Abstract
Stra6 is the retinoic acid (RA)-inducible gene encoding the cellular receptor for holo-retinol binding protein. This transmembrane protein mediates the internalization of retinol, which then upregulates RA-responsive genes in target cells. Here, we show that Stra6 can be upregulated by DNA damage in a p53-dependent manner, and it has an important role in cell death responses. Stra6 expression induced significant amounts of apoptosis in normal and cancer cells, and it was also able to influence p53-mediated cell fate decisions by turning an initial arrest response into cell death. Moreover, inhibition of Stra6 severely compromised p53-induced apoptosis. We also found that Stra6 induced mitochondria depolarization and accumulation of reactive oxygen species, and that it was present not only at the cellular membrane but also in the cytosol. Finally, we show that these novel functions of Stra6 did not require downstream activation of RA signalling. Our results present a previously unknown link between the RA and p53 pathways and provide a rationale to use retinoids to upregulate Stra6, and thus enhance the tumour suppressor functions of p53. This may have implications for the role of vitamin A metabolites in cancer prevention and treatment.
Keywords: apoptosis, p53, reactive oxygen species, retinoic acid, Stra6
Human cells are constantly subjected to stresses that damage their DNA. This activates a series of mechanisms aimed at repairing the damage or eliminating severely affected cells before they divide and endanger the homoeostasis of the organism. A master controller of these cellular responses is p53, which functions as a ‘guardian of the genome'.1 The majority of cancers show loss of p53 function,2, 3, 4, 5 underscoring its importance in tumour suppression. As a transcription factor, p53 targets the expression of a variety of genes involved in specific cellular responses, which include cell cycle arrest, senescence and apoptosis.1, 4, 6, 7, 8, 9, 10 The factors determining cell fate decisions after p53 upregulation are still not well understood.11
Vitamin A is a liposoluble factor necessary for most life forms, which animals cannot synthesize.12 In humans, retinol, a form of vitamin A, is obtained from the diet and usually stored in the liver. A retinol binding protein (RBP) delivers it to other organs by forming a complex with it (holo-RBP) and transporting it through the blood. Once inside the cell, retinol is transformed into active retinoic acid (RA) by the action of enzymes such as ADH and RALDH.13 In target cells, RA signals through its specific nuclear receptors (RARs and RXRs)14, 15 to induce a series of RA-responsive genes that have important roles in embryonic development, organogenesis and maintenance of epithelia, as well as the physiological functions of the immune system, the brain, the eye and the reproductive system.12, 16 RA also participates in cell growth, differentiation and death. It can induce arrest in the G1 phase of the cell cycle by upregulating p21,16 and apoptosis through an increase in death receptors such as FAS, DR4 and DR5.16
The complexity of RA signalling is exemplified by the fact that retinoids are known teratogens, but they can also inhibit tumour cell growth.16 One of the main active effectors of the pathway is all-trans RA (ATRA), which can regulate the expression of a large number of genes through binding and activation of RAR/RXR. For instance, ATRA can induce senescence, a tumour suppressor mechanism, through the activation of p16, p21 and p27.17 Of special interest is the effect of ATRA on acute promyelocytic leukaemia, which blocks the PML–RARα fusion protein responsible for the disease.18 Although RA-based treatments have been successfully used against certain types of tumours, its mechanisms of action are not clear.16
Stra6 was first identified in mouse as a RA-responsive gene.19 It encodes a transmembrane protein that is widely expressed during development and also in most adult organs.20 Recently, it was discovered that Stra6 is the specific membrane receptor for RBP.21 The existence of such a receptor was previously discovered in the 1970s,22 but its identification remained elusive. It has been shown that Stra6 binds to holo-RBP and is necessary for the cellular uptake of retinol, therefore having an important upstream role in RA signalling. This was confirmed by the fact that Stra6 mutations observed in humans are responsible for familial syndromes likely to be related to the role of vitamin A in embryonic development,21 which include severe malformations such as anophtalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary displasia, lung hypoplasia and mental retardation.23 Consistent with this, a Stra6 knockout mouse model showed several ocular defects similar to those described in humans.24 Moreover, after binding to holo-RBP, Stra6 is phosphorylated when retinol is internalized and transferred to CRBP-I.25 This phosphorylation is needed for retinol uptake.25 Phosphorylated Stra6 also acts as a cytokine receptor that activates the JAK2/STAT5 pathway.25, 26 This inhibits insulin signalling and enhances lipid accumulation, consistent with the association of certain SNPs in the Stra6 gene with type 2 diabetes.27 Because of this, it has been proposed that Stra6 should be considered the first member of a new group of ‘cytokine signalling transporters'.25
Here, we show a novel function of Stra6 as part of the cellular responses to damage, and a previously unknown crosstalk between the p53 and RA pathways. We found that Stra6 participates in p53-induced cell death, likely by engaging the mitochondrial pathway of apoptosis and elevating intracellular reactive oxygen species (ROS). This makes Stra6 able to influence p53-mediated cell fate decisions by favouring apoptosis over arrest. We also found that this is independent of the downstream activation of RA targets. Our results define Stra6 as a new member of the p53 pathway and suggest that inducing Stra6 with retinoids could have a positive effect on the antineoplastic defences of the cell.
