengeographie auf physiologischer Grundlage (in part translated in 1903 as Plant Geography on a Physiological Basis). In this book, Schimper (1898) distinguished between topsoil and subsoil properties. Trees with their vast root systems, capable of using deep sources of water, would therefore be capable of surviving long droughts in arid climates. In contrast, grasses are shallow-rooted plants and hence “Moisture in the subsoil has little influence on the covering of grass; only moisture in the superficial soil is important to it” [his emphasis, not ours] (Schimper 1903). Schimper (1903, p. 174) further went on to say “Almost immaterial for grassland are the following:- Moisture in the subsoil (except when the superficial soil has a great power of capillary conduction)…” [his emphasis, not ours]. Although Schimper (1898) had focused on resource partitioning in the soil, his explanations for grassland or woodland dominance were primarily related to climate, leading him to call those biome physiognomies “climatic or district formations, the character of whose vegetation is governed by atmospheric precipitations” which he differentiated from “the edaphic or local formations, whose vegetation is chiefly determined by the nature of the soil” [his emphasis, not ours] (Schimper 1903, p. 161). Schimper (1903, pp. 165–166) further stated that “The depth of their root-system renders it possible for trees to thrive in areas where long seasons of drought accompanied by great heat recur periodically, …” and that “It is neither frequent atmospheric precipitation nor a rainy vegetative season that is of importance to tree-growth, but it is the continuous presence of a supply of water within reach of the extremities of the roots, and therefore at a considerable depth in the soil. It is immaterial during what season this supply is renewed.” [his emphasis, not ours].
During his field trips to Southwest Africa (now Namibia), the German ecologist Heinrich Walter took specific interest in the mechanisms leading to tree–grass codominance in savannas (Walter 1939). Walter developed his competition–equilibrium theory between trees and grasses only for natural, dry savannas with level topography and average, fine-textured sandy soils and an annual precipitation of just 100–600 mm (Walter 1954, 1971). He argues that in this (very restrictive) type of “climatic savanna”, tree–grass ratios would directly reflect a rainfall gradient. Up to 250 mm annual precipitation, grasses (all C4 grasses in these ecosystems) would dominate nearly exclusively because all the water influx would be rapidly taken up by the intensively transpiring grass layer with its dense, shallow root system. Tree seedlings would be unable to establish, because under such arid conditions grasses would be the superior competitors for soil water due to their greater water-use efficiency. When annual precipitation increases above 250 mm, woody plants would increase in density with increasing rainfall because not all rain water may be taken up by the grasses (Ward and Ngairorue 2000). An increasing amount of water may penetrate into the deeper subsoil where trees would have the majority of their roots and hence be the superior competitor for water. Up to 500 mm mean annual precipitation (MAP), Walter (1939) considered grasses to be functionally dominant and trees are merely tolerated by the herbaceous stratum. Above 500 mm MAP, Walter (1939) noted that woody plants become dominant, and grasses would be tolerated by the trees.
According to Walter (1939), this rainfall-controlled tree–grass equilibrium works in climatic savannas but not in edaphic and secondary or anthropogenic savannas where other factors such as stony, two-layered or nutrient-deficient soils (=“edaphic savannas”), topography and catenas, termite mounds, waterlogging, regular fires and/or grazing impact from cattle ranching (=“anthropogenic savannas”) are primary determinants of the structure of the system. In Walter’s (1973a) classic book, Die Vegetation der Erde in ökophysiologischer Betrachtung (translated as Vegetation of the Earth in relation to climate and the eco-physiological conditions; Walter 1973b), the correlation between increasing tree-grass ratios and increasing rainfall reflects a competitive equilibrium that only exists in a small proportion of the world’s “natural” savannas, namely in Southwest Africa (now known as Namibia) and in central Argentina (Walter 1973b, p. 71). This is important for later considerations, when studies from other climatic systems make reference to the two-layer hypothesis. Walter’s (1939) subdivision into climatic, edaphic and anthropogenic savannas (e.g. where there was a grazing-induced impact) has largely been replaced by a distinction between arid,mesic and/or humid savannas (e.g. Sankaran et al. 2005; Higgins et al. 2010—there is some debate over the thresholds between these categories), with climatic savannas being a subgroup of arid savannas.
We note that Walter never explained vertical resource partitioning solely via the root system. He stressed the differences in the water economy of woody and herbaceous plants, and therefore above-ground properties, as an “antagonistic” cause of tree–grass differentiation (e.g. Walter 1954, 1973a). He argued that the cause of the antagonism is to be sought in the differences in (1) their root systems and (2) their water economy (1973b, p. 68). Grasses would transpire permanently and intensively until necrosis and dormancy start in the dry season. Therefore, grasses would grow best in tropical, wet summer savannas and hence, would constitute the continuous layer in this biome. In contrast, woody plants would regulate their osmotic pressure much more precisely through closing or opening their stomata with their loss of and demand for water being comparatively higher in the dry season. The grasses that Walter (1973b) refers to are C4 grasses that are adapted to arid ecosystems due to their greater water-use efficiency at high temperatures than C3 grasses (Ehleringer and Monson 1993).
Walter (1973b) devoted over an entire page (pp. 68–69) to the differences in their root systems and to the transpiration characteristics of grasses and trees: “But it is still the grasses which are the dominating partner, and they determine how much water is left over for the woody plants” (p. 69). Thus, transpiration differences between grasses and trees were perceived by Walter (1939) to be the ultimate cause of vertical resource partitioning and differences in the root system merely the proximate cause (see Knoop and Walker’s (1985) ‘superior competitor’ concept below). Walter never viewed vertical rooting differences as a single plant property in explaining the two-layer hypothesis. However, the two-layer hypothesis has often been referred to as a self-explanatory hypothesis of below-ground aspects without taking Walter’s (1971) holistic descriptions and geographical restrictions into account. In fact, it was Walker and Noy-Meir (1982) who first reduced the holistic complexity of the hypothesis to its current interpretation (Fig. 1). Essentially, these authors developed a series of models to study stability and resilience of savannas, including the “minimum model for a savanna” (Walker and Noy-Meir 1982), which was Walter’s two-layer hypothesis (the term was first coined by Walker et al. 1981). Knoop and Walker (1985) further modified this concept and derived the notion of the ‘superior competitor’ for savanna tree–grass interactions, where one functional group (usually grasses) was competitively superior to the other. The ‘superior competitor’ does not require there to be complete separation of grass and tree roots but only requires that one is more efficient than the other in water and, perhaps, nutrient uptake.
Field data and theoretical models have produced conflicting evidence regarding Walter’s two-layer hypothesis. Several field studies have shown the increase of shrub or tree abundance under heavy grazing which is expected under Walter’s (1939) model because grasses are removed, allowing trees to occupy the vacated space (e.g. van Vegten 1983; Skarpe 1990a, b; Perkins and Thomas 1993; Grellier et al. 2012). However, recruitment in honey mesquite (Prosopis glandulosa), a bush-encroaching tree in North America, is unrelated to herbaceous biomass or density, indicating that release from competition with grasses is not required for mass tree recruitment to occur (Brown and Archer 1989, 1999). Similarly, while some models have shown that the two-layer hypothesis may indeed lead to tree–grass coexistence (Walker et al. 1981; Walker and Noy-Meir 1982), a spatially explicit simulation model by Jeltsch et al. (1996) showed that rooting niche separation might not be sufficient to warrant coexistence under a range of climatic situations.
With this review, we collate evidence for and against vertical resource partitioning from 36 core studies that have investigated vertical resource partitioning (see “Materials and methods” for details of keywords used for selecting publications). We analyse how much simplification of the hypothesis is appropriate and under what ecological conditions the hypothesis may be valid. We made the following predictions:
Walter’s two-layer hypothesis would adequately predict root-niche partitioning in dry savannas and in desert systems but may not do so effectively for moist savannas because of the potential effects of disturbances such as grazing and fire (Sankaran et al. 2005).
The notion of the ‘superior competitor’ (sensu Knoop and Walker 1985) may be sufficient to explain cases of partial root overlap.
Studies that have rejected the two-layer hypothesis may have done so under conditions where Walter’s criteria did not hold.
Materials and methods
In total, there are 36 studies in this global review although there are 35 separate papers on the two-layer hypothesis, with one study (Belsky 1994) being suitable for separate consideration because it investigated tree–grass codominance for a dry and a moist savanna site. We used the keywords “Walter”, “two-layer hypothesis”, “root niche partitioning”, as well as “woody plant encroachment”, “shrub encroachment” and “bush encroachment” to search for appropriate papers. Deviations of these publications from the two-layer hypothesis in terms of their actual findings or in terms of their interpretation will be listed in the “Results” and evaluated in the “Discussion”. In the “Discussion”, we focus particularly on those cases where there was partial support for the two-layer hypothesis. We also included studies that were inconsistent with Walter’s original description (e.g. they were not topographically flat) as long as they investigated rooting depths appropriately.
