Abstract
INTRODUCTION
Distal gastrectomy with lymph node dissection is the standard treatment for gastric cancer. Remnant gastric necrosis after distal gastrectomy is very rare and fatal complication.
PRESENTATION OF CASE
A-78-year-old male diagnosed with advanced gastric cancer underwent distal gastrectomy with lymph node dissection. Postoperative gastric remnant necrosis occurred following splenic infarction. There was thought to be an insufficient blood supply to the gastric remnant due to the lymph node dissection along the proximal splenic artery during the initial surgery. Non-contrast abdominal computed tomography did not reveal any necrosis in the remnant stomach. An endoscopic examination confirmed this diagnosis. Total remnant gastrectomy was performed, and the patient thereafter successfully recovered.
DISCUSSION
Careful management of blood vessels and lymph node dissection above the pancreas should be performed to avoid restricting the blood flow and also to prevent gastric remnant necrosis.
CONCLUSION
The knowledge of this fatal complication is crucial for management of postoperative complication. For early and accurate diagnosis, upper gastrointestinal endoscopy is necessary in case of remnant gastric necrosis.
Keywords: Remnant gastric necrosis, Splenic infarction, Distal gastrectomy
1. Introduction
Distal gastrectomy with lymph node dissection is the standard treatment for gastric cancer. The incidence of mortality is quite low in Japan.1 The stomach can resist ischemic necrosis, and therefore gastrectomy can be safely performed, and gastric remnant necrosis is a rare postoperative complication. This report presents a case of gastric remnant necrosis followed by spleen infarction after distal gastrectomy with lymph node dissection.
2. Case report
A 78-year-old male with hypertension, hyperlipidemia and chronic kidney disease presented with anorexia and dysphagia. He had been treated stenting of the left common iliac artery for arteriosclerosis obliterans 2 years earlier. He had no history of smoking or diabetes. Endoscopic examination of the stomach revealed a type 3 tumor circumferentially located in the antrum of the stomach. A histological examination of biopsied specimens showed poorly differentiated adenocarcinoma. The results of blood tests, including the tumor marker levels (CEA and CA19-9) were within the normal limits except for creatinine, which was 1.36 mg/dl. Computed tomography (CT) of the abdomen revealed wall thickening of the antrum and calcification of the splenic artery due to arteriosclerosis (Fig. 1). There was no evidence of metastasis in the liver, other organs and lymph nodes. Distal gastrectomy and lymph node dissection (D2) and resection of the posterior layer of the transverse mesocolon was performed due to invasion through the posterior layer of the transverse mesocolon. A Billroth I reconstruction was performed and a J-VAC drain® was placed under the anastomosis. A histological examination revealed poorly differentiated adenocarcinoma that had invaded the serosa of the gastric wall with lymph node metastasis. The TNM stage of the tumor was T4aN2M0.
Fig. 1.
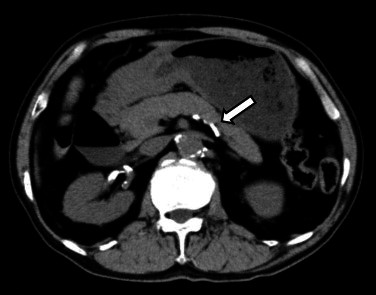
Calcification of the splenic artery due to arteriosclerosis is recognized on preoperative non-contrast CT (white arrow).
High fever and left upper quadrant pain occurred on postoperative day 3. Laboratory data demonstrated leukocytosis (WBC count: 27,900/μl) and a high C-reactive protein level (26.76 mg/dl). Computed tomography of the abdomen revealed fluid collection anterior to the spleen, around the tail of pancreas. In addition, Doppler ultrasound could not demonstrate the presence of a blood flow to the spleen. He was diagnosed to have spleen infarction. The fluid collection was found to be reactive abdominal fluid. We therefore continued to observe the patient under total parenteral nutrition. The high fever and inflammation (WBC count: 18,700/μl and high C-reactive protein level: 16.39 mg/dl) continued, but the left upper quadrant pain gradually subsided. An upper gastrointestinal series revealed good passage through the anastomosis and no leakage on postoperative day 8 (Fig. 2). Oral intake was permitted.