Results
Stra6 is induced by genotoxic stress in a p53-dependent manner
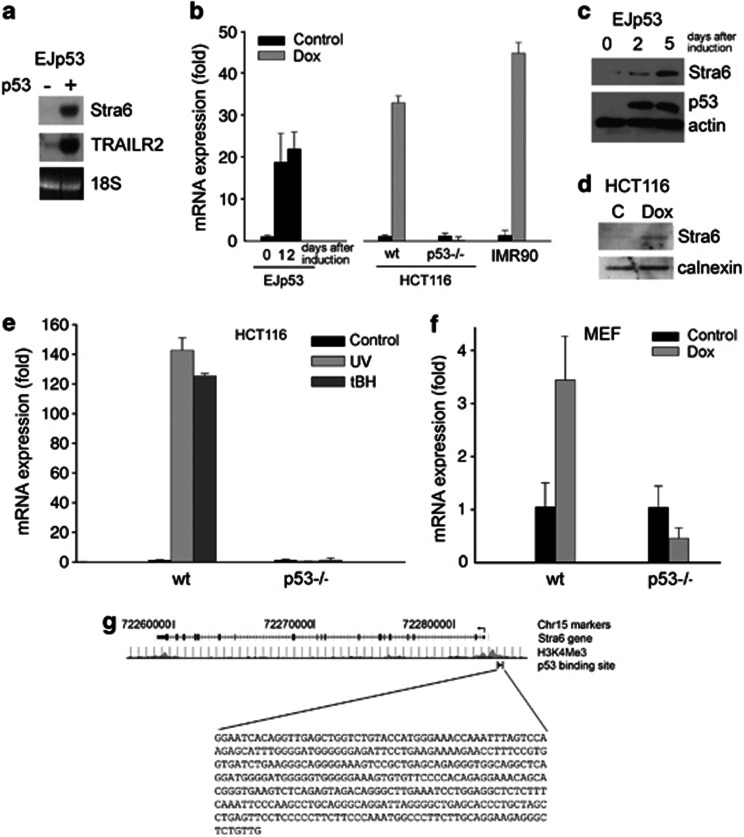
We identified Stra6 as a p53-induced gene in a cDNA expression array that we have used to characterize several new p53 targets.28, 29, 30 Using a bladder cancer cell line with a tetracycline (tet)-regulatable p53 expression system, EJp53,31 we observed by northern blot (Figure 1a) and quantitative real-time PCR (qRT-PCR) (Figure 1b, left panel) that Stra6 mRNA was upregulated in the presence of p53. As expected, this was accompanied by an increase in Stra6 protein levels (Figure 1c). Moreover, we found that Stra6 was induced in response to DNA damaging agents such as doxorubicin (Figure 1b, right panel, and 1d), UV radiation or oxidants (Figure 1e). This physiological response was observed in both normal human fibroblasts (IMR90) and colon cancer cells expressing wt p53 (HCT116). However, genotoxic stress was not able to induce Stra6 in the absence of p53 (Figures 1b and e). This was also the case in MEFs from p53-null mice (Figure 1f). Using the GIS Chip-PET data32 (available via the UCSC Genome Browser), we observed that p53 binds on chromosome 15q21.2, at a region rich in H3K4Me3 that is likely to be the promoter of Stra6 (Figure 1g). Thus, our data indicates that Stra6 is induced after genotoxic stress in a p53-dependent manner, and suggests that Stra6 is likely to be a novel target gene that has a role in p53-mediated responses to stress.
Figure 1.
Stra6 is a p53 target gene induced after DNA damage. (a) Northern blot analysis of Stra6 mRNA levels in uninduced EJp53 (−) and EJp53 induced to express p53 by tet removal for 2 days (+). A probe against TRAILR2 (a direct p53 target gene) was used as a positive control, and a picture of ethidium bromide-stained 18S ribosomal RNA as loading control. (b) qRT-PCR of Stra6 mRNA expression in EJp53 (0, 1 or 2 days after p53 induction by tet removal), and HCT116, HCT116p53−/− and IMR90 treated for 24 h with 1.4 μM doxorubicin, compared with untreated cells. Expression was normalized to that of TBP. Mean values and S.D. of three experiments are plotted. (c) Western blot showing protein expression levels of Stra6 and p53 in EJp53 cells (0, 1 or 2 days after p53 induction by tet removal). β-Actin is used as loading control. (d) Western blot showing protein expression levels of Stra6 in lysates containing membranes of control HCT116 (c) and HCT116 treated for 24 h with 1.4 μM doxorubicin (Dox). Calnexin is used as loading control. (e) qRT-PCR showing Stra6 mRNA expression in HCT116 and HCT116p53−/− after 24 h of being treated with 400 μM tBH (for 2 h) or 50 mJ/cm2 UV radiation, compared with untreated cells. Expression was normalized to that of TBP. Mean values and S.D. of triplicate experiments are plotted. (f) Same, with wt and p53−/− MEFs treated for 24 h with 1.4 μM doxorubicin. (g) Graphic representation of the Stra6 gene obtained using the ar. 2006 (NCBI36/hg18) assembly database in UCSC Genome Browser (genome.ucsc.edu). Data from GIS Chip-PET (obtained from a p53 Ab used on 5FU- treated HCT116 cells32) show p53 binding on position chr15:74,475,962-74,511,081 (15q21.2), close to the promoter-associated histone mark H3K4Me3 (determined by ENCODE data on 9 cell lines)
Stra6 contributes to p53-induced apoptosis
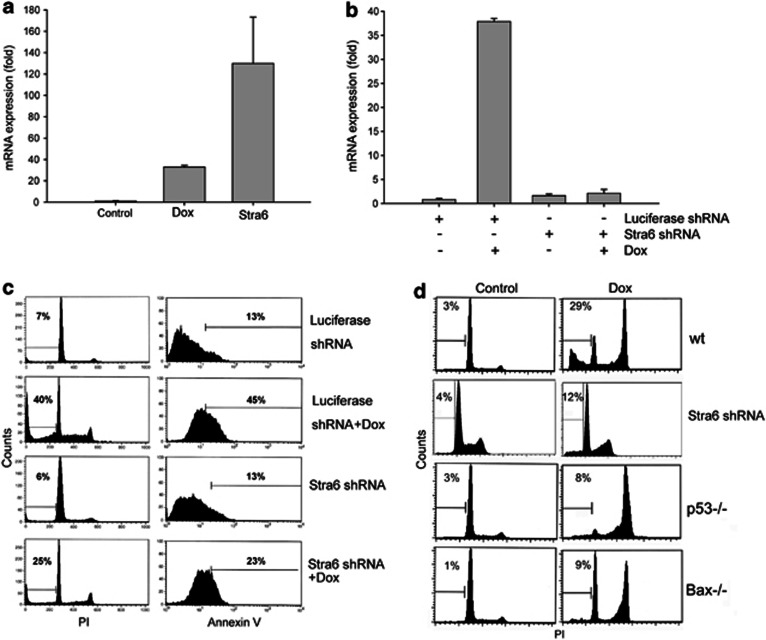
In order to elucidate the role of Stra6 in DNA damage responses, we transfected cells with a vector encoding Stra6 (Figures 2a and b). Annexin V staining showed that Stra6 triggered a moderate apoptotic response in IMR90 and HCT116 (Figure 2c), despite the high expression levels achieved in transfection (see Figure 2b). However, it induced a substantial amount of apoptosis in ovarian cancer cells PA-1 and A1847. Cells that showed the greatest sensitivity to Stra6 were also more sensitive to DNA damage. Of note PA-1 showed high sensitivity to Stra6 even though the expression levels were the lowest (see Figure 2b). These data suggest that cell specific factors determine sensitivity to Stra6 and establish that Stra6 has pro-apoptotic activity. To further test this, we transfected Stra6 into EJp53. We have previously shown that upregulation of p53 in these cells triggers cell cycle arrest without significant apoptosis.31 Although Stra6 did not induce cell death in EJp53 in the absence of p53, when p53 was expressed, Stra6 shifted the cellular response from arrest to apoptosis, as represented by a significant increase in the percentage of dead cells (Figure 2d). This shows that Stra6 can influence p53-mediated cell fate decisions and supports the hypothesis that it has a role in p53-induced apoptosis.