We separated dry and moist tropical/subtropical savannas from the temperate biome. All dry savannas will be those arid to semi-arid savannas that receive 100–650 mm mean annual precipitation (MAP) and all moist savannas will be those subhumid (mesic) to humid systems that receive more than 650 mm rainfall per year (Huntley 1982; Sankaran et al. 2005). A review of 854 sites across Africa has shown that maximum woody cover in savannas receiving a MAP of <650 mm is constrained by, and increases linearly with, MAP (Sankaran et al. 2005). These dry savannas are considered ‘stable’ because fire, herbivory, and soil properties interact to merely reduce woody cover below the MAP-controlled upper bound. However, there is no clear cut-off line between dry and moist savannas because the actual value at which rainfall ceases to have an overriding effect on primary production depends on soil texture (Bell 1982; East 1984). Furthermore, Higgins et al. (2010) have noted that fire can only be confidently considered to affect savanna structure at MAP >820 mm. We note that Lehmann et al. (2009) have also shown that the rainfall threshold for a switch between rainfall-dependent tree densities and biotic dependence differs across savannas in South America, Africa and Australia.
The typical climate of savannas is seasonal, with wet, warm to hot summers alternating with dry, warm to cool winters (Johnson and Tothill 1985). As a biome of the tropics and subtropics, savannas are distinguished from the seasonal climates of the temperate zone by a hot wet season of 4–8 months and a dry season for the rest of the year. This strong seasonal climate unifies all global savannas by allowing dry grass fuels to accumulate and to regularly burn (Scholes 1997; Getzin 2007), except where there is insufficient rainfall in arid savannas (Meyer et al. 2005).
Revisiting and evaluating the validity of Walter’s two-layer hypothesis requires the exclusion of both edaphic and anthropogenic savannas (e.g. Ward et al. 2004; Wiegand et al. 2005; Wu and Archer 2005). We explicitly excluded edaphic savannas because, for example, shallow soils on bedrock prevent vertical soil–water partitioning in the arid savanna of Namibia (Wiegand et al. 2005). While fine-textured soils usually favour grasses with their shallow roots (Nano and Clarke 2010) and coarse-textured soils favour trees with their extensive and deeper-reaching root systems (Ward and Esler 2011), these tree–grass ratios may be reversed if topographic redistribution of water overrides soil texture effects (Wu and Archer 2005). In Van Wijk’s (2009) ecohydrological model, roots were predicted to be deeper with higher rainfall (consistent with Jackson et al.’s 1996 and Schenk and Jackson’s 2002 empirical data) because of a shift in the importance of evaporation relative to drainage. As rainfall increased, drainage was more important than evaporation, resulting in an optimal distribution of roots at greater depths. Van Wijk (2009) also found that this result was consistent with the pattern resulting from increasing coarseness (sandiness) of the soil because more water is lost from drainage in coarse soils (see also empirical data from Kambatuku et al. 2012). We note that there may be some overlap between climatic and edaphic savannas, as recognized by Walter (1939, 1970).
We must also exclude those cases where grasses have been compared to other herbaceous plants (e.g. Walter 1973b; Fargione and Tilman 2005) and where woody plants have been compared to other woody plants (e.g. Smith and Walker 1983; Holdo and Timberlake 2008; Meyer et al. 2008; Sher et al. 2010). We are not aware of any phylogenetic bias in the choice of woody plant species as these ranged from gymnosperms to a variety of different angiosperm families (Electronic Supplement). We will discuss evidence for and against the two-layer hypothesis for the different vegetation systems (cf. Electronic Supplement) separately. The two-layer hypothesis can be rejected if grasses compete equally with tree roots in the subsoil or if tree roots do not grow deeper than grass roots. However, in terms of vertical niche partitioning and water-use efficiency of both functional types, the concept of the ‘superior competitor’ (Knoop and Walker 1985) in the soil layer is sufficient to meet Walter’s (1973b) idea of niche partitioning by competitive advantage. Conversely, the requirement of partitioning of the rooting space based on a strict threshold is a sufficient but not a necessary condition for the two-layer hypothesis to be valid. It seems clear that taproots are not necessarily sufficient to supply the transpiration needs of a complete tree canopy (Scholes 1997) and that topsoil–subsoil boundaries are not sharp in a plant-physiological sense (see, e.g., Kambatuku et al. 2012). We also recognize that, while tree seedlings are growing their roots deeper, they are likely to compete with established grasses (Weltzin and McPherson 1997; Ward 2005; Nano and Clarke 2010; Ward and Esler 2011). Thus, we focus here on adult plants.
Results
Overview of study sites
Of the 36 study sites, 21 could be classified as savannas (Electronic Supplement). Eight of these were dry and 13 were moist savannas, the latter receiving a MAP of more than 650 mm (following Sankaran et al. 2005). Of the 8 dry savanna sites, only 5 were savannas in a strict sense (Scholes 1997) with a hot wet season in summer and no rainfall in winter. These 5 savanna sites were located in sub-Saharan Africa. The 3 dry savannas with winter precipitation were located in the southern United States. Eleven of the 13 moist savannas had summer rainfall only and occurred predominantly in Africa (2 were in Australia). The 2 moist savannas with partly winter rainfall were in the southern United States.
Of the 13 dry non-savanna sites (MAP <650 mm), 2 were subtropical deserts, 3 were temperate semi-deserts, 7 were temperate steppes, and 1 was a temperate (dry) sandhills. All these were located in the New World with the exception of 2 desert subtropical sites in South Africa (February et al. 2011; Shiponeni et al. 2011) which also differed in their seasonality of rainfall (Electronic Supplement). An additional 2 temperate non-savanna sites received more than 650 mm MAP.
Overview of root measurements
At 25 study sites, vertical resource partitioning was assessed based on root excavations. In 23 studies, vertical resource partitioning was based on measurements of soil water or plant water potentials, soil water content or stable isotope ratios (note that root excavations and assessments of soil water or plant water potentials are not mutually exclusive; Electronic Supplement). At 9 study sites, roots were excavated down to a depth of 40–70 cm (plus 1 site where bins were used; Kambatuku et al. 2012), at 8 sites to a depth of >70–120 cm, and at 7 sites to a depth of >120–270 cm. A clear differentiation between tree–grass ratios within fine-root samples (diameter <2 mm) using the 13C isotopic approach was done for four study sites only (Electronic Supplement).
Overview of evidence for vertical resource partitioning
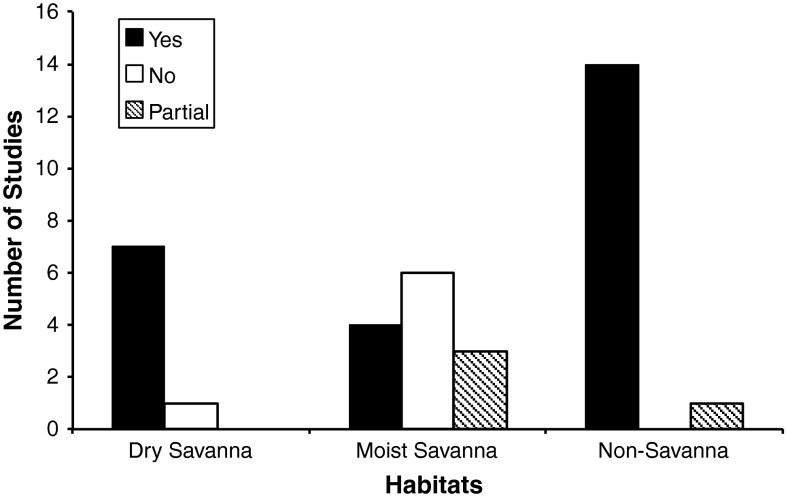
For 32 out of 36 study sites, authors claimed to have investigated the two-layer hypothesis. However, 28 of these 36 study sites belonged to moist savanna, semi-desert, desert or temperate vegetation systems to which the original two-layer hypothesis of Walter (1954, 1971) did not refer. Based on these individual studies, we found (for the 8 dry savannas) 7 study sites with evidence supporting the two-layer hypothesis, and 1 with evidence against it (Fig. 2). For the 13 moist savannas, we found 4 study sites with evidence supporting the two-layer hypothesis, 3 with partial evidence, and 6 with evidence against it. For the 15 non-savanna studies, we found 14 study sites with evidence supporting the two-layer hypothesis and 1 with partial evidence in support of the two-layer hypothesis (Electronic Supplement).
Fig. 2.
Summary of conclusions from survey of literature testing Walter’s (1939) two-layer hypothesis. Yes supported two-layer hypothesis; No did not support; Partial inconsistent support (details in “Discussion”)
Discussion
Of the 36 studies regarding Walter’s two-layer hypothesis that were reviewed, 25 supported the two-layer hypothesis, 4 showed partial support and 7 were inconsistent with the two-layer hypothesis. The two-layer hypothesis is also consistent with indirect observations that grass removal allows tree roots to occupy the subsoil layer. We cite five examples here:
A study by Schultz et al. (1955) in California showed that there was a negative correlation between brush seedling survival (mostly Ceanothus cuneatus, C. leucodermis and Arctostaphylos mariposa) and herbaceous plant density.
