Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF receptor ligand family that was discovered in the mid-nineties.1, 2 The TRAIL system comprises four transmembrane TRAIL receptors, two of which are agonistic receptors that signal to cell death (i.e., TRAIL-R1, TRAIL-R2), while the other two are antagonistic receptors (i.e., TRAIL-R3, TRAIL-R4) that do not transmit a death signal and thus can confer resistance toward TRAIL-induced apoptosis.3 As TRAIL can bind to any of these four receptors, relative expression levels of TRAIL receptors on the cell's surface determine, at least in part, the outcome of the TRAIL response. Thus, based on this organization of the TRAIL receptors, there is already an inbuilt dichotomy in the TRAIL system, which has been implicated in the higher susceptibility of cancer versus normal non-malignant cells to TRAIL-induced cell death.
Besides this heterogeneity of the TRAIL system comprising both agonistic and antagonistic cell surface receptors, there has been accumulated evidence over the last years indicating an additional level of dichotomy within the TRAIL signaling network in human cancers (Figure 1). On one side, TRAIL activates caspase-dependent apoptosis or non-apoptotic cell death pathways in TRAIL-sensitive tumor cells. On the other side, there are now numerous reports showing that TRAIL can also stimulate non-apoptotic pathways via TRAIL receptors. The engagement of these survival signaling cascades not only interferes with TRAIL-induced apoptosis, thereby conferring resistance toward TRAIL, but can also elicit several additional biological effects that contribute to the malignant phenotype of human cancers, including proliferation, invasion, migration and metastasis.4 Importantly, this dichotomy implies that the agonistic TRAIL receptors TRAIL-R1 and TRAIL-R2 can also engage cell survival pathways, depending on the cellular context. Thus, despite its name, that is, apoptosis-inducing ligand, and despite the fact that TRAIL belongs to the family of death receptor ligands, the induction of apoptosis is only one of the biological effects that is elicited by TRAIL in cancer cells.
Figure 1.
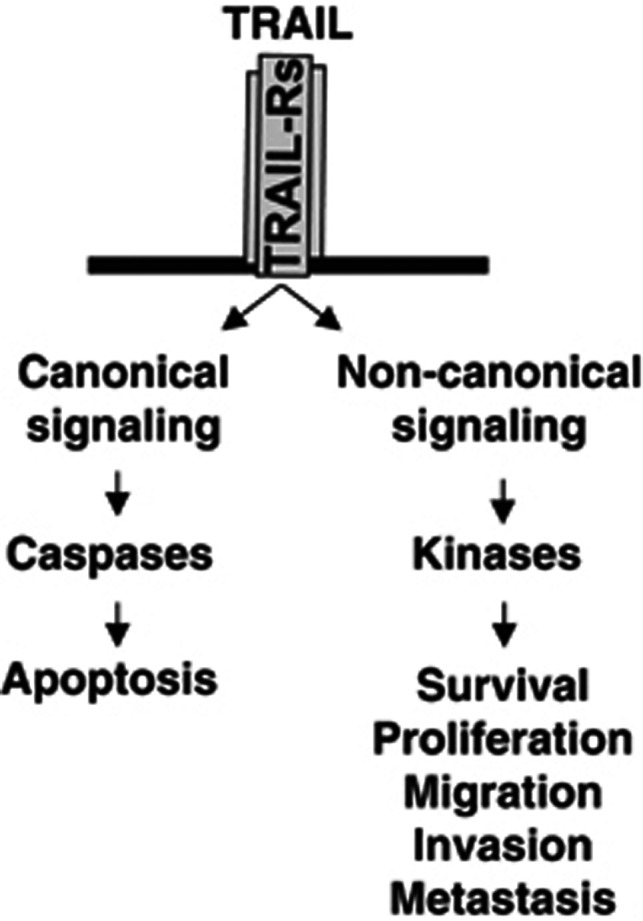
Dichotomy of TRAIL signaling. Binding of TRAIL to agonistic TRAIL receptors either activates canonical signal-transduction pathways resulting in caspase activation and apoptosis or alternatively can engage non-canonical signaling cascades, leading to increased proliferation, invasion, migration and metastasis
The review by Azijli5 in this issue of Cell Death & Differentiation focuses on this ‘dark' side of the death ligand TRAIL, namely the engagement of non-canonical survival signaling pathways, which is less well studied compared with the cell death-inducing properties of TRAIL. The authors provide a comprehensive review of the molecular mechanisms that are involved in the regulation of this part of the tumor biology of TRAIL. In particular, they discuss non-canonical kinase signaling events including various kinases such as RIP1, IκB/ NF-κB, MAPK p38, JNK, ERK1/2, MAP3K TAK1, PKC, PI3K/Akt and Src.