Figure 2.
Stra6 expression induces apoptosis. (a) Western blot showing protein expression levels of Stra6 in 293 transfected with an empty vector (control) or a Stra6 plasmid for 48 h. (b) qRT-PCR data showing relative Stra6 mRNA expression in IMR90, HCT116 and PA-1 transfected with an empty vector (Control) or Stra6 for 48 h. ACTB was used as control. Results show mean of three experiments and error bars represent S.D.. (c) Representative FACS analysis of Annexin V-stained IMR90, HCT116, PA-1 and A1847. Cells where either transfected with an empty vector (Control and Dox) or a Stra6 vector (Stra6 and Stra6+Dox). Cells were treated 24 h after transfection with 1.4 μM doxorubicin for 48 h. Percentages indicate Annexin V-positive cells (apoptotic). (d) Representative cell cycle profiles of PI-stained EJp53 transfected for 3 days with an empty vector (Control) or a Stra6 plasmid in the presence or absence of p53 (induced by tet removal)
Of note, the levels of Stra6 expression achieved by transfection were at least ∼3–4 fold higher than those observed after DNA damage (Figure 3a). To better explore the physiological relevance of our findings, we generated HCT116 cells stably expressing shRNA against Stra6. This shRNA was able to completely block Stra6 induction after exposure to DNA damaging agents (Figure 3b). Using these cells, we observed that the absence of Stra6 impaired apoptosis after genotoxic stress, with less than half the amount of cell death being induced after exposure to doxorubicin when measured by either propidium iodide (PI) or Annexin V staining (Figure 3c). The effects of Stra6 inhibition on p53-induced apoptosis were similar to those observed in cells lacking Bax, one of the main pro-apoptotic p53 target genes (Figure 3d). In view of these results, we concluded that Stra6 participates in the physiological responses to damage mediated by p53 by contributing to the induction of apoptosis.
Figure 3.
Stra6 expression is important for p53-mediated cell death. (a) qRT-PCR comparing the expression of Stra6 in HCT116 transfected with an empty vector (Control, Dox) or Stra6, treated or not with 1.4 μM doxorubicin for 24 h (Dox). (b) qRT-PCR measuring Stra6 mRNA levels in cells stably expressing shRNA against luciferase or Stra6, treated with 1.4 μM doxorubicin (Dox) for 24 h. Expression was normalized to that of TBP. Mean values and S.D. of three experiments are plotted. (c) Left column: Representative cell cycle profiles of HCT116 expressing shRNA against luciferase or Stra6, stained with PI (left column) or AnnexinV (right column). Cells were treated with 1.4 μM doxorubicin or 400 μM tBH for 48 h. Numbers indicate percentage of events in SubG1 (left, dead cells) or Annexin V-positive cells (right, apoptotic cells). (d) Representative cell cycle profiles of PI-stained HCT116 (wt), HCT116 stably expressing shStra6 (shStra6), HCT116p53−/− (p53−/−) and HCT116Bax−/− (Bax−/−) treated with 1.4 μM doxorubicin for 48 h. Numbers indicate percentage of SubG1 events (dead cells)
Stra6 increases intracellular ROS levels and mitochondrial depolarization
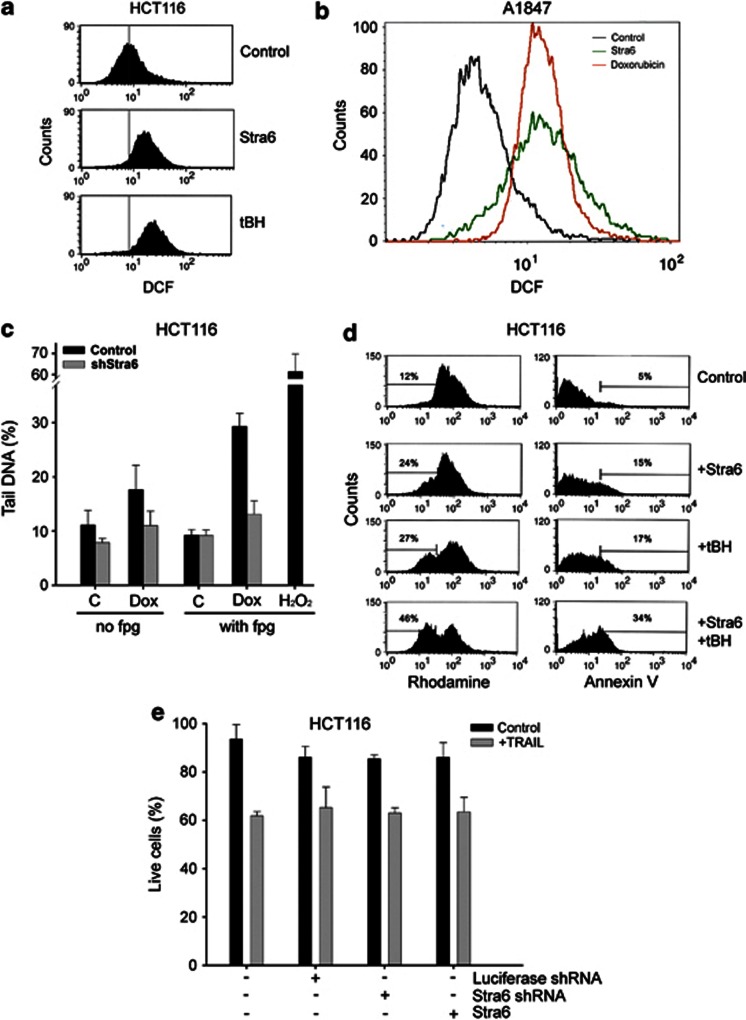
The mechanisms by which Stra6 triggers and/or enhances apoptosis are not immediately obvious from its known role as a cellular gatekeeper of RA signalling. We reasoned that Stra6 could be increasing intracellular ROS, which are known to have an important role in p53-induced apoptosis and determine cell fate decisions after p53 upregulation.33, 34 This would be consistent with results shown in Figure 2a, as doxorubicin partially induces apoptosis through ROS, and cancer cells are usually more sensitive to oxidative stress.35 Indeed, a DCF (2′,7′-dichlorodihydrofluorescein diacetate) staining showed that Stra6 expression was sufficient to elevate intracellular ROS in HCT116 and A1847 to levels similar to those observed after exposure to oxidants such as tert-butyl-hydroxyperoxide (tBH) or doxorubicin (Figures 4a and b). An increase in intracellular ROS was also observed in normal human fibroblasts transfected with Stra6 (Supplementary Figure 1A). The contribution of Stra6 to ROS induction was confirmed by comet assay analysis, which showed that cells in which Stra6 had been knocked down suffered less induced oxidative damage to their DNA in response to doxorubicin (Figure 4c). This shows that Stra6 contributes to the generation of ROS in DNA damage responses and suggests that it may act on the intrinsic pathway of apoptosis, in which ROS and mitochondria are central. Consistent with this, we observed a marked mitochondria depolarization after Stra6 expression in normal and cancer cells, as measured by rhodamine staining (Figure 4d and Supplementary Figure 1B), concomitant to the increase in apoptosis. This was comparable with the amount of depolarization and apoptosis exerted by tBH (Figure 4d), which supports the hypothesis that Stra6-mediated induction of cell death depends on ROS increases. Of note, Stra6 did not have a part in apoptosis induced by TRAIL, which acts through the extrinsic pathway, as a shRNA against Stra6 did not ameliorate it (Figure 4e). All these data together indicate that Stra6 participates in p53-induced apoptosis, probably through the intrinsic pathway by affecting mitochondria polarization and increasing ROS accumulation.