Walter (1973b, p. 71) noted “Such a labile equilibrium in the savanna is readily disrupted by man when he begins to utilize the land for grazing purposes. Water losses due to transpiration cease when the grass is eaten off, so that more water remains in the soil to the advantage of the woody plants (mostly Acacia species), which can subsequently develop luxuriantly…” [his emphasis, not ours].
Stuart-Hill and Tainton (1989) removed grasses from the vicinity of Acacia karroo trees in the Eastern Cape province of South Africa and found that there was a 20 % increase in soil water as a consequence (MAP = 422 mm).
Ward and Esler (2011) found that clipping grass had a significant positive effect on the recruitment of Acacia mellifera trees in the Northern Cape province of South Africa (MAP = 380 mm).
Grellier et al. (2012) found that competition with grass, as well as the presence of fire, had a significant effect on recruitment of Acacia sieberiana (MAP = 745 mm) in the KwaZulu-Natal province of South Africa; more A. sieberiana recruited when grasses were clipped or when there was fire. Essentially, this means that bare space leads to recruitment of these species.
The second criterion for evaluating the two-layer hypothesis is the concept of the ‘superior competitor’ (Knoop and Walker 1985) in the soil layer, which is more important than niche partitioning based on a strict threshold. Even if tree and grass roots overlap to a certain extent, the two-layer model can still operate, provided grasses and trees are the superior competitors in their layers, for example due to different relative distributions at variable depths (Knoop and Walker 1985; Dodd et al. 1998; Van Langevelde et al. 2003; Sankaran et al. 2004; Kambatuku et al. 2012).
Non-savanna vegetation
At the (predominantly temperate) non-savanna sites, support for the validity of the two-layer hypothesis was high (Electronic Supplement). Most of these studies took place in dry sites (13 of 15 studies). In 2 of the 15 studies, the criterion of 650 mm MAP (see “Materials and methods” above; Higgins et al. 2010) was exceeded, yet both of these studies (Nippert and Knapp 2007; Asbjornsen et al. 2008) supported Walter’s two-layer hypothesis. Most of these results were based on measurements of water use by plants, with 4 studies based on root excavation only (5 studies employed both root excavation and water use measures). Excavation studies showed that grass roots dominate in the upper 15 cm but shrub roots dominate below 35 cm in the Patagonian steppe (Soriano and Sala 1983). In the short-grass steppe of Colorado, grasses had 50 % of their roots in the upper 30 cm, but few had roots below 50 cm, whereas shrubs had a greater root proportion below 50 cm depth (Lee and Lauenroth 1994). In the Chihuahuan Desert, root density was highest in the topsoil for all three shrub species tested and for the grass species, but root density decreased fastest with depth for grasses whereas shrubs had roots down to 70 cm and even below (Montaña et al. 1995). Although there was root overlap in these studies, grasses and woody plants were either the superior or exclusive competitors in their respective soil layers. Studies based on water use show similar findings (Sala et al. 1989; Baldocchi et al. 2004; Nippert and Knapp 2007). For example, in the short-grass steppe of Colorado, the dominant zone of soil water acquisition of grasses is the upper 15 cm, but little is used below, whereas shrubs (Atriplex canescens) also use subsoil and ground water (Dodd et al. 1998).
In the single study on non-savanna vegetation with partial evidence supporting the two-layer hypothesis, two grass species and one deciduous shrub (Prosopis caldenia) displayed a pattern consistent with the two-layer hypothesis, although an evergreen shrub species (Condalia microphylla) primarily used topsoil water (Peláez et al. 1994). There was root overlap between grasses and the evergreen shrub in the topsoil, but the authors assume little competition between both life forms because evergreen shrubs from arid areas have inherently slow growth rates and low capacities to photosynthesise and absorb nutrients.
The concept of the ‘superior competitor’ is essential for the two-layer hypothesis at all those sites that have a very low MAP or where rainfall occurs biannually during both the warm and the cool season. The superiority in water uptake becomes crucial for tree–grass codominance when only grasses are capable of using small amounts of precipitation. This is important, especially at dry sites with sparse summer rain such as the Chihuahuan Desert, where as little as 15 mm rainfall only raised the xylem water potential of grasses but not of shrubs, indicating the importance of small rainfall events and shallow roots for the survival of grasses (Montaña et al. 1995; Ward 2009). This also applies to sites with biannual rainfall. According to Sala et al. (1989), “water in the upper layers has different seasonality and shorter residence time than deep water, and plant traits which confer an advantage in shallow-water use are not usually optimal for use of deep water” (see also Fig. 1). In such systems, grasses are favoured to use the summer rain during the growing season but, in their dormant phase in winter, water recharges deeper soil layers and woody plants are advantaged (Sala et al. 1997; Schenk and Jackson 2002; Schwinning et al. 2005). This result was supported by a field study in Utah, where only the grass species but not the shrub species responded to fluctuations in water content of the first few centimetres in the uppermost soil layer (Schwinning et al. 2002, 2005). The ability to convert water uptake from light recent summer rains into carbon was greatest for the grass species, but the opposite occurred when shrubs benefited from water uptake from deeper percolating winter rains. Schwinning et al. (2005) found that, after a very dry summer, only the deepest-rooted tree (Ceratoides lanata) could take up water, which is consistent with the two-layer hypothesis. Ehleringer et al. (1991) proposed that an increase in the proportion of summer rain relative to winter rain would lead to an increase in the frequency of grasses at the expense of woody plants, although summer droughts may reduce grass frequency (see Schwinning et al. 2005). This result demonstrates that tree–grass ratios in areas with biannual precipitation depend on the relative amounts of seasonal rain. Consequently, an isolated consideration of tree–grass ratios with respect to total summer rain only must lead to imprecise interpretation of Walter’s two-layer hypothesis (Ogle and Reynolds 2004).
Schenk and Jackson’s (2002) claim that the two-layer hypothesis is applicable to dry areas with wet winters only is limited to the indirect evidence that there is a greater differentiation between mean root depths of herbaceous plants and shrubs habitats with wet winters than with dry winters. We believe this not to be crucial evidence because it ignores the role of ‘superior competitor’ (sensu Knoop and Walker 1985), i.e. it is not the degree of difference in mean root depths that is crucial but rather that trees or shrubs can take water from below the herbaceous plants. Additional evidence to support this claim comes from the study by Golluscio et al. (1998) in Patagonia who showed that the poor response of deep-rooted shrubs to summer rainfalls could have occurred because rain water either does not reach deep soil layers, the plants are in a dormant phenological status, and/or deep soil layers have a high water potential. Golluscio et al. (1998) claim that the dormant phenological status and high water potentials of deep soil layers may cause high deep-drainage losses, leading to the low response of aboveground net primary production to precipitation during wet years. In conclusion, the two-layer hypothesis model has been consistently shown to apply in dry summer–winter rainfall areas and dry areas with wet winters.
We also highlight the importance of light rainfall events for vertical niche partitioning in dry regions that experience summer rain only (Sala and Lauenroth 1985). No general statement on the validity of the two-layer hypothesis in non-savanna vegetation systems in the subtropics and tropics is possible (2 studies only: February et al. 2011; Shiponeni et al. 2011—both of these winter-rainfall studies supported the two-layer hypothesis).
Moist savannas
There was inconsistent evidence for Walter’s two-layer hypothesis in moist savannas. In six studies (n = 13 studies) performed in moist savannas, there was no support for the two-layer hypothesis, four supported the two-layer hypothesis and three studies showed partial support. Small rainfall events become of less relevance for resource partitioning in humid savannas because precipitation is not (but fire intensity is) a primary limiting factor in such disturbance-driven systems (Higgins et al. 2000, 2010; Sankaran et al. 2005; Bond 2008; Murphy and Bowman 2012; Staver and Levin 2012). For example, Verweij et al. (2011) performed an experiment in South Africa (MAP = 740 mm) where Terminalia sericea trees either had their taproots cut (“trenching”) or their lateral roots were cut about 1 m from the trunk. They found that there was a stronger negative effect on water potential and growth of T. sericea when the lateral roots were cut than when the taproot was cut. From this result, they inferred that Walter’s two-layer hypothesis did not work in their situation. Their result may be due to nutrient availability being more limiting than water in moist savannas (see, e.g., Cramer 2012). Trees and grasses may both counteract the drainage loss of nutrients to deeper soil layers by concentrating their roots in the topsoil layer. A similar result was obtained in the Lamto savanna in West Africa, where the shallow and overlapping tree and grass roots were most abundant in the upper 20 cm, yet they co-occurred down to 60 cm (Le Roux et al. 1995; Mordelet et al. 1997).
Three moist savanna studies showed partial support for the two-layer hypothesis:
At a MAP of 1,000 mm in Ghana, tree and grass roots were concentrated in the upper 30 cm (Lawson et al. 1968). Unfortunately, all fine roots with a diameter <2 mm were classified as grass roots in this study. Hence, the authors concluded that only grass roots would dominate in the upper 20 cm and tree roots at a depth of 20–30 cm. This study poses a major caveat consistent with almost all root investigations because there is a problem regarding the underestimation of fine root biomass (Jackson et al. 1997). It could well have been correct that a considerable proportion of the fine roots in the upper 20 cm belonged to woody plants and not just to grasses; thus root overlap may have occurred (see also Belsky 1994).
