Abstract
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) of the alimentary canal are malignant tumors with <1% of cases diagnosed in esophagus. These cases require special consideration given their close proximity to vital structures and propensity to be highly aggressive. Management of patients with GISTs has been transformed since the introduction of tyrosine kinase inhibitors. In this report, we present an unusual case of GIST with spontaneous esophageal perforation.
PRESENTATION OF CASE
A 39-year-old Caucasian male presented to our hospital with complaints of severe chest and abdominal pain. A diagnostic chest radiograph revealed a moderate right-sided pleural effusion. Subsequently, an esophagram demonstrated a perforation proximal to an elongated stricture in the distal esophagus. A left thoracotomy was performed whereby a large mediastinal mass firmly attached to the esophagus and gastroesophageal junction was encountered. The neoplasm involved proximal one-third of the stomach and perforated into the right hemithorax. Histopathological evaluation of the tumor led to a diagnosis of GIST.
DISCUSSION
GISTs of the gastroesophageal junction are uncommon and may rarely present with esophageal perforation. The standard of care for treating GIST at present includes tyrosine kinase inhibitors. This pharmacologic agent, along with improved surgical techniques and understanding of molecular markers for accurate diagnosis, will assuredly continue to improve overall survival of patients with GISTs.
CONCLUSION
When stricture or achalasia is detected on imaging, GIST should be considered in the differential diagnosis for individual patients. Additionally, chest and abdomen CT scans of may be performed to confirm presence of a tumor mass, thereby ruling out achalasia.
Keywords: GIST, Gastroesophageal junction, KIT, Esophageal perforation, Imatinib
1. Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the alimentary canal that account for 0.1–3% of all gastrointestinal malignancies. Lesions can occur anywhere along the gastrointestinal tract, with the majority arising in the stomach (60%) and small intestine (30%). Primary GIST of the esophagus, however, is rare and constitutes less than 1% of all tumors of its kind.1 GIST of the gastroesophageal junction presenting with esophageal perforation has seldom been described in the literature. We report here an unusual presentation of GIST with spontaneous esophageal perforation, followed by a discussion of the unique aspects of prognosis and surgical management of esophageal GIST in comparison to lesions in other primary anatomic sites.
2. Case report
A 39-year-old male presented to our hospital with severe chest and abdominal pain after a few months of progressive dyspnea and odynophagia. Recently, he had complaints of emesis after meals and eventually became intolerant of solids and liquids. Additionally, the patient admitted to an approximately 100 lb weight loss over the past few years. Upon arrival, he was hypotensive and septic, with hemoglobin of 11.9 g/dL and white blood cell count of 18.6 thou/cu mm. After resuscitation and stabilization, a diagnostic chest radiograph revealed a moderate right-sided pleural effusion that was drained via tube thoracostomy with an output of 1200 mL serosanguineous fluid. A subsequent esophagram demonstrated a perforation proximal to an elongated stricture in the distal esophagus.
Due to the chronic nature of his symptoms, we made the presumptive diagnosis of achalasia with perforation from a possible food bolus proximally and a surgical intervention was deemed necessary. Our operative plan was to perform a left thoracotomy and if necessary, dissect the diaphragm to mobilize the stomach. A surgical approach from the right chest was decided to be a less ideal by the surgical team due to the difficulties of mobilizing the intra-abdominal esophagus and upper stomach.
A left thoracotomy was performed on this patient, during which a large mediastinal mass firmly attached to the esophagus and gastroesophageal junction was encountered. The visible portion of the mass measured approximately 6 cm and extended down through the diaphragmatic hiatus. During the initial exploration, the esophagus was mobilized with the affixed neoplasm. At this point, the surgical team observed the perforation of the tumor into the patient's right hemithorax. Subsequently, the diaphragm was dissected laterally to expose the intra-abdominal esophagus and stomach. The tumor involved the proximal one-third of the stomach. Since it was impossible to gain distal control of the mass, a midline abdominal exploration was performed followed by a proximal gastrectomy and distal esophagectomy with reconstruction via gastric pull-up and esphago-gastrostomy in the left chest. The diaphragm was then closed around the gastric pull-up. The abdomen and thoracotomy were closed, and chest tubes were placed.
Pathologic analysis of the fibrous neoplasm removed from the patient measured 19 cm × 15 cm × 14 cm and weighed 1291 g (Fig. 1). Histopathological evaluation of the tumor was consistent with a mixed spindle and epitheloid cell GIST. There was necrosis present in approximately 10% of the tumor with a 6 cm break in the pseudocapsule. The mitotic index was 11 per 50 high-power-field. The malignant cells exhibited dense cellularity and marked atypia with a pushing tumor border (Fig. 2). Immunohistochemical studies revealed tumor neoplastic cells that stained positive for KIT (CD117) and CD34, while smooth muscle actin and S-100 protein were negative (Fig. 3).