Figure 4.
Stra6 induces intracellular ROS accumulation and mitochondrial depolarization. (a) Representative plots of DCF-stained HCT116 transfected with an empty vector (Control, tBH) or Stra6 for 48 h, and 24 h after being treated with 100 μM tBH for 2 h (tBH). Plots show mean fluorescence intensity. Vertical grey bar indicates averages basal fluorescence of Control cells. (b) Same with A1847 and 1.4 μM doxorubicin. (c) Comet assay of HCT116 expressing shRNA against luciferase (Control) or Stra6 (shStra6), treated with 0.7 μM doxorubicin. Positive control are cells treated with 200 μM H2O2 for 10 min on ice right before analysis. Experiments were performed in the absence or presence of fpg to determine specific damage due to oxidation (the difference in tail DNA percentage between the two conditions). Controls show results using fpg buffer. Mean percentage of tail DNA is plotted. Error bars represent S.D.. Experiments were performed twice in triplicates. (d) Representative plots of rhodamine 123- (left) and Annexin V- (right) stained HCT116 cells, transfected with an empty vector (first and third rows) or Stra6 (second and fourth rows), and 24 h after being treated with 200 μM tBH for 2 h. Percentages indicate cells with depolarized mitochondria (left) or apoptotic (right). (e) Percentage of live HCT116 expressing a shRNA against luciferase or Stra6, or transfected with Stra6 for 24 h, all after treatment with 20 ng/ml TRAIL for 48 h, as measured by Annexin V staining. Results show mean of three experiments and error bars represent S.D.
The subcellular localization of Stra6
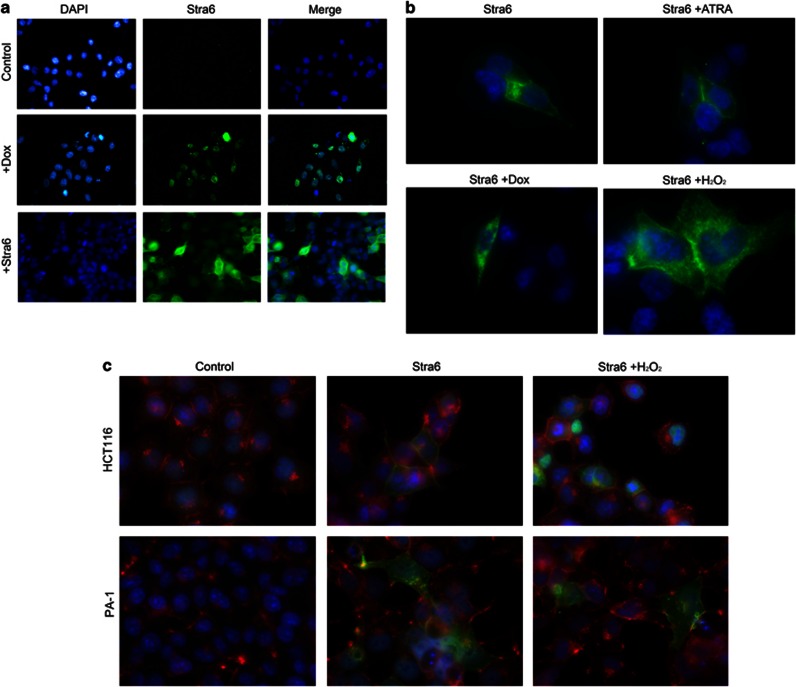
In order to better understand the role of Stra6 in the intrinsic pathway of apoptosis, we next studied its intracellular localization. We first confirmed by immunofluorescence microscopy that doxorubicin upregulated endogenous Stra6 in HCT116 cells (Figure 5a). Basal levels were virtually undetectable, consistent with our previous results (see Figure 1). Moreover, we found that, after damage, Stra6 was not exclusively located at the membrane in these cells, but also present in the cytosol. This suggests that DNA damage could change the subcellular localization of Stra6. To further explore this possibility, we used HCT116 with transfected Stra6 (Figure 5b). In the absence of DNA damage, most Stra6 could be detected in the cellular membrane, although some was already located in the cytosol (top left panel). Consistent with previous reports,36 treatment with ATRA maintained its predominant membrane localization (Figure 5b, top right panel). Of note, treatment with either doxorubicin or H2O2 increased the presence of transfected Stra6 in the cytosol (Figure 5b, bottom panels, and Figure 5c). This was also the case in PA-1 cells (Figure 5c). This difference in the balance between membrane-associated and cytosolic Stra6 in the presence of DNA damage is indicative of Stra6 having separate functions in the RA and p53 pathways.
Figure 5.