Seghieri (1995) found in Cameroon (MAP = 800 mm) that, in some soils, Walter’s two-layer hypothesis worked and in others it did not. In heavily degraded soils, water did not infiltrate deeply and, consequently, grass and tree roots were limited to the upper horizons (see also Wiegand et al. 2005 for an example in an arid savanna). On the other types of soil, root distribution depended on the soil profile and Walter’s two-layer hypothesis was supported. Thus, this study displays the “edaphic savanna” type to which Walter’s (1939) hypothesis did not apply.
In an Australian moist savanna, Zerihun et al. (2006) found woody and grassy fine roots co-occurred throughout the soil profile. Qualitatively, these authors found evidence supporting the two-layer hypothesis because coarser woody roots with a diameter of 2–15 mm dominated at a depth of 15–30 cm. However, quantitatively, these authors questioned the contribution of these coarser and often suberized roots to water uptake and highlighted the importance of fine woody roots for shaping savanna structure. The importance of fine tree roots in the upper soil horizon has been demonstrated for blue oak Quercus douglasii in moist sites in California (Millikin and Bledsoe 1999). Here, the smallest fine roots with a diameter <0.5 mm had both higher nitrogen concentrations and, in the area outside the central root system, greater biomass than large fine (0.5–2.0 mm) or coarse (>2.0 mm) roots. In Millikin and Bledsoe’s (1999) study, the smallest fine roots accounted for 71 % of total root biomass, large fine roots for 25 % and coarse roots for 4 % from 0 to 20 cm depth. Fine roots also have higher turnover rates. As structures transporting nutrients and water, coarse roots often act indirectly, providing connections between shoots and fine roots, while fine roots directly absorb nutrients and water (Eissenstat 1992; Gordon and Jackson 2000). Eissenstat (1992) also noted that coarse roots could perform an important role in the uptake of water.
To answer questions about resource uptake and plant competition, information about fine root length density and spatial distribution is important (Millikin and Bledsoe 1999). Consequently, most empirical studies that have investigated Walter’s two-layer hypothesis must be interpreted with caution because the excavation techniques that were applied, such as separating roots from soil with pressurized water, almost certainly underestimate fine roots (Jackson et al. 1996). In our view, the study of Mordelet et al. (1997) was path-breaking because they assessed 13C abundance for fine roots that allowed the separation of C4-grasses and C3-trees through the difference in their ‘signatures’ (isotopic values). The studies of Le Roux et al. (1995) and Weltzin and McPherson (1997) were also important approaches to investigating the two-layer hypothesis in savannas; they were the first to use stable isotopes of water [18O and 2H (deuterium)] in savannas. Meanwhile, techniques for molecular identification of fine tree and grass roots at species level and relative species abundances have been developed (e.g. Bohrer et al. 2007; Mommer et al. 2008), but have not yet been used to investigate the two-layer hypothesis.
Towards the drier end of the moist savannas, the two-layer hypothesis was found to be applicable in a mesquite (Prosopis) savanna of Texas (Brown and Archer 1990). At a MAP of 680 mm, only grasses but not the woody plants were capable of using light summer rain showers in the topsoil. In contrast, rapid development of taproots of Prosopis seedlings enabled these trees to utilize deeper subsoil water. A similar result was obtained by Weltzin and McPherson (1997) using stable isotopes of oxygen and hydrogen. Evidence against the two-layer hypothesis was provided by Kulmatiski et al. (2010) who found, using D2O tracer techniques, that Sclerocarya birrea trees (MAP = 746 mm) absorbed very little water below 20 cm, even when there were no early-season rains. This species used stored water to produce early leaves, as supported by evidence of decreases in stem diameter. Kulmatiski et al. (2010) also found that there was little to no stomatal conductance by late-season leaves of another savanna tree, T. sericea, at this study site. This result contrasts with earlier claims (Walter 1971; Scholes and Archer 1997) that leaf retention in the dry season indicates that trees use deep soil water.
Dry savannas
All but one study (Hipondoka et al. 2003) supported the two-layer hypothesis in dry savannas. As was the case at the temperate non-savanna sites with summer–winter precipitation, the two-layer hypothesis was also applicable to dry North American savannas with biannual precipitation. At a MAP of 600 mm, woody plants used summer rain mainly at depths of 60–90 cm but they used winter rain at depths of 130–150 cm because the dormant grasses with their main root biomass in the upper 20 cm did not compete with the woody plants for winter rain (Weltzin and McPherson 1997). At a drier savanna site, grass roots were most dense in the upper 15 cm but shrub roots were most dense below and had additional taproots, supporting the two-layer hypothesis (Cable 1969).
At the dry African savanna sites experiencing summer rain only, grass root densities mostly peaked in the upper 10–30 cm of topsoil and then rapidly declined with depth (Knoop and Walker 1985; Belsky 1994). Although grasses grew down to 120 cm, these studies highlight that the two-layer hypothesis is applicable due to different relative root distributions, e.g. grass roots would take up more of the topsoil water. Kambatuku et al. (2012) also found that there was considerable overlap in the roots of A. mellifera trees and grasses but that, in general, A. mellifera had deeper roots than the grasses and used deeper water when competing with grasses, supporting the two-layer hypothesis.
In the single dry savanna study that did not support the predictions of the two-layer hypothesis, Hipondoka et al. (2003) were unable to detect separation of tree and grass roots in deep sands. These authors indicated that water sources were too deep for the tree roots to reach and, consequently, shallow-rooted trees such as Acacia mellifera predominated there. We note that this is not universally true; the world record root depth of 68 m (Acacia erioloba and Boscia albitrunca) occurs in the deep Kalahari sands of southern Africa (Schulze et al. 1998; see also Seymour 2008).
Functional topsoil–subsoil boundaries with plant-physiological relevance may shift upwards with decreasing annual precipitation because shallower roots are more capable of using the limited water (Schenk and Jackson 2002). For example, the proportion of shallow lateral roots of the shrub T. sericea increased at dry savanna sites with decreasing MAP of 500 mm towards sites with only 250 mm MAP (Hipondoka and Versfeld 2006). Grasses may also have shallower rooting depths with increasing aridity. An extreme is Stipagrostis sabulicola of the Namib desert (Namibia) that has an extensive mat of superficial roots which radiate many metres from the parent plant to absorb dew from the top few centimetres of soil (Yeaton 1988). In arid savannas of the Middle East, North America, Australia and southern Africa, rainfall is (apart from a few larger rainfalls of >50 mm) characterized by localized events ranging from 5 to 15 mm, which is sufficient for grasses to grow (Evenari et al. 1982; Sala and Lauenroth 1985; Caughley and Gunn 1993; Lovegrove 1993). These small precipitation events only wet the upper centimetres of the soil and are therefore usually inaccessible to the roots of shrubs (Electronic Supplement). Grasses are much more effective in water uptake from the uppermost centimetres of the topsoil (Scholes and Archer 1997). Hence, with decreasing mean annual precipitation, a shallower topsoil–subsoil boundary may explain vertical resource partitioning between grasses and woody plants.
In dry regions, woody plants may use the few stronger rainfall pulses more effectively (see, e.g., Chesson et al. 2004; Ogle and Reynolds 2004). Deep-rooted trees such as certain Acacia or Prosopis species are most often found in water-limited systems (Jackson et al. 1996; Moustakas et al. 2006; Seymour 2008). One of the most important facilitative functions performed by deep-rooted trees is hydraulic lift, where water is taken up to the surface from deep roots and passes by osmosis into the surface soil at night (see, e.g., Richards and Caldwell 1987; Ludwig et al. 2003). This water may benefit herbaceous plants and shrubs. However, we note that Ludwig et al. (2004) showed that the facilitative effects of hydraulic lift were outweighed by the competitive effects between grass and tree roots. Ludwig et al. (2003) recorded that individual Acacia tortilis trees in Tanzania (MAP = 650 mm) may use hydraulic lift to bring 75–225 l of water each night to an area of more than 300 m2. In a subsequent paper, Ludwig et al. (2004) found that root trenching led to increased soil water content under A. tortilis trees. This meant that trees were taking up more water from the topsoil than they exuded via hydraulic lift. Consequently, these trees were heavier in trenched plots than in controls, probably because of reduced competition for water. These authors also used stable isotope analyses of plant and source water to show that grasses in trenched plots used a smaller proportion of deep water than those grasses that competed with trees, which is consistent with the notion that Walter’s two-layer hypothesis worked at this site. We note that there was a hard layer at 70 cm in the Ludwig et al. (2004) study which limits the application of the two-layer hypothesis in this study (i.e. it is essentially an “edaphic savanna”). An additional benefit of deep roots occurs in deep sands where trees may use ‘inverse hydraulic lift’ to transport the few heavier rainfall events from the upper roots down to the deepest roots in dry subsoil layers (Schulze et al. 1998).