Fig. 1.
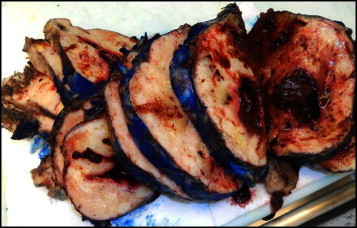
Malignant gastrointestinal tumor (GIST) of the esophagus, clinically ruptured, gross appearance. This gross photograph of the 19 cm tumor demonstrates the typical fleshy appearance of a GIST. There is an area of cystic degeneration and necrosis, supporting malignancy, which contributed to the clinical rupture of this tumor. The external surface has been marked with blue ink to facilitate evaluation of the tumor.
Fig. 2.
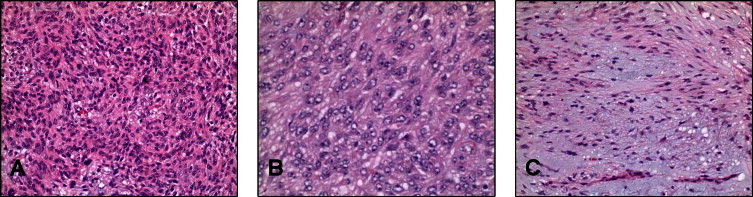
Malignant gastrointestinal tumor (GIST) of the esophagus, microscopic appearance. The tumor demonstrated (A) overall dense cellularity, a mixture of (B) epithelioid and (C) spindled areas growth, and brisk (11/50 high power fields) mitotic activity with about 10% necrosis (not illustrated), all of which contribute to this tumor being designated as malignant.
Fig. 3.
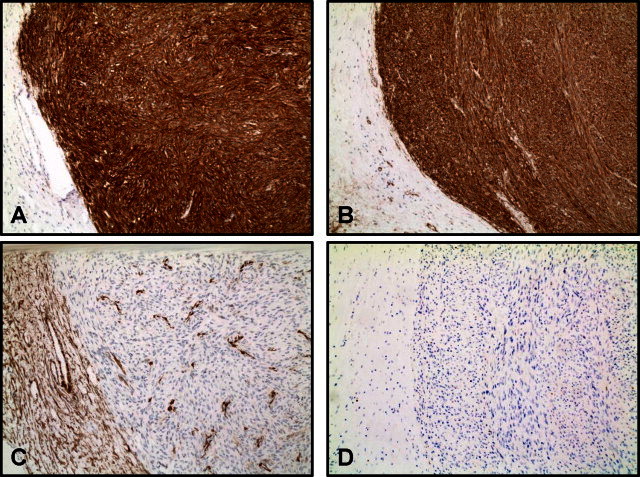
Malignant gastrointestinal tumor (GIST) of the esophagus, immunohistochemical staining. The tumor showed dense positive reactivity with antibodies directed against (A) CD117 (cKit) and (B) CD34, with lack of reactivity with antibodies against (C) smooth muscle actin (SMA) and (D)S100. These results support this being a GIST rather than leiomyosarcoma or malignant nerve sheath tumor.
Postoperatively, the patient initially had an uneventful recovery with no evidence of leak from his anastomosis. He was then started on a diet. Subsequently, he developed an empyema on post-operative day 7 in the left chest requiring an operative decortication from which he recovered completely. The patient did have poor overall fitness for someone of his age. His hospital course was prolonged with on overall length of stay of 15 days due to sepsis and malnutrition.
When the patient had satisfactorily recovered from his hospitalization to tolerate medical treatment, his case was presented at our multidisciplinary tumor conference. The recommendation was made that he receives imatinib for an extended period of time, which would be determined by the status of his overall health and response to the medication.