Stra6 intracellular localization after DNA damage. (a) Representative immunofluorescence images of HCT116 treated with 0.7 μM doxorubicin for 24 h, with a primary antibody against Stra6 and counterstained with DAPI. Magnification: × 20 (b) Same, with HCT116 transfected with Stra6 and treated with 1 μM ATRA, 0.7 μM doxorubicin or 10 μM H2O2 for 24 h. Magnification: × 100. (c) Representative immunofluorescence images of HCT116 and PA-1 treated with H2O2 for 24 h, with primary antibodies against Stra6 (green) and calnexin (red, a membrane marker), counterstained with DAPI (blue). Magnification: × 100
RA signalling is not necessary for the induction of apoptosis by Stra6
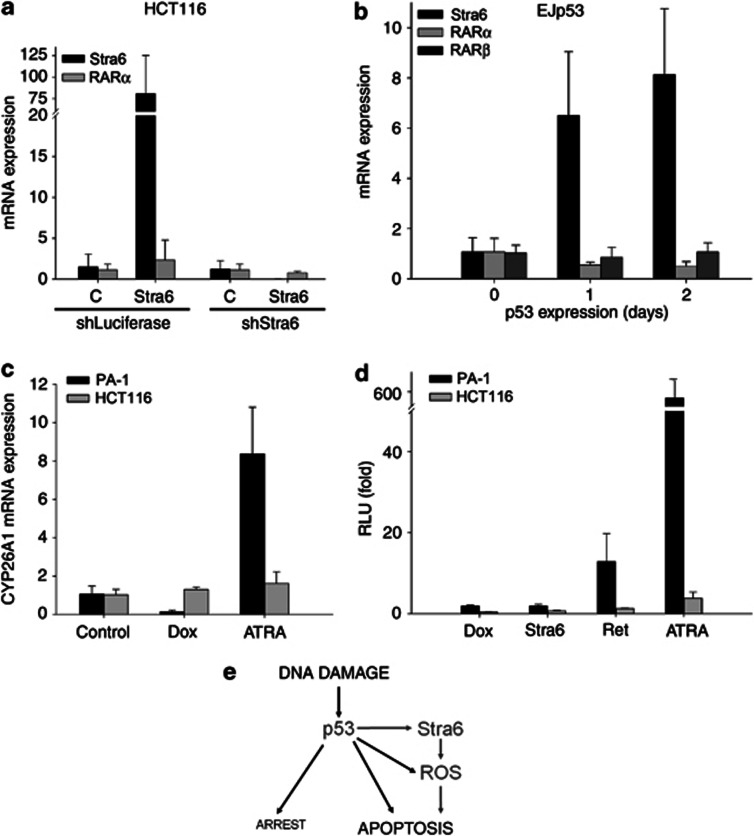
Our results provide a novel link between the RA and p53 pathways through Stra6. As RA signalling can itself trigger apoptosis,16 one could hypothesize that Stra6 could be engaging this pathway to contribute to p53-mediated cell death. On the other hand, the subcellular localization of Stra6 suggests that its pro-apoptotic functions could be independent of its role in the RA pathway. This is supported by the fact that although HCT116 are RA-resistant due to a deficiency in the expression of RARs,37 Stra6 expression can trigger apoptosis (see Figure 2b), ROS accumulation (see Figure 4a) and mitochondria depolarization (see Figure 4d). To clarify this, we first measured whether Stra6 expression resulted in induction of downstream targets of RA. As expected, transfection of Stra6 into HCT116 did not affect the levels of the RA target gene, RARα (Figure 6a). p53 was able to induce Stra6 mRNA in EJp53 cells, as previously shown, but not RA target genes RARα or RARβ (Figure 6b). We also measured the induction of another RA target gene, CYP26A1, in HCT116 and PA-1, a cell with intact RA signalling. As shown in Figure 6c, DNA damage did not induce the expression of CYP26A1 in any of these cell lines. As expected, ATRA induced CYP26A1 in PA-1, but not in RA-insensitive HCT116.
Figure 6.
Stra6 expression does not activate the RA pathway. (a) qRT-PCR data showing Stra6 and RARα mRNA expression in HCT116 stably expressing shRNA against luciferase or Stra6, transfected with a control plasmid or Stra6 for 48 h. ACTB was used as control. Results show mean of three experiments and error bars represent S.D. (b) Same as above in EJp53 induced to express p53 for 1 or 2 days by tet removal and measuring expression of RARα and RARα mRNA. (c) Same as above in PA-1 and HCT116 treated with 0.7 μM doxorubicin (Dox) or 1 μM ATRA for 24 h and measuring expression of CYP26A1. (d) Luciferase reporter assay with a RA reporter. PA-1 and HCT116 were transfected with a Stra6 plasmid or an empty vector and then treated with 0.7 μM doxorubicin (Dox), 10 μM retinol (Ret) or 1 μM ATRA (ATRA) for 24 h. Results show mean of three experiments and error bars represent S.D. (e) Graphic summary of our findings (in grey) in the context of p53 DNA damage responses
We next used a luciferase reporter assay driven by a RA-responsive promoter, which confirmed that neither DNA damage nor transfected Stra6 induced RA signalling in HCT116 (Figure 6d and Supplementary Figure 2A). This was also the case in PA-1, in which both retinol and ATRA were able to activate the reporter (Figure 6d and Supplementary Figure 2B). These results together strongly suggest that the role of Stra6 in the p53 pathway is independent of the activation of downstream RA signalling.
Discussion
In this study, we describe a novel function of the RA-responsive gene, Stra6, in p53-mediated apoptosis. p53 is a transcription factor that in response to different kinds of stress elicits cellular responses like arrest or death through upregulation of its target genes.38 Several p53-induced genes with pro-apoptotic capabilities have already been described, but due to the complexity of the p53 pathway,39 it is likely that many are still uncharacterized.
We provide insights as to previously unknown modulation of p53 functions by genes involved in RA signalling. We show that Stra6 levels increase in a p53-dependent manner after exposure to different kinds of damaging agents, both in normal and cancer cells. Its importance in p53-induced apoptosis is shown by the fact that Stra6 inhibition severely reduced the percentage of dead cells after stress, similar to what happens when other key apoptotic effectors of p53 are suppressed (see Figure 3d). This shows the apoptotic potential of Stra6, and links it to tumour suppressor mechanisms, consistent with the fact that Stra6 mutations have been found in the stomach, breast, skin and brain cancers (according to the Sanger Institute's COSMIC database), and that Stra6 is downregulated in the breast, lung and colon cancers, among others (according to databases accessed through Oncomine). More data will be needed to explore the possible antitumoural role of Stra6.
Stra6 is a transmembrane protein that binds to holo-RBP and internalizes retinol. Thus, the potential mechanisms of the action of Stra6 as part of the apoptotic machinery were not evident. A logical hypothesis was that it could be acting through the death receptors located at the cellular membrane, which have been shown to be activated both by RA16 and p53 (see Figure 1a). However, we found that Stra6 had no effect on TRAIL-induced apoptosis. Instead, it affected the polarization of mitochondria, a key step in the cascade of events that lead to apoptosis via the intrinsic pathway, and the intracellular levels of ROS.