Fig. 2.

An upper gastrointestinal series shows good passage through the anastomosis.
The high fever and inflammation gradually decreased and his appetite recovered to a normal level (Fig. 3). However, high fever occurred again on postoperative day 22 (C-reactive protein level: 12.03 mg/dl). The structure of the gastric remnant wall was confirmed on non-contrast abdominal CT (Fig. 4). Furthermore, the upper gastrointestinal series showed the leakage of contrast medium from the greater curvature to the abdominal cavity. Gastrointestinal endoscopy was performed to observe the mucosa of gastric remnant and confirm whether the cause of the perforation was ischemia. Endoscopy revealed shedding and necrotic change of the gastric remnant mucosa, with circumferential involvement from the oral side to the site of anastomosis (Fig. 5).
Fig. 3.

The high fever and inflammation gradually decreased.
Fig. 4.
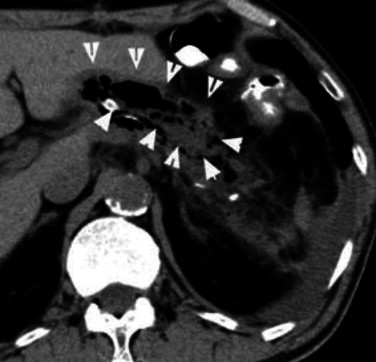
The structure of the wall of the gastric remnant (white arrow) was observed on non-contrast abdominal CT.
Fig. 5.

Mucosal necrosis of the gastric remnant with circumferential involvement from the oral side to the anastomosis (the outside of dotted line). The mucosa of the duodenum was normal.
Emergency laparotomy was performed on the same day under a diagnosis of necrosis of the remnant stomach. The laparotomy revealed necrosis of the entire gastric remnant from the esophageal-gastric junction to the anastomosis. The remnant stomach adhered to the inferior side of the lateral liver. A total gastrectomy with Roux-en-Y reconstruction and abscess drainage was performed (Fig. 6). The histopathology revealed total mucosal necrosis in the gastric remnant. He recovered and was discharged with no problems 46 days after the reoperation.
Fig. 6.

The macroscopic findings of the gastric remnant. All mucosa showed necrosis (▵: oral side, ▿: anal side).
3. Discussion
The stomach can resist ischemic necrosis, because of its rich vascular supply and extensive submucosal plexus.2 There are a few reports of gastric necrosis from various etiologies in the literature. These include bulimia,3 abdominal trauma,4 and infectious diseases.5 Vascular causes include therapy transcatheter embolization,6 infusion of vasopressin,7 splenectomy,8 and splenic vein thrombosis accompanying splenic infarction.9 Remnant gastric necrosis after distal gastrectomy is very rare. There are few case reports of remnant gastric necrosis after gastrectomy.10–14
An interruption of the blood flow to the gastric remnant may have caused necrosis of the gastric remnant. The etiology of such an interruption of the blood flow can be caused by arteriosclerosis, atrial fibrillation, sepsis, or venous thrombosis.10 Smoking, diabetes, hypertension, and hyperlipidemia are also risk factors of these conditions. In one case, the authors mentioned that the anatomical variation of the gastric arterial supply was a risk factor. The deficient common hepatic artery (Adachi type VI15) and the short gastric artery noted by abdominal enhanced CT, may be related to an imbalance of the blood supply after gastrectomy.12 In this regard, it is important to confirm the presence of any anatomical variation before the operation in order to prevent the development of necrosis. The patient had a history of arteriosclerosis obliterans, hyperlipidemia, and calcification of the splenic artery due to arteriosclerosis was recognized on preoperative abdominal CT (Fig. 1). In this case, calcification of the artery may have been risk factors for the observed reduction of the blood flow. In addition, lymph node dissection above the pancreas may have induced the reduction of the blood flow. Lymph node dissection above the pancreas was performed around the splenic artery, so the possibility of stenosis, overextension or spasm of the artery was also considered during the surgical management.