3. Discussion
Since 1992, there has been a considerable increase in the reported incidence of gastrointestinal stromal tumors. This surge in the number of diagnoses per year may be attributed to greater awareness and improved histopatholologic detection of this neoplasm.2 It is now believed that GISTs arise from pluripotential mesenchymal stem cells programmed to differentiate into interstitial cells of Cajal in the myenteric plexus.3 A gain-of-function mutation in the juxtamembrane domain of the c-kit gene with constitutive, ligand-independent activation of KIT receptor tyrosine kinase is thought to cause tumorgenesis. Autophosphorylation of the receptor, most often KIT (CD117) or platelet-derived growth factor receptor alpha (PDGFRA), leads to constitutive activation of the receptor and tumor growth.4
The immunohistochemical profile of GISTs is characteristic, with nearly 95% of tumors staining positive for KIT (CD117). Often the staining is strongly diffuse in the cell cytoplasm. Less commonly, a membranous or perinuclear pattern is observed. The intensity of KIT staining can be inconsistent. A proper titer of the KIT/CD117 antibody must be used in order to avoid over-staining that could mislabel other neoplasms as GIST. Staining intensity, however, has no predictive value of tumor response to treatment.5 KIT-positivity can also be found in other tumors, including melanoma, angiosarcoma and neuroblastoma, and therefore is not sufficient to make a diagnosis of GIST.6 A more convincing diagnosis can be made with concordance of tumor morphology and immunophenotype. Additional expressed markers in gastrointestinal tumors include CD34 antigen (70%), smooth muscle actin (30–40%), desmin (<5%) and S100 protein (~5%). The immunophenotype varies according to anatomic site. GIST of the esophagus, gastric region and rectum are often CD34 positive.
For the few GISTs that are KIT-negative, mutations of the PDGFRA gene can be helpful in ruling out other lesions of mesenchymal origin. Recent advances have resulted in the identification of DOG1, a calcium-dependent receptor activated chloride channel protein, which seems to be expressed in GIST regardless of mutation type.7,8 Although DOG1 testing is not recommended for KIT-positive lesions, this marker can be utilized in categorization of suspected cases of GIST not confirmed by KIT and PDGFRA testing.
Histopathological features of GISTs generally fall into one of three categories.9 Spindle cell morphology is seen in nearly 70% of cases and shows cells arranged in short fascicles or whorls with uniform cytology, nuclei with fine chromatin and inconspicuous nucleoli. Approximately 20% of lesions are epithelioid type, marked by a nested architecture and more nuclear atypia compared to the spindle type. Combined phenotypes of the previous two morphologies are observed in about 10% of patients.9 The case presented in this report comes under this rare category of mixed spindle and epitheloid cell GIST.
Fletcher et al. in 2002 outlined a classification scheme to define the relative risk for malignant behavior in GISTs.10 The authors proposed stratification of patients with GISTs into low-, intermediate- and high-risk groups based on tumor size and mitotic index. Although the grading system is predictive in a majority of cases, small lesions with low mitotic rates however have been reported to metastasize or become locally aggressive in rare instances.11
GISTs can present clinically in a myriad of ways. Smaller lesions, which can in the millimeter range, are usually asymptomatic and diagnosed incidentally or found at autopsy. Masses can grow as large as 35 cm, with a median size at presentation of 5 cm.12 In comparison to other malignant tumors, GISTs generally do not metastasize lymphatics and therefore lymphadenectomy is typically not indicated.13 The propensity of a GIST to become malignant depends on the primary anatomic site. Esophageal GISTs are rather aggressive malignancies due to their malignant potential, high recurrence, and mortality rates.14 Historically, a majority of patients with esophageal GIST have a late diagnosis and consequently are associated with worse outcomes.15 In an epidemiological study analyzing SEER data, the relative five-year survival after diagnosis of primary GIST of the esophagus compared to overall GIST at any location was 17% versus 45% respectively.16
According to the National Comprehensive Cancer Network, surgery should be the initial therapy for a primary GIST with no metastasis located in an anatomic site with a tolerable morbidity risk related to a surgical procedure. Bearing in mind the adjacent vital structures, this risk is relatively high for esophageal GISTs, and the preferred mode of resection is controversial in the literature. Removal of the tumor can be attempted via simple enucleation or esophagectomy. Indications for each modality remain unclear with regard to tumor size. Although esophagectomy carries higher risks of morbidity and mortality, certain experts in this field argue that excision of larger lesions without complete local resection is ineffective.17Simple enucleation of larger tumors may result in greater positive microscopic margins and recurrence rates. Tumors with large volumes (i.e. >10 cm) which are in close proximity to the gastroesophageal junction are especially difficult to resect with adequate margins.18 Still, the attainment of negative microscopic margins has not been shown to consistently increase long-term survival. Further investigation and multicenter data on surgical strategies for esophageal GISTs are needed. The advent of tyrosine kinase inhibitors has changed the standard of care for GISTs and as a direct consequence, survival of patients with GISTs has increased since the introduction of FDA approval of imatinib mesylate in 2002.2 Imatinib mesylate is a potent and selective inhibitor of a family of structurally related tyrosine kinases, including KIT, BCR-ABL and PDGFRA. Neo-adjuvant treatment with imatinib may improve resectability by down staging the tumor. Adjuvant therapy is recommended for large tumors, marginally resectable small tumors, and unresectable primary localized GISTs. Drug resistance is not infrequent and serum level surveillance has not been shown to impact management of patients who experience resistance. For these patients and those who cannot tolerate imatinib, the newer multikinase inhibitor sunitinib malate may delay median time to tumor progression.19
After a comprehensive literature review, the current case report might be the first reported case to describe resection of a GIST of the gastroesophageal junction that presented with pseudoachalasia and esophageal perforation. The authors hope that the summarization of their clinical experience with this intriguing case may assist with the management of such rare malignant entities.