We hypothesize that Stra6-induced ROS are central to its ability to trigger apoptosis (Figure 6e). This is supported by our results showing that cells that were more sensitive to doxorubicin (a known oxidant) also responded to Stra6 with higher levels of cell death (see Figure 2c). Moreover, Stra6 expression could turn an initial arrest response to p53 into cell death (see Figure 2d), similar to what can be achieved with oxidants.33 Indeed, we have previously shown that ROS can influence cell fate decisions after p53 upregulation, and we proposed that ROS increases can convert an arrest response into apoptosis when a threshold of oxidative damage is reached.33 This model would also explain the mechanism by which Stra6 has a role in p53 cell fate decisions: by increasing intracellular oxidants, it would ensure the commitment to the cell death pathway. On its own, Stra6 would induce apoptosis preferably in cells that are more sensitive to oxidants (such as PA-1 and A1847), because their threshold of sensitivity to oxidative stress would be lower. Given the fact that Stra6 can affect mitochondria functions (see Figure 4d), these oxidants are likely to be released from the mitochondria, the main source of intracellular ROS. Further studies will be essential to test this hypothesis.
It is important to note that the induction of apoptosis by Stra6 did not require the activation of downstream RA target genes. This was supported by all the experiments performed in HCT116, a cell that is known to be RA-insensitive,37 and by the fact that Stra6 was not able to induce the expression of downstream RA target genes or a RA reporter neither in these nor in cells with normal RA responses (see Figure 6). Thus, the roles of Stra6 in the RA and p53 pathways are likely to be independent. We propose that these pleiotropic functions of Stra6 could be determined by its subcellular localization. According to this model, when in the cellular membrane, Stra6 would play a role in RA signalling, being the receptor responsible for the internalization of retinol, and triggering the cascade of events that lead to the induction of RA-responsive genes. However, after damage, the pool of Stra6 present in the cytosol would participate in p53-mediated responses by acting on the mitochondria and increasing ROS levels (Figure 5e). It remains to be seen whether cytoplasmic Stra6 is its full-length form, a truncated one or even a different isoform. Characterization of the proteins that co-operate with Stra6 in different cellular locations would also help define how it interacts with mitochondria and the other components of the intrinsic pathway in order to perform its pro-apoptotic activity.
We believe that our findings not only help clarify the intricate biological processes activated after genotoxic stress, but also propose new therapeutic avenues for cancer. Our data suggest that cells with wild-type p53 could be primed to undergo apoptosis in response to chemotherapeutic agents, by increasing the expression of Stra6. As Stra6 is a RA-responsive gene, this could be achieved in target cells by treatment with retinoids. In fact, it has already been shown that exposure of colon cancer cells to RA results in accumulation of Stra6.36 The same is true in normal fibroblasts treated with ATRA (Supplementary Figure 1C). Moreover, oral vitamin A supplements induce Stra6 expression in mice.40 Treating mice harbouring wnt-1-expressing breast tumours with RA, resulted in increased Stra6 mRNA in cancer but not in normal cells, possibly due to a wnt1-dependent enhancement of RA signalling.41 According to our results, Stra6 could be more effective as an enhancer of apoptosis in cancer cells with higher sensitivity to oxidants, consistent with our previous results showing that cell-specific sensitivity to oxidative stress can determine the induction of cell death instead of arrest.42 Therefore, all these data suggests that retinoids could potentially be used as adjuvants of chemo- and radiotherapy in tumours that retain responsiveness to RA and a certain functionality of the p53 pathway. This also anticipates that vitamin A, a relatively cheap and safe compound, could be successfully used in chemoprevention, as it would increase the basal levels of Stra6 expression and thus enhance the tumour suppressor functions of p53.
Cell fate decisions after p53 induction are determined by a sophisticated system of interacting factors that is not completely understood.39 Our results add another element to this network, but more importantly, they provide a novel pathway of modulation of p53 functions by vitamin A, and its active metabolites that may be exploited in cancer therapy and prevention in the future.
Materials and Methods
Cell Culture
EJ bladder cancer cells with a tet-regulated expression system (EJp5331) were maintained in DMEM supplemented with 10% FBS, penicillin–streptomycin (50 units/ml), hygromycin (100 μg/ml) and geneticin (750 μg/ml), plus 1 μg/ml tet to repress the expression of p53. EJp53 were provided by Dr. Stuart Aaronson (Mount Sinai School of Medicine, New York, NY, USA). HCT116, HCT116 p53−/−, HCT116 Bax−/−, PA-1, A1847, 501T, IMR90 and 293 were maintained in DMEM supplemented with 10% FBS and penicillin–streptomycin (50 units/ml). Fresh medium was added at least every 3 days. Cells were treated with different concentrations of doxorubicin (Dox, Sigma-Aldrich, St Louis, MO, USA), tert-butyl-hydroperoxide (tBH, Sigma-Aldrich), H2O2 (Sigma-Aldrich), ATRA (Sigma-Aldrich), retinol (Sigma-Aldrich) or TNF-related apoptosis-inducing ligand (TRAIL, recombinant, Sigma-Aldrich). Chemicals were added to the culture media, and were not removed until analysis was performed, or when media was changed, as specified. For UV treatments, medium was removed and cells were kept under a UV lamp for a total of 50 mJ/cm2.
Modulation of Stra6 expression
A full-length Stra6 cDNA clone in a pCMV6-XL4 vector was obtained from Origene, Rockville, MD, USA. Transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), following manufacturer's specifications. HCT116 stably expressing a Luciferase or a Stra6 shRNA were prepared using shRNA HuSH plasmids from Origene, following manufacturer's protocols. Selection of stable clones was performed with 1 μg/ml puromycin for 2 weeks.
FACS Analysis
Fluorescent stained cells were transferred to polystyrene tubes with cell-strainer caps (Falcon) and subjected to FACS (Becton, Dickinson, Franklin Lakes, NJ, USA) FACSCalibur or FACSCanto II). Cell Quest 3.2 or FACSDiva 6.0 software (Becton Dickinson) was used for acquisition and analysis.
Cell cycle analysis
Cells were stained with PI, using the CycleTEST Plus DNA reagent kit (Becton Dickinson), following the instructions provided by manufacturer, and then analysed by FACS. SubG1 events were considered to represent dead cells.
Annexin/PI fluorescent staining
Cells were washed with PBS and stained with the Anenxin-V-Fluos Staining kit (Boehringer Manheim, Basel, Switzerland), following instructions provided by manufacturer, and then analysed by FACS. Cells positive for Annexin V were considered apoptotic.
Measurement of intracellular oxidation
Cells were incubated with 5 μg/ml 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen) for 30 min at 37 °C, then washed with PBS, trypsinized and collected in 0.5 ml of PBS, followed by FACS analysis. Values of mean fluorescence intensity were plotted.