In the mopane [Hardwickia (formerly Colophospermum) mopane] woodlands of the Limpopo Province of South Africa, these H. mopane trees typically grow on poorly drained soils that have a high runoff potential. Smit and Rethman (2000) investigated soil–water partitioning between trees and grasses along a tree-thinning gradient from a non-cleared natural woodland stand with virtual absence of herbaceous plants to a fully cleared grass plot. In those plots where dense woodland dominated, incidental water loss from five rain showers >10 mm each ranged from over 70 % to over 80 %. The fully cleared grass plot, however, had a water loss of just over 10 %. In this plot, the higher water quantities infiltrated down to >45 cm depth although grasses predominantly used soil water from the top 45 cm. However, in the natural dense woodland plot with high water loss, the smaller water quantities infiltrated only to a depth of 45 cm and the shallow mopane roots took up most of this water in the upper 30 cm. This means that, along this experimental tree-thinning gradient, grasses and trees competed with each other in the same shallow soil layers because, under natural woodland conditions, the water cannot infiltrate deeply. Thus, the two-layer hypothesis was not applicable (i.e. it is also essentially an “edaphic savanna”). Interestingly, in typical mopane woodland, this leads to the often-observed absence of grasses because the mopane trees with their shallow roots and higher water-use efficiency outcompeted the grasses (Smit and Rethman 2000). Dye and Walker (1980) have shown that very shallow-rooted trees such as H. mopane do not coexist with grass unless sufficient moisture is available below the soil dominated by grass roots. In our view, this study provides indirect support for the two-layer hypothesis because only where vertical partitioning of soil water is possible, may trees and grasses coexist with each other.
Increasing global CO2 levels
Increasing global CO2 levels are predicted to cause a change from dominance of C4 plants to C3 dominance because of the greater net photosynthetic efficiency of C3 plants (mostly trees) relative to the C4 grasses that are dominant in many savannas (Polley et al. 1997; Bond 2008; Ward 2010; Bond and Midgley 2012). An additional effect, reviewed by Körner (2006) and Leakey and Lau (2012), was that trees are likely to become more water-use efficient owing to increased carbon efficiency, although both sets of authors are at pains to note that nutrients might ultimately limit the benefits of increased growth by C3 plants—initial growth increases that are experienced by many C3 species were followed by returns to conventional growth rates after a few years. Clearly, Walter (1939) derived his hypothesis long before there was concern over rising global CO2 levels and an understanding of the differences between C3 and C4 plant physiology. Bond and Midgley (2012) consider increasing CO2 to be less of a problem in dry savannas, where they consider “overgrazing” to be more of a problem. If heavy grazing is more of a problem, as these authors claim, then Walter’s (1939) two-layer hypothesis would still be valid because grasses are removed, leading to encroachment by trees (Walter 1954, 1973b, Ward 2010). However, Ward (2010) disagrees and considers rising CO2 levels to be a problem regardless of savanna type, although extreme rainfall events would be required for tree recruitment to occur in dry regions (Kraaij and Ward 2006; Ward and Esler 2011).
Conclusions
Our review has shown that the two-layer hypothesis is an adequate model to explain soil–water partitioning and tree–grass codominance in dry savannas, as long as it is carefully appreciated in its original context and with all its subtleties (Walter 1939, 1954, 1971, 1989). Beyond this, it is also applicable to dry temperate systems such as steppes or semi-deserts (Schenk and Jackson 2002). The two-layer hypothesis was never proposed for moist savannas because, in these biotic feedback-dominated systems, tree–grass codominance is primarily mediated by disturbance. The sources of disturbance in moist savannas may be fire, herbivory and physical soil disturbance (Ward 2009), and do not necessarily include niche partitioning (DeAngelis and Waterhouse 1987; Higgins et al. 2000; Sankaran et al. 2005). Nonetheless, we note that the two-layer hypothesis did work in some moist savannas and tallgrass prairies.
We propose a revised form of Walter’s (1939) two-layer hypothesis that is appropriate for dry savannas (<650 mm MAP) and more arid regions regardless of the rainfall season (see also Schimper 1903), we recognize that Walter’s two-layer hypothesis is more likely to occur with biannual rain. We are aware that tree–grass codominance in dry ecosystems is also mediated by temporal niche partitioning such as intra-annual variation in rainfall (Rodriguez-Iturbe et al. 1999), patch dynamics (Wiegand et al. 2006; Moustakas et al. 2006; Meyer et al. 2007), spatial heterogeneities in ecosystem processes (Jeltsch et al. 2000) or by soil moisture differences between canopy and inter-canopy patches (Breshears and Barnes 1999). However, we have purposely focused on vertical resource partitioning because the two-layer model has not yet (until now) been substantially reviewed in its strict and original context. Of course, the ultimate causes for soil–water partitioning by trees and grasses are directly related to differences in transpiration and osmotic regulation (Walter 1971; Van Wijk and Rodriguez-Iturbe 2002; Van Wijk 2009). We highlight the importance of topsoil–subsoil boundaries in the upper soil layers for tree–grass coexistence in dry ecosystems because rainfall pulses occur in subtle but crucial quantities (Sala and Lauenroth 1985; Chesson et al. 2004; Ward 2009). We believe that a broader view of savannas needs to incorporate soil–climate interactions and not focus so strongly on fire–climate restrictions. Several authors (Sankaran et al. 2005; Higgins et al. 2010; Staver and Levin 2012) have noted that it is only in moist savannas and especially in savannas at the savanna-forest ecotone (MAP = 1,000–2,000 mm) that fire is particularly important. We emphasize that the concept of the ‘superior competitor’ (Knoop and Walker 1985; Kambatuku et al. 2012) is useful and important in explaining coexistence using Walter’s (1939) two-layer hypothesis model. Then, Walter’s two-layer hypothesis has been truly revisited—back to the roots!
Electronic supplementary material
Acknowledgments
We thank Eduardo Zea for fruitful discussions during the initial phase of this project, Tobias Rütting for help with screening the literature and the Volkswagen Foundation (to K.W. and D.W.) and the National Research Foundation (to D.W.) for financial support. D.W. thanks the University of KwaZulu-Natal for granting him sabbatical leave, the University of Göttingen International Centre for the guest professorship while on sabbatical leave and Esther Lauer for many kindnesses to him and his family. K.W. was partly funded by the State of Lower Saxony (Ministry of Science and Culture; Cluster of Excellence “Functional Biodiversity Research”). We are grateful to the two anonymous reviewers and the editor whose comments considerably improved this manuscript.