Table 1 shows the clinical findings of gastric remnant necrosis reported in Japan. In many cases, the necrosis occurred about 2–3 weeks after surgery. Only four of 10 previous cases were diagnosed before surgery. The possibility of gradually developed necrosis was suggested, however, there was no direct evidence. The current patient developed necrosis 22 days following surgery.
Table 1.
The clinical findings of gastric remnant necrosis in Japan.
| Case no. | Year | Age | Sex | Reconstruction | Post-op (days) | Pre-operative diagnosis | Treatment | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | 1991 | n.d. | n.d. | n.d. | 26 | Necrosis | Conservative | Died |
| 2 | 1995 | 61 | F | B-I | 7 | Peritonitis | Total gastrectomy | Survived |
| 3 | 1996 | 49 | M | B-I | 14 | Peritonitis necrosis | Total gastrectomy | Survived |
| 4 | 1996 | 60 | M | B-I | 8 | Peritonitis | Total gastrectomy | Survived |
| 5 | 1998 | 55 | M | B-I | 25 | Peritonitis | n.d. | n.d. |
| 6 | 1999 | 53 | M | B-I | 20 | Peritonitis | Total gastrectomy | Died |
| 7 | 2004 | 72 | M | R-Y | 15 | Peritonitis | Total gastrectomy | Survived |
| 8 | 2006 | 68 | M | B-I | 7 | Peritonitis | Total gastrectomy | Died |
| 9 | 2008 | 72 | M | B-I | 14 | Peritonitis | Total gastrectomy | Died |
| 10 | 2009 | 50 | M | B-I | 26 | Necrosis | Total gastrectomy | Survived |
| 11 | 2011 | 64 | M | R-Y | 21 | Necrosis | Total gastrectomy | Survived |
| Our case | 2011 | 78 | M | B-I | 22 | Necrosis | Total gastrectomy | Survived |
n.d.: not described; B-I: Billroth I; R-Y: Roux-en Y; and post-op: post-operative days.
The infarction is often diagnosed with contrast-enhanced CT.13 However, the diagnosis of necrosis of the gastric remnant is difficult if the blood flow is maintained partially. The blood flow to the gastric remnant is partially maintained in some cases.14 Early diagnosis requires observation of mucosa of the gastric remnant by gastrointestinal endoscopy,11,12 which reveals necrotic change of the gastric remnant mucosa.
In general, anastomotic leakage would be considered in the differential diagnosis of cases like ours, so we should be examined at first by the upper gastrointestinal series. However, early diagnosis the gastric remnant necrosis could not be confirmed in the current case, because patient did not show peritonitis and splenic infarction. The mortality rate of remnant gastric necrosis is 40–70%,12,14 so it is important to carefully observe the postoperative progress for prevention and early diagnosis of this complication. In this case, the leakage of contrast medium was confirmed from the greater curvature, so we suspected unusual types of leakage. This is the reason why we cannot avoid endoscopic examination. Endoscopic examinations should therefore be performed to make an early diagnosis of gastric remnant necrosis. As to gastric remnant necrosis, total gastrectomy and drainage was required treatment.
Careful management of blood vessels and lymph node dissection above the pancreas should be performed to avoid restricting the blood flow and also to prevent gastric remnant necrosis.
4. Conclusion
Upper gastrointestinal endoscopy is necessary to make an accurate diagnosis of remnant gastric necrosis. It is important to observe the blood flow in the remnant gastric mucosa in patients that develop splenic infarction and this should be confirmed by upper gastrointestinal endoscopy.
Conflict of interest statement
None of the authors have any conflict of interest to declare.
Funding
None.
Ethical approval statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
All authors contributed to this study.