Measurement of mitochondrial membrane potential
Cells were incubated with 5 μg/ml rhodamine 123 (Invitrogen) for 30 min at 37 °C, then washed with PBS, trypsinized and collected in 0.5 ml of PBS, followed by FACS analysis. Values of mean fluorescence intensity were plotted.
qRT-PCR
Medium was aspirated from the plates and cells were washed once with ice-cold PBS. Total RNA was extracted using TRIzol (Invitrogen), following manufacturer's protocols. RNA was resuspended in RNase-free water by passing the solution several times through a pipette tip. Total RNA was quantified using a Nanodrop ND8000 (Thermo Scientific, Waltham, MA, USA). cDNA was prepared from 1 μg of RNA with the Precision qScript Reverse Transcription kit (PrimerDesign, Southampton, UK) using oligo-dT primers, following manufacturer's instructions. The cDNA was diluted 1 : 10 and 5 μl were used for each reaction. Experiments were all performed in triplicate. Custom designed real-time PCR assay from PrimerDesign Ltd was used for the qRT-PCR with 2 × Precision Mastermix (PrimerDesign Ltd), following manufacturer's instructions. Reactions were carried out on a Roche Light Cycler 480 (Roche, Penzberg, Germany) under the following conditions: enzyme activation for 10 min at 95 °C, followed by 50 cycles of denaturation for 15 s at 95 °C and data collection for 60 s at 60 °C. A post PCR run melting curve was used to probe the specificity of the primers. Primers: ACTB (provided by PrimerDesign), TBP (5′-CACGAACCACGGCACTGATT-3′, 5′-TTTTCTTGCTGCCAGTCTGGAC-3′), Stra6 (5′-TCCTGCCTACCATCCTCCT-3′, 5′-AGACAGACCTCCACCCAAC-3′), RARα (5′-GGGCAAATACACTACGAACAAC-3′, 5′-GGCGAACTCCACAGTCTTAAT-3′), RARβ (5′-CTAAATACACCACGAATTCCAGTGCTGA-3′, 5′-CAGACGTTTAGCAAACTCCACGATCTTA-3′) and CYP26A1 (5′-TCACTTACCTGGGGCTCTACC-3′, 5′-ACTTGTTGTCTTGATTGCTCTTGC-3′).
Northern blot analysis
Total RNA was extracted using TRIzol (Invitrogen), following manufacturer's instructions. 20 μg RNA were loaded into a 1.6% agarose-formaldehyde gel with ethidium bromide for electrophoresis, and then transferred to a nylon membrane and crosslinked in a UV crosslinker at 125 mJ. A 32P-labelled Stra6 or TRAILR2 cDNA, isolated from the plasmid and purified through BioSpin columns (BioRad, Hercules, CA, USA), was used as a probe for hybridization, following standard protocols.
Immunoblot analysis
Cells were washed twice with ice-cold PBS and lysed using 500 μl of ProteoJET Mammalian Cell Lysis Reagent (Fermentas, Waltham, MA, USA) to extract cellular membranes or as described before,43 in the presence of 1 μg/ml Protease Inhibitor Cocktail Set III (Calbiochem, Darmstadt, Germany). Lysates were cleared by centrifugation at maximum speed for 15 min at 4 °C. Protein concentrations were then determined using Bradford protein assay (Fermentas). Twenty micrograms of total cell protein per sample were subjected to 10 or 6% SDS-PAGE and transferred to Immobilon-P membrane (Millipore, Darmstadt, Germany). An ECL detection system (Thermo Scientific) was used. p53 was detected with the 1801 antibody (ab28, Abcam, Cambridge, UK) and Stra6 with a rabbit polyclonal antibody (ab73490, Abcam). Levels of β-actin (ab3280, Abcam) or calnexin (rabbit polyclonal, Cell Signalling) were used as loading controls.
Measurement of oxidative damage to DNA
The relative levels of oxidative purine base damage were monitored using the human formamidopyrimidine DNA glycosylase (fpg) comet assay, as described previously for the human 8-oxoguanine DNA glycosylase 1 (hOGG1) comet assay (hOGG1 comet)44 with the following modifications: after lysis the slides were washed once with distilled water and immersed in three changes of enzyme digestion buffer (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA and 0.2 mg/ml bovine serum albumin (pH 8.0)), for 5 min each time, at room temperature. Fpg (Sigma-Aldrich) was added to the gel (50 μl/gel) at 1/500 or 1/1000 dilutions; gels were covered with a cover slip and incubated in a humidified chamber at 37 °C for 30 min; the cover slips were removed and the slides were placed in a horizontal electrophoresis tank. From this step onwards, the assay was performed as described.45 DNA damage was expressed as the percentage of DNA in the comet tails.
Immunofluorescence
Cells were split into 6-well plates containing sterile coverslips. Cells were transfected as described above with 4 μg of either Stra6 plasmid or an empty vector. After 24 h, media was aspirated from the plates and cells were washed three times with 1 × PBS. Cells were fixed using 1 ml of 4% paraformaldehyde for 30 min with gentle shaking. After fixing, cells were washed three times with 1 × PBS and permeabilized with 1 ml 0.1% Triton X-100 for 10 min. Cells were then washed three times with 1 × PBS and blocked with 1% BSA for 30 min. Coverslips were incubated with 100 μl 1 : 100 primary antibody (Stra6, Abcam; calnexin, Cell Signalling) overnight at 4 °C. The following day, coverslips were washed three times with 1 × PBS and incubated with 100 μl secondary anti-rabbit and anti-mouse antibody (Alexa Fluor 488 and 594, Invitrogen) for 45 min in the dark. After incubation, coverslips were washed three times with 1 × PBS and stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Invitrogen) for 10 min. Slides were labelled and the coverslips were mounted and sealed with transparent nail varnish. Slides were analysed using a Nokia TE300 semi-automatic microscope.
Luciferase assay
Cells were grown into 24-well plates and transfected with a pGL2 Promoter vector containing five DR1 consensus RAREs46 (generously provided by D. Wotton, University of Virginia) and co-transfected with a β-galactosidase plasmid as a transfection control. Medium was removed from the wells and cells were washed once with 1 × PBS. One hundred and forty microlitres of 1 × lysis buffer was added to each well and the plates were incubated on the shaker for 2 h at room temperature or for 30 min at −80 °C. For β-galactosidase, 80 μl of each cell lysate were transferred into a 96-well plate and 100 μl of β-galactosidase substrate was added to each well and incubated at 37 °C for 15 min. The absorbance was measured at 405 nm. For the luciferase assay, 20 μl of each cell lysate were transferred into a white 96-well plate. 50 μl of luciferase substrate (Promega, Madison, WI, USA) was dispensed into each well and light emission measurements were taken after 30 s.