References
- Asbjornsen H, Shepherd G, Helmers M, Mora G. Seasonal patterns in depth of water uptake under contrasting annual and perennial systems in the Corn Belt region of the midwestern U.S. Plant Soil. 2008;308:69–92. doi: 10.1007/s11104-008-9607-3. [DOI] [Google Scholar]
- Baldocchi DD, Xu L, Kiang N. How plant functional-type, weather, seasonal drought, and soil physical properties alter water and energy fluxes of an oak–grass savanna and an annual grassland. Agric For Meteorol. 2004;123:13–39. doi: 10.1016/j.agrformet.2003.11.006. [DOI] [Google Scholar]
- Bell RHV. The effect of soil nutrient availability on community structure in African ecosystems. In: Huntley BJ, Walker BH, editors. Ecology of tropical savannas. Berlin: Springer; 1982. pp. 193–216. [Google Scholar]
- Belsky AJ. Tree/grass ratios in East African savannas: a comparison of existing models. J Biogeogr. 1990;17:483–489. doi: 10.2307/2845380. [DOI] [Google Scholar]
- Belsky AJ. Influences of trees on savanna productivity: tests of shade, nutrients, and tree–grass competition. Ecology. 1994;75:922–932. doi: 10.2307/1939416. [DOI] [Google Scholar]
- Bohrer G, Beck G, Ward D, Roth-Bejerano N, Kagan-Zur V. Arbuscular mycorrhizae and plant environment interactions in a wild host, Vangueria infausta, from the Kalahari Desert, South Africa. In: Montaño NM, Camargo-Ricalde SL, Garcia-Sánchez R, Monroy-Ata A, editors. Arbuscular mycorrhizae in arid and semi-arid ecosystems. Mexico City: Mundi-Prensa; 2007. pp. 268–304. [Google Scholar]
- Bond WJ. What limits trees in C4 grasslands and savannas? Annu Rev Ecol Evol Syst. 2008;39:641–659. doi: 10.1146/annurev.ecolsys.39.110707.173411. [DOI] [Google Scholar]
- Bond WJ, Midgley GF. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philos Trans R Soc Lond B. 2012;367:601–612. doi: 10.1098/rstb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breshears DD, Barnes FJ. Interrelationships between plant functional types and soil moisture heterogeneity for semiarid landscapes within the grassland/forest continuum: a unified conceptual model. Landsc Ecol. 1999;14:465–478. doi: 10.1023/A:1008040327508. [DOI] [Google Scholar]
- Brown JR, Archer SR. Woody plant invasion of grasslands: establishment of honey mesquite (Prosopis glandulosa var. glandulosa) on sites differing in herbaceous biomass and grazing history. Oecologia. 1989;80:19–26. doi: 10.1007/BF00789926. [DOI] [PubMed] [Google Scholar]
- Brown JR, Archer SR. Water relations of a perennial grass and seedling vs. adult woody plants in a subtropical savanna. Oikos. 1990;57:366–374. doi: 10.2307/3565966. [DOI] [Google Scholar]
- Brown JR, Archer SR (1999) Shrub invasion of grassland: recruitment is continuous and not regulated by herbaceous biomass or density. Ecology 80:2385–2396
- Cable DR. Competition in the semi-desert grass–shrub type as influenced by root systems, growth habitats, and soil moisture extraction. Ecology. 1969;50:27–38. doi: 10.2307/1934659. [DOI] [Google Scholar]
- Caughley G, Gunn A. Dynamics of large herbivores in deserts: kangaroos and caribou. Oikos. 1993;67:47–55. doi: 10.2307/3545094. [DOI] [Google Scholar]
- Chesson P, Gebauer RLE, Schwinning S, Huntley N, Wiegand K, Ernest MSK, Sher A, Novoplansky A, Weltzin JF. Resource pulses, species interactions and diversity maintenance in arid and semi-arid environments. Oecologia. 2004;141:236–253. doi: 10.1007/s00442-004-1551-1. [DOI] [PubMed] [Google Scholar]
- Cramer MD. Unravelling the limits to tree height: a major role for water and nutrient trade-offs. Oecologia. 2012;169:61–72. doi: 10.1007/s00442-011-2177-8. [DOI] [PubMed] [Google Scholar]
- DeAngelis DL, Waterhouse JC. Equilibrium and nonequilibrium concepts in ecological models. Ecol Monogr. 1987;57:1–21. doi: 10.2307/1942636. [DOI] [Google Scholar]
- Dodd MB, Lauenroth WK, Welker JM. Differential water resource use by herbaceous and woody plant life-forms in a shortgrass steppe community. Oecologia. 1998;117:504–512. doi: 10.1007/s004420050686. [DOI] [PubMed] [Google Scholar]
- Dye PJ, Walker BH. Vegetation–environment relations on sodic soils of Zimbabwe-Rhodesia. J Ecol. 1980;68:599–606. doi: 10.2307/2259424. [DOI] [Google Scholar]
- East R. Rainfall, soil nutrient status and biomass of large African savanna mammals. Afr J Ecol. 1984;22:245–270. doi: 10.1111/j.1365-2028.1984.tb00700.x. [DOI] [Google Scholar]
- Eggemeyer KD, Awada T, Harvey FE, Wedin DA, Zhou X, Zanner CW. Seasonal changes in depth of water uptake for encroaching trees Juniperus virginiana and Pinus ponderosa and two dominant C4 grasses in a semiarid grassland. Tree Physiol. 2008;29:157–169. doi: 10.1093/treephys/tpn019. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu Rev Ecol Syst. 1993;24:411–439. doi: 10.1146/annurev.es.24.110193.002211. [DOI] [Google Scholar]
- Ehleringer JR, Phillips SL, Schuster WSF, Sandquist DR. Differential utilization of summer rains by desert plants. Oecologia. 1991;88:430–434. doi: 10.1007/BF00317589. [DOI] [PubMed] [Google Scholar]
- Eissenstat D. Costs and benefits of constructing roots of small diameter. J Plant Nutr. 1992;15:763–782. doi: 10.1080/01904169209364361. [DOI] [Google Scholar]
- Evenari M, Shanan L, Tadmor N. The Negev: the challenge of a desert. Cambridge: Harvard University Press; 1982. [Google Scholar]
- Fargione J, Tilman D. Niche differences in phenology and rooting depth promote coexistence with a dominant C4 bunchgrass. Oecologia. 2005;143:598–606. doi: 10.1007/s00442-005-0010-y. [DOI] [PubMed] [Google Scholar]
- February EC, Allsopp N, Shabane T, Hattas D. Coexistence of a C4 grass and a leaf succulent shrub in an arid ecosystem. The relationship between rooting depth, water and nitrogen. Plant Soil. 2011;349:253–260. doi: 10.1007/s11104-011-0867-y. [DOI] [Google Scholar]
- Getzin S (2007) Structural fire effects in the world’s savannas. A synthesis for biodiversity and land-use managers. VDM, Saarbrücken. ISBN: 978-3-8364-3664-9
- Golluscio RA, Sala OA, Lauenroth WK. Differential use of large summer rainfall events by shrubs and grasses: a manipulative experiment in the Patagonian steppe. Oecologia. 1998;115:17–25. doi: 10.1007/s004420050486. [DOI] [PubMed] [Google Scholar]
- Gordon WS, Jackson RB (2000) Nutrient concentrations in fine roots. Ecology 81:275–280
- Grellier S, Barot S, Janeau J-L, Ward D. Grass competition is more important than seed ingestion by livestock for Acacia recruitment in South Africa. Plant Ecol. 2012 [Google Scholar]
- Higgins SI, Bond WJ, Trollope WSW. Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J Ecol. 2000;88:213–229. doi: 10.1046/j.1365-2745.2000.00435.x. [DOI] [Google Scholar]
- Higgins SI, Scheiter S, Sankaran M. The stability of African savannas: insights from the indirect estimation of the parameters of a dynamic model. Ecology. 2010;91:1682–1692. doi: 10.1890/08-1368.1. [DOI] [PubMed] [Google Scholar]
- Hipondoka MHT, Versfeld WD. Root system of Terminalia sericea shrubs across rainfall gradient in a semi-arid environment of Etosha National Park, Namibia. Ecol Indic. 2006;6:516–524. doi: 10.1016/j.ecolind.2005.07.004. [DOI] [Google Scholar]
- Hipondoka MHT, Aranibar JN, Chirara C, Lihavha M, Macko SA. Vertical distribution of grass and tree roots in arid ecosystems of southern Africa: niche differentiation or competition? J Arid Environ. 2003;54:319–325. doi: 10.1006/jare.2002.1093. [DOI] [Google Scholar]
- Holdo R, Timberlake J. Rooting depth and above-ground community composition in Kalahari sand woodlands in western Zimbabwe. J Trop Ecol. 2008;24:169–176. doi: 10.1017/S0266467408004835. [DOI] [Google Scholar]
- Huntley BJ. Southern African savannas. In: Huntley BJ, Walker BH, editors. Ecology of tropical savannas. Berlin: Springer; 1982. pp. 101–119. [Google Scholar]
- Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze E-D. A global analysis of root distributions for terrestrial biomes. Oecologia. 1996;108:389–411. doi: 10.1007/BF00333714. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Mooney HA, Schulze E-D. A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch F, Milton SJ, Dean WRJ, Van Rooyen N. Tree spacing and coexistence in semiarid savannas. J Ecol. 1996;84:583–595. doi: 10.2307/2261480. [DOI] [Google Scholar]
- Jeltsch F, Weber GE, Grimm V. Ecological buffering mechanisms in savannas: a unifying theory of long-term tree–grass coexistence. Plant Ecol. 2000;161:161–171. doi: 10.1023/A:1026590806682. [DOI] [Google Scholar]