References
- 1.Sasako M., Inoue M., Lin J.T., Khor C., Yang H.K., Ohtsu A. Gastric cancer working group report. Japanese Journal of Clinical Oncology. 2010;40:i28–i37. doi: 10.1093/jjco/hyq124. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson E.D. The circulation of the stomach. Gastroenterology. 1965;48(January):85–109. [PubMed] [Google Scholar]
- 3.Abdu R.A., Garritano D., Culver O. Acute gastric necrosis in anorexia nervosa and bulimia. Two case reports. Archives of Surgery. 1987;122:830–832. doi: 10.1001/archsurg.1987.01400190096021. [DOI] [PubMed] [Google Scholar]
- 4.Garfinkle S.E., Matolo N.M. Gastric necrosis from blunt abdominal trauma. Journal of Trauma. 1976;16:405–407. [PubMed] [Google Scholar]
- 5.Patel R.M., Desoto-LaPaix F., Mallaiah L.R. Gastric infarction: a complication of endocarditis due to Staphylococcus aureus. Journal of Clinical Gastroenterology. 1983;5:159–163. [PubMed] [Google Scholar]
- 6.Brown K.T., Friedman W.N., Marks R.A. Gastric and hepatic infarction following embolization of the left gastric artery: case report. Radiology. 1989;172:731–732. doi: 10.1148/radiology.172.3.2788892. [DOI] [PubMed] [Google Scholar]
- 7.Bradley E.L., 3rd, Goldman M.L. Gastric infarction after therapeutic embolization. Surgery. 1976;79:421–424. [PubMed] [Google Scholar]
- 8.Martinez C.A., Waisberg J., Palma R.T., Bromberg S.H., Castro M.A., Santos P.A. Gastric necrosis and perforation as a complication of splenectomy. Case report and related references. Arquivos de Gastroenterologia. 2000;37:227–230. doi: 10.1590/s0004-28032000000400008. [DOI] [PubMed] [Google Scholar]
- 9.Kanetaka K., Azuma T., Ito S., Yamaguchi S., Matsuo S., Kanematsu T. Gastric necrosis after an infarction of the spleen: report of a case. Surgery Today. 2003;33:867–869. doi: 10.1007/s00595-003-2598-z. [DOI] [PubMed] [Google Scholar]
- 10.Casey K.M., Quigley T.M., Kozarek R.A., Raker E.J. Lethal nature of ischemic gastropathy. American Journal of Surgery. 1993;165:646–649. doi: 10.1016/s0002-9610(05)80453-2. [DOI] [PubMed] [Google Scholar]
- 11.Morita Y., Azumi Y., Nakamoto M. A case report of ischemic necrosis of the proximal gastric remnant following distal gastrectomy. Nichirinnge. 2004;65:1539–1543. [Google Scholar]
- 12.Nonaka T., Hidaka S., Fukuoka H., Abo T., Takeshita H., Nanashima A. A case report of necrosis of gastric remnant after distal gastrectomy. Japanese Journal of Gastroenterological Surgery. 2011;44:829–835. [Google Scholar]
- 13.Kim H.J., Lee K.H., Kim Y.H., Kim H.H., Kim S.H., Lee H.J. Gastric remnant infarction following laparoscopy-assisted distal gastrectomy: CT diagnosis in two cases. Abdominal Imaging. 2007;32:290–292. doi: 10.1007/s00261-006-9071-6. [DOI] [PubMed] [Google Scholar]
- 14.Isabella V., Marotta E., Bianchi F. Ischemic necrosis of proximal gastric remnant following subtotal gastrectomy with splectomy. Journal of Surgical Oncology. 1984;25:124–132. doi: 10.1002/jso.2930250215. [DOI] [PubMed] [Google Scholar]
- 15.Adachi B. Das areteriensystem der Japanar. Band II. Maruzen Co.; Kyoto: 1928. Anatomie der Japaner I; pp. 11–73. [Google Scholar]