Acknowledgments
We thank S A Aaronson for his help in the initial stages of this project. We thank S Cowley and M J Dyer for useful discussions and critical reading of the manuscript. We also thank K Straatman and the Advanced Image Facilities for assistance with immunofluorescence experiments. This work was funded by the MRC (www.mrc.ac.uk, New Blood Lectureship Research Support Funding). S Carrera held a predoctoral fellowship from CONACYT (www.conacyt.mx, fellow no.200721). Work in the lab of GDDJ is supported by the European Union (ECNIS, www.ecnis.org), Hope Against Cancer (www.hfcr.org) and Cancer Research UK (www.cancerresearchuk.org). S Cuadrado was a predoctoral fellowship holder from Junta de Castilla y León, Consejería de Educación (www.educa.jcyl.es). This work was also partially supported by grants from Fondo de Investigaciones Sanitarias (FIS, PI08/1813) from the Spanish Government (www.mineco.gob.es/) to E.V.
Glossary
- ATRA
all-trans retinoic acid
- DAPI
4′,6-diamidino-2-phenylindole, dihydrochloride
- DCF
2′,7′-dichlorodihydrofluorescein diacetate
- Dox
doxorubicin
- Fpg
formamidopyrimidine DNA glycosylase
- hOGG1
human 8-oxoguanine DNA glycosylase 1
- PI
propidium iodide
- RA
retinoic acid
- RBP
retinol binding protein
- ROS
reactive oxygen species
- tBH
tert-butyl-hydroxyperoxide
- TRAIL
TNF-related apoptosis-inducing ligand
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by RA Knight
Supplementary Material
References
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE, Werbin H. Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Bond J, Haughton M, Blaydes J, Gire V, Wynford-Thomas D, Wyllie F. Evidence that transcriptional activation by p53 plays a direct role in the induction of cellular senescence. Oncogene. 1996;13:2097–2104. [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Vousden KH. p53: death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored. Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. The molecular and genetic dissection of the retinoid signalling pathway. Gene. 1993;135:223–228. doi: 10.1016/0378-1119(93)90069-f. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nature reviews. Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- Park SH, Lim JS, Jang KL. All-trans retinoic acid induces cellular senescence via upregulation of p16, p21, and p27. Cancer Lett. 2011;310:232–239. doi: 10.1016/j.canlet.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Asou N. 2. All-trans retinoic acid in the treatment of acute promyelocytic leukemia. Intern Med. 2007;46:91–93. doi: 10.2169/internalmedicine.46.1780. [DOI] [PubMed] [Google Scholar]
- Taneja R, Bouillet P, Boylan JF, Gaub MP, Roy B, Gudas LJ, et al. Reexpression of retinoic acid receptor (RAR) gamma or overexpression of RAR alpha or RAR beta in RAR gamma-null F9 cells reveals a partial functional redundancy between the three RAR types. Proc Natl Acad Sci USA. 1995;92:7854–7858. doi: 10.1073/pnas.92.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud C, Bouillet P, Oulad-Abdelghani M, Dolle P. Restricted expression of a novel retinoic acid responsive gene during limb bud dorsoventral patterning and endochondral ossification. Dev Genet. 1996;19:66–73. doi: 10.1002/(SICI)1520-6408(1996)19:1<66::AID-DVG7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Heller J. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J Biol Chem. 1975;250:3613–3619. [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, et al. Retinoid content, visual responses and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci. 2012;53:3027–3039. doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, O'Byrne SM, Vreeland AC, Blaner WS, Noy N. Cross-talk between signalling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol Cell Bio. 2012;32:3164–3175. doi: 10.1128/MCB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci USA. 2011;108:4340–4345. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AK, Sugunan D, Kumar H, Anilkumar G. Case-control analysis of SNPs in GLUT4, RBP4 and STRA6: association of SNPs in STRA6 with type 2 diabetes in a South Indian population. PloS One. 2010;5:e11444. doi: 10.1371/journal.pone.0011444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- Munoz-Fontela C, Macip S, Martinez-Sobrido L, Brown L, Ashour J, Garcia-Sastre A, et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 2008;205:1929–1938. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungamuri SK, Benson EK, Wang S, Gu W, Lee SW, Aaronson SA. p53-mediated heterochromatin reorganization regulates its cell fate decisions. Nat Struct Mol Biol. 2012;19:478–484. doi: 10.1038/nsmb.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue MM, Shin DY, Lee SW, Aaronson SA. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA. 1997;94:9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis [see comments] Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, et al. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–4205. [PubMed] [Google Scholar]
- Sonneveld E, van den Brink CE, van der Leede BM, Schulkes RK, Petkovich M, van der Burg B, et al. Human retinoic acid (RA) 4-hydroxylase (CYP26) is highly specific for all-trans-RA and can be induced through RA receptors in human breast and colon carcinoma cells. Cell Growth Differ. 1998;9:629–637. [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nature reviews. Mol Cell Bio. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- Tan L, Wray AE, Ross AC. Oral vitamin A and retinoic acid supplementation stimulates antibody production and splenic Stra6 expression in tetanus toxoid-immunized mice. J Nutr. 2012;142:1590–1595. doi: 10.3945/jn.112.161091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice DA, Szeto W, Soloviev I, Rubinfeld B, Fong SE, Dugger DL, et al. Synergistic induction of tumor antigens by Wnt-1 signaling and retinoic acid revealed by gene expression profiling. J Biol Chem. 2002;277:14329–14335. doi: 10.1074/jbc.M200334200. [DOI] [PubMed] [Google Scholar]
- Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, et al. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem. 2012;287:9845–9854. doi: 10.1074/jbc.M111.250357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera S, de Verdier PJ, Khan Z, Zhao B, Mahale A, Bowman KJ, et al. Protection of cells in physiological oxygen tensions against DNA damage-induced apoptosis. J Biol Chem. 2010;285:13658–13665. doi: 10.1074/jbc.M109.062562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte TL, Cooke MS, Jones GD. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic Biol Med. 2009;46:78–87. doi: 10.1016/j.freeradbiomed.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Duarte TL, Almeida GM, Jones GD. Investigation of the role of extracellular H2O2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol Lett. 2007;170:57–65. doi: 10.1016/j.toxlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bartholin L, Powers SE, Melhuish TA, Lasse S, Weinstein M, Wotton D. TGIF inhibits retinoid signalling. Mol Cell Biol. 2006;26:990–1001. doi: 10.1128/MCB.26.3.990-1001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.