- Johnson RW, Tothill JC. Definition and broad geographic outline of savanna lands. In: Tothill JC, Mott JJ, editors. Ecology and management of the world’s savannas. Canberra: Australian Academy of Science; 1985. pp. 1–13. [Google Scholar]
- Kambatuku JR, Cramer MD, Ward D. Overlap in soil water sources of savanna woody seedlings and grasses. Ecohydrol. 2012 [Google Scholar]
- Knoop WT, Walker BH. Interactions of woody and herbaceous vegetation in a southern African savanna. J Ecol. 1985;73:235–253. doi: 10.2307/2259780. [DOI] [Google Scholar]
- Körner C. Plant CO2 responses: an issue of definition, time and resource supply. New Phytol. 2006;172:393–411. doi: 10.1111/j.1469-8137.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- Kraaij T, Ward D. Effects of rain, nitrogen, fire and grazing on tree recruitment and early survival in bush-encroached savanna, South Africa. Plant Ecol. 2006;186:235–246. doi: 10.1007/s11258-006-9125-4. [DOI] [Google Scholar]
- Kulmatiski A, Beard KH, Verweij RJT, February EC. A depth-controlled technique measures vertical, horizontal and temporal patterns of water use by trees and grasses in a subtropical savanna. New Phytol. 2010;88:199–209. doi: 10.1111/j.1469-8137.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- Lawson GW, Jenik J, Armstrong-Mensah KO. A study of a vegetation catena in guinea savanna at Mole Game Reserve (Ghana) J Ecol. 1968;56:505–522. doi: 10.2307/2258248. [DOI] [Google Scholar]
- Le Roux X, Bariac T, Mariotti A. Spatial partitioning of the soil-water resource between grass and shrub components in a West-African humid savanna. Oecologia. 1995;104:147–155. doi: 10.1007/BF00328579. [DOI] [PubMed] [Google Scholar]
- Leakey A, Lau JA. Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2] Philos Trans R Soc Lond B. 2012;367:613–629. doi: 10.1098/rstb.2011.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Lauenroth WK. Spatial distributions of grass and shrub root systems in the shortgrass steppe. Am Midl Nat. 1994;132:117–123. doi: 10.2307/2426206. [DOI] [Google Scholar]
- Lehmann CER, Ratnam J, Hutley L. Which of these continents is not like the other? Comparisons of tropical savanna systems: key questions and challenges. New Phytol. 2009;181:508–511. doi: 10.1111/j.1469-8137.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- Lovegrove B. The living deserts of southern Africa. Vlaeberg: Fernwood; 1993. [Google Scholar]
- Ludwig F, Dawson TE, De Kroon H, Berendse F, Prins HHT. Hydraulic lift in Acacia tortilis trees on an East African savanna. Oecologia. 2003;134:293–300. doi: 10.1007/s00442-002-1119-x. [DOI] [PubMed] [Google Scholar]
- Ludwig F, Dawson TE, Prins HHT, Berendse F, De Kroon H. Belowground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol Lett. 2004;7:623–631. doi: 10.1111/j.1461-0248.2004.00615.x. [DOI] [Google Scholar]
- Meyer KM, Ward D, Moustakas A, Wiegand K. Big is not better: small Acacia mellifera shrubs are more vital after fire. Afr J Ecol. 2005;43:131–136. doi: 10.1111/j.1365-2028.2005.00559.x. [DOI] [Google Scholar]
- Meyer KM, Wiegand K, Ward D, Moustakas A. The rhythm of savanna patch dynamics. J Ecol. 2007;95:1306–1315. doi: 10.1111/j.1365-2745.2007.01289.x. [DOI] [Google Scholar]
- Meyer KM, Ward D, Wiegand K, Moustakas A. Multi-proxy evidence for competition between savanna woody species. Perspect Plant Ecol Evol Syst. 2008;10:63–72. doi: 10.1016/j.ppees.2007.09.002. [DOI] [Google Scholar]
- Millikin CS, Bledsoe CS. Biomass and distribution of fine and coarse roots from blue oak (Quercus douglasii) trees in the northern Sierra Nevada foothills of California. Plant Soil. 1999;214:27–38. doi: 10.1023/A:1004653932675. [DOI] [Google Scholar]
- Mommer L, Wagemaker CAM, De Kroon H, Ouborg NJ. Unravelling below-ground plant distributions: a real-time polymerase chain reaction method for quantifying species proportions in mixed root samples. Mol Ecol Res. 2008;8:947–953. doi: 10.1111/j.1755-0998.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- Montaña C, Cavagnaro B, Briones O. Soil water use by co-existing shrubs and grasses in the southern Chihuahuan Desert, Mexico. J Arid Environ. 1995;31:1–13. doi: 10.1006/jare.1995.0043. [DOI] [Google Scholar]
- Mordelet P, Menaut JC, Mariotti A. Tree and grass rooting patterns in an African humid savanna. J Veg Sci. 1997;8:65–70. doi: 10.2307/3237243. [DOI] [Google Scholar]
- Moustakas A, Guenther M, Wiegand K, Mueller K-H, Ward D, Meyer KM, Jeltsch F. Long-term mortality patterns of the deep-rooted Acacia erioloba: the middle class shall die! J Veg Sci. 2006;17:473–480. [Google Scholar]
- Murphy BP, Bowman DMJS. What controls the distribution of tropical forest and savanna? Ecol Lett. 2012;15:748–758. doi: 10.1111/j.1461-0248.2012.01771.x. [DOI] [PubMed] [Google Scholar]
- Nano CEM, Clarke PJ. Woody–grass ratios in a grassy arid system are limited by multi-causal interactions of abiotic constraint, competition and fire. Oecologia. 2010;162:719–732. doi: 10.1007/s00442-009-1477-8. [DOI] [PubMed] [Google Scholar]
- Nippert JB, Knapp AK. Soil water partitioning contributes to species coexistence in tallgrass prairie. Oikos. 2007;116:1017–1029. doi: 10.1111/j.0030-1299.2007.15630.x. [DOI] [Google Scholar]
- Ogle K, Reynolds JF. Plant responses to precipitation in desert ecosystems: integrating functional types, pulses, thresholds, and delays. Oecologia. 2004;141:282–294. doi: 10.1007/s00442-004-1507-5. [DOI] [PubMed] [Google Scholar]
- Peláez DV, Distel RA, Bóo RM, Elia OR, Mayor MD. Water relations between shrubs and grasses in semi-arid Argentina. J Arid Environ. 1994;27:71–78. doi: 10.1006/jare.1994.1046. [DOI] [Google Scholar]
- Perkins JS, Thomas DSG. Spreading deserts or spatially confined environmental impacts? Land degradation and cattle ranching in the Kalahari desert of Botswana. Land Degrad Rehabil. 1993;4:179–194. doi: 10.1002/ldr.3400040307. [DOI] [Google Scholar]
- Polley HW, Mayeux HS, Johnson HB, Tischler CR. Atmospheric CO2, soil water, and shrub/grass ratios on rangelands. J Range Manag. 1997;50:278–284. doi: 10.2307/4003730. [DOI] [Google Scholar]
- Richards JH, Caldwell MM. Hydraulic lift: substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia. 1987;73:486–489. doi: 10.1007/BF00379405. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe I, D’Odorico P, Porporato A, Ridolfi L. On the spatial and temporal links between vegetation, climate, and soil moisture. Water Resour Res. 1999;35:3709–3722. doi: 10.1029/1999WR900255. [DOI] [Google Scholar]
- Sala OE, Lauenroth WK. Root profiles and the ecological effect of light rainshowers in arid and semiarid regions. Am Midl Nat. 1985;114:406–408. doi: 10.2307/2425617. [DOI] [Google Scholar]
- Sala OE, Golluscio RA, Lauenroth WK, Soriano A. Resource partitioning between shrubs and grasses in the Patagonian steppe. Oecologia. 1989;81:501–505. doi: 10.1007/BF00378959. [DOI] [PubMed] [Google Scholar]
- Sala OE, Lauenroth WK, Golluscio RA. Plant functional types in temperate semi-arid regions. In: Smith TM, Shugart HH, Woodward FI, editors. Plant functional types: their relevance to ecosystem properties and global change. New York: Cambridge University Press; 1997. pp. 217–233. [Google Scholar]
- Sankaran M, Ratnam J, Hanan NP. Tree–grass coexistence in savannas revisited—insights from an examination of assumptions and mechanisms invoked in existing models. Ecol Lett. 2004;7:480–490. doi: 10.1111/j.1461-0248.2004.00596.x. [DOI] [Google Scholar]
- Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS, Gignoux J, Higgins SI, Le Roux X, Ludwig F, Ardo J, Banyikwa F, Bronn A, Bucini G, Caylor KK, Coughenour MB, Diouf A, Ekaya W, Feral CJ, February EC, Frost PGH, Hiernaux P, Hrabar H, Metzger KL, Prins HHT, Ringrose S, Sea W, Tews J, Worden J, Zambatis N. Determinants of woody cover in African savannas. Nature. 2005;438:846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
- Sarmiento G. The ecology of Neotropical savannas. Cambridge: Harvard University Press; 1984. [Google Scholar]
- Scheiter S, Higgins SI. Partitioning of root and shoot competition and the stability of savannas. Am Nat. 2007;170:587–601. doi: 10.1086/521317. [DOI] [PubMed] [Google Scholar]
- Schenk HJ, Jackson RB. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol. 2002;90:480–494. doi: 10.1046/j.1365-2745.2002.00682.x. [DOI] [Google Scholar]
- Schimper AFW. Pflanzengeographie auf physiologischer Grundlage. Jena: Gustav Fischer; 1898. [Google Scholar]