Conflicts of interest
The authors report that there are no conflicts of interests.
Funding
None.
Ethical approval statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Phayvanh P. Sjogren – study design, data collections, writing, manuscript final approval.
Nilanjana Banerji – study design, data collections, data analysis, writing, manuscript final approval.
Kenneth P Batts – data analysis, writing, image procurement, manuscript final approval.
Matthew J. Graczyk – study design, manuscript final approval.
Daniel H. Dunn – study design, writing, manuscript final approval.
References
- 1.Demetri G.D., von Mehren M., Antonescu C.R., DeMatteo R.P., Ganjoo K.N., Maki R.G. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. Journal of the National Comprehensive Cancer Network. 2010;8(Suppl. (2)):S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steigen S.E., Eide T.J. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006;114:192–200. doi: 10.1111/j.1600-0463.2006.apm_261.x. [DOI] [PubMed] [Google Scholar]
- 3.Kindblom L.G., Remotti H.E., Aldenborg F., Meis-Kindblom J.M. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. American Journal of Pathology. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota S., Isozaki K., Moriyama Y., Hashimoto K., Nishida T., Ishiguro S. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 5.Chirieac L.R., Trent J.C., Steinert D.M., Choi H., Yang Y., Zhang J. Correlation of immunophenotype with PFS in patients with gastrointestinal stromal tumors treated with imatinib mesylate. Cancer. 2006;107:2237–2244. doi: 10.1002/cncr.22226. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M., Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Applied Immunohistochemistry & Molecular Morphology. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 7.West R.B., Corless C.L., Chen X., Rubin B.P., Subramanian S., Montgomery K. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. American Journal of Pathology. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa I., Lee C.H., Kim M.K. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. American Journal of Surgical Pathology. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M., Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Archives of Pathology & Laboratory Medicine. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher C.D., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B.J. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Human Pathology. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 11.Gouveia A.M., Pimenta A.P., Lopes J.M., Capelinha A.F., Ferreira S.S., Valbuena C. Esophageal GIST: therapeutic implications of an uncommon presentation of a rare tumor. Dis Esophagus. 2005;18:70–73. doi: 10.1111/j.1442-2050.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 12.Stamatakos M., Douzinas E., Stefanaki C., Safioleas P., Polyzou E., Levidou G. Gastrointestinal stromal tumor. World Journal of Surgical Oncology. 2009 Aug 1;7:61. doi: 10.1186/1477-7819-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staiger W.I., Ronellenfitsch U., Kaehler G., Schildhaus H.U., Dimitrakopoulou-Strauss A., Schwarzbach M.H. The Merendino procedure following preoperative imatinib mesylate for locally advanced gastrointestinal stromal tumor of the esophagogastric junction. World Journal of Surgical Oncology. 2008;6:37. doi: 10.1186/1477-7819-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang P., Jiao Z., Han B., Zhang X., Sun X., Su J. Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. European Journal Cardio-Thoracic Surgery. 2010;38:223–227. doi: 10.1016/j.ejcts.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M., El-Rifai W.H.L., Sobin L., Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Human Pathology. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 16.Tran T., Davila J.A., El-Serag H.B. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1458 cases from 1992 to 2000. American Journal of Gastroenterology. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 17.Blum M.G., Bilimoria K.Y., Wayne J.D., de Hoyos A.L., Talamonti M.S., Adley B. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Annals of Thoracic Surgery. 2007;84:1717–1723. doi: 10.1016/j.athoracsur.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 18.Gervaz P., Huber O., Morel P. Surgical management of gastrointestinal stromal tumours. British Journal of Surgery. 2009;96:567–578. doi: 10.1002/bjs.6601. [DOI] [PubMed] [Google Scholar]
- 19.Rajendra R., Pollack S.M., Jones R.L. Management of gastrointestinal stromal 21 tumors. Future Oncology. 2013 Feb;9(2):193–206. doi: 10.2217/fon.12.178. [DOI] [PubMed] [Google Scholar]