- Schimper AFW. Plant geography on a physiological basis. Oxford: Clarendon; 1903. [Google Scholar]
- Scholes RJ. Savanna. In: Cowling RM, Richardson DM, Pierce SM, editors. Vegetation of southern Africa. Cambridge: Cambridge University Press; 1997. pp. 258–277. [Google Scholar]
- Scholes RJ, Archer SR. Tree–grass interactions in savannas. Annu Rev Ecol Syst. 1997;28:517–544. doi: 10.1146/annurev.ecolsys.28.1.517. [DOI] [Google Scholar]
- Schultz AM, Launchbaugh JL, Biswell HH. Relationship between grass density and brush seedling survival. Ecology. 1955;36:226–238. doi: 10.2307/1933228. [DOI] [Google Scholar]
- Schulze E-D, Caldwell MM, Canadell J, Mooney HA, Jackson RB, Parson D, Scholes R, Sala OE, Trimborn P. Downward flux of water through roots (i.e. inverse hydraulic lift) in dry Kalahari sands. Oecologia. 1998;115:460–462. doi: 10.1007/s004420050541. [DOI] [PubMed] [Google Scholar]
- Schwinning S, Davis K, Richardson L, Ehleringer JR. Deuterium enriched irrigation indicates different forms of rain use in shrub/grass species of the Colorado Plateau. Oecologia. 2002;130:345–355. doi: 10.1007/s00442-001-0817-0. [DOI] [PubMed] [Google Scholar]
- Schwinning S, Starr BI, Ehleringer JR. Summer and winter drought in a cold desert ecosystem (Colorado Plateau) part I: effects on soil water and plant water uptake. J Arid Environ. 2005;60:547–566. doi: 10.1016/j.jaridenv.2004.07.003. [DOI] [Google Scholar]
- Seghieri J. The rooting patterns of woody and herbaceous plants in a savanna; are they complementary or in competition? Afr J Ecol. 1995;33:358–365. doi: 10.1111/j.1365-2028.1995.tb01045.x. [DOI] [Google Scholar]
- Seymour CL. Grass, rainfall and herbivores as determinants of Acacia erioloba (Meyer) in an African savanna. Plant Ecol. 2008;197:131–138. doi: 10.1007/s11258-007-9366-x. [DOI] [Google Scholar]
- Sher AA, Wiegand K, Ward D. Do Acacia and Tamarix trees compete for water in the Negev desert? J Arid Environ. 2010;74:338–343. doi: 10.1016/j.jaridenv.2009.09.007. [DOI] [Google Scholar]
- Shiponeni N, Allsopp N, Carrick PJ, Hoffman MT. Competitive interactions between grass and succulent shrubs at the ecotone between an arid grassland and succulent shrubland in the Karoo. Plant Ecol. 2011;212:795–808. doi: 10.1007/s11258-010-9864-0. [DOI] [Google Scholar]
- Skarpe C. Shrub layer dynamics under different herbivore densities in an arid savanna, Botswana. J Appl Ecol. 1990;27:873–885. doi: 10.2307/2404383. [DOI] [Google Scholar]
- Skarpe C. Structure of the woody vegetation in disturbed and undisturbed arid savanna. Vegetatio. 1990;87:11–18. doi: 10.1007/BF00045650. [DOI] [Google Scholar]
- Smit GN, Rethman NFG. The influence of tree thinning on the soil water in a semi-arid savanna of southern Africa. J Arid Environ. 2000;44:41–59. doi: 10.1006/jare.1999.0576. [DOI] [Google Scholar]
- Smith TM, Walker BH. The role of competition in the spacing of savanna trees. Proc Grassl Soc S Afr. 1983;18:159–164. [Google Scholar]
- Soriano A, Sala OE. Ecological strategies in a Patagonian arid steppe. Vegetatio. 1983;56:9–15. doi: 10.1007/BF00036131. [DOI] [Google Scholar]
- Staver AC, Levin SA. Integrating theoretical climate and fire effects on savanna and forest systems. Am Nat. 2012;180:211–224. doi: 10.1086/666648. [DOI] [PubMed] [Google Scholar]
- Stuart-Hill G, Tainton NM. Water utilization patterns around isolated Acacia karroo trees in the false thornveld of the eastern Cape. J Grassl Soc S Afr. 1989;6:195–204. doi: 10.1080/02566702.1989.9648188. [DOI] [Google Scholar]
- Van Langevelde F, van de Vijver CADM, Kumar L, van de Koppel J, de Ridder N, van Andel J, Skidmore AK, Hearne JW, Stroosnijder L, Bond WJ, Prins HHT, Rietkerk M (2003) Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84:337–350
- Van Vegten JA. Thornbush invasion in eastern Botswana. Vegetatio. 1983;56:3–7. doi: 10.1007/BF00036129. [DOI] [Google Scholar]
- Van Wijk M. Understanding plant rooting patterns in semi-arid systems: an integrated model analysis of climate, soil type and plant biomass. Glob Ecol Biogeogr. 2009;20:331–342. doi: 10.1111/j.1466-8238.2010.00601.x. [DOI] [Google Scholar]
- Van Wijk M, Rodriguez-Iturbe I. Tree–grass competition in space and time: insights from a simple cellular automata model based on ecohydrological dynamics. Water Resour Res. 2002;38:18-1–18-15. doi: 10.1029/2001WR000768. [DOI] [Google Scholar]
- Verweij RJT, Higgins SI, Bond WJ, February EC. Water sourcing by trees in a mesic savanna: responses to severing deep and shallow roots. Environ Exp Bot. 2011;74:229–236. doi: 10.1016/j.envexpbot.2011.06.004. [DOI] [Google Scholar]
- Walker BH. Structure and function of savannas: an overview. In: Tothill JC, Mott JJ, editors. Ecology and management of the world’s savannas. Canberra: Australian Academy of Science; 1985. pp. 83–91. [Google Scholar]
- Walker BH, Noy-Meir I. Aspects of the stability and resilience of savanna ecosystems. In: Huntley BJ, Walker BH, editors. Ecology of tropical savannas. Berlin: Springer; 1982. pp. 556–590. [Google Scholar]
- Walker BH, Ludwig D, Holling CS, Peterman RM. Stability of semi-arid savanna grazing systems. J Ecol. 1981;69:473–498. doi: 10.2307/2259679. [DOI] [Google Scholar]
- Walter H. Grasland, Savanne und Busch der arideren Teile Afrikas in ihrer ökologischen Bedingtheit. Jahrb Wiss Bot. 1939;87:750–860. [Google Scholar]
- Walter H. Die Verbuschung, eine Erscheinung der subtropischen Savannengebiete und ihre ökologischen Ursachen. Vegetatio. 1954;5–6:6–10. doi: 10.1007/BF00299544. [DOI] [Google Scholar]
- Walter H. Vegetationenszonen und Klima. Stuttgart: Eugen Ulmer; 1970. [Google Scholar]
- Walter H. Ecology of tropical and subtropical vegetation. Edinburgh: Oliver & Boyd; 1971. [Google Scholar]
- Walter H. Die Vegetation der Erde in ökophysiologischer Betrachtung. Jena: Gustav Fischer; 1973. [Google Scholar]
- Walter H. Vegetation of the earth in relation to climate and the eco-physiological conditions. New York: Springer; 1973. [Google Scholar]
- Walter H. Bekenntnisse eines Ökologen. Stuttgart: Gustav Fischer; 1989. [Google Scholar]
- Ward D. Do we understand the causes of bush encroachment in African savannas? Afr J Range Forage Sci. 2005;22:101–105. doi: 10.2989/10220110509485867. [DOI] [Google Scholar]
- Ward D. The biology of deserts. Oxford: Oxford University Press; 2009. [Google Scholar]
- Ward D. A resource ratio model of the effects of changes in CO2 on woody plant invasion. Plant Ecol. 2010;209:147–152. doi: 10.1007/s11258-010-9731-z. [DOI] [Google Scholar]
- Ward D, Esler KJ. What are the effects of substrate and grass removal on recruitment of Acacia mellifera seedlings in a semi-arid environment? Plant Ecol. 2011;212:245–250. doi: 10.1007/s11258-010-9818-6. [DOI] [Google Scholar]
- Ward D, Ngairorue BT. Are Namibia’s grasslands desertifying? J Range Manag. 2000;53:138–144. doi: 10.2307/4003273. [DOI] [Google Scholar]
- Ward D, Saltz D, Ngairorue BT (2004) Spatio-temporal rainfall variation and stock management in arid Namibia. J Range Manag 57:130–140
- Weltzin JF, McPherson GR. Spatial and temporal soil moisture partitioning by trees and grasses in a temperate savanna, Arizona, USA. Oecologia. 1997;112:156–164. doi: 10.1007/s004420050295. [DOI] [PubMed] [Google Scholar]
- Wiegand K, Ward D, Saltz D. Multi-scale patterns and bush encroachment in an arid savanna with a shallow soil layer. J Veg Sci. 2005;16:311–320. doi: 10.1111/j.1654-1103.2005.tb02369.x. [DOI] [Google Scholar]
- Wiegand K, Saltz D, Ward D. A patch-dynamics approach to savanna dynamics and woody plant encroachment—insights from an arid savanna. Perspect Plant Ecol Evol Syst. 2006;7:229–242. doi: 10.1016/j.ppees.2005.10.001. [DOI] [Google Scholar]
- Wu XB, Archer SR. Scale-dependent influence of topography-based hydrologic features on patterns of woody plant encroachment in savanna landscapes. Landsc Ecol. 2005;20:733–742. doi: 10.1007/s10980-005-0996-x. [DOI] [Google Scholar]
- Yeaton RI. Structure and function of the Namib dune grasslands: characteristics of the environmental gradients and species distributions. J Ecol. 1988;76:744–758. doi: 10.2307/2260571. [DOI] [Google Scholar]
- Zerihun A, Montagu KD, Hoffmann MD, Bray SG. Patterns of below- and aboveground biomass in Eucalyptus populnea woodland communities of northeast Australia along a rainfall gradient. Ecosystems. 2006;9:501–515. doi: 10.1007/s10021-005-0155-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.