It is important to note that the so-called agonistic TRAIL receptors TRAIL-R1 and TRAIL-R2 not only trigger apoptosis in TRAIL-sensitive cells but are also capable to activate survival pathways in tumor cells that resist the induction of cell death upon exposure to TRAIL. The fact that the very same receptors can signal to both cell death and cell survival depending on the cellular context highlights that additional factors beyond the mere presence of agonistic TRAIL receptors on the cell surface eventually determine the outcome of signaling events. At present, little is yet known about the molecular events that control cell death versus survival signaling downstream of agonistic TRAIL receptors.
The functional dichotomy of the TRAIL system has important implications for cancer therapy with TRAIL. In principle, recombinant soluble TRAIL or fully humanized TRAIL receptor antibodies are considered as promising strategies for the treatment of cancer, as they can preferentially engage cell death programs in cancer cells while sparing normal, non-malignant cells.6 However, the engagement of survival signaling cascades, for example, in TRAIL-resistant cancers, is obviously counterproductive for cancer therapy with TRAIL, as it can not only confer treatment resistance but may also elicit a variety of additional, potentially harmful effects, for example, increased proliferation, invasion, migration and metastasis. The fact that under certain conditions the agonistic TRAIL receptors TRAIL-R1 and TRAIL-R2 can mediate these tumor-promoting activities of TRAIL implies that these unwanted effects are not restricted to soluble TRAIL that can bind to all four TRAIL receptors, but also apply to antibodies that are specifically designed to target one of the two apoptosis-inducing TRAIL receptors. Therefore, the use of TRAIL receptor-specific antibodies will likely not be sufficient to avoid these tumor-promoting activities of TRAIL. Moreover, it is currently unclear whether TRAIL triggers such survival signaling events exclusively in cancers that are resistant toward TRAIL-induced apoptosis. Alternatively, TRAIL may simultaneously engage cell death and survival pathways also in tumors that are in principle sensitive toward TRAIL. The latter possibility would imply that the TRAIL-stimulated engagement of survival pathways in a subpopulation of cells within a tumor may facilitate the development of acquired resistance toward TRAIL that may eventually contribute to tumor relapse. Another implication relates to the therapeutic window of TRAIL receptor agonists for the treatment of cancer. So far, the preferential induction of apoptosis in malignant versus non-malignant cells has been considered as a particular strength of TRAIL-based therapeutics that could be exploited for cancer therapy with TRAIL, as it may provide a therapeutic window to preferentially kill tumor cells with little toxicity against normal cells and tissues. However, as similar non-canonical signal-transduction pathways are activated by TRAIL in non-transformed normal cells as in TRAIL-resistant tumor cells, disruption with these survival pathways to augment the antitumor activity of TRAIL may in parallel also increase toxic side effects in normal cells, thereby diminishing the therapeutic window of TRAIL-based therapeutics. While data, so far, indicate that combination therapies that interfere with TRAIL-stimulated survival pathways may preferentially sensitize cancer but not normal cells for TRAIL, the underlying molecular mechanisms for this differential sensitization have not yet been unraveled. This indicates that much more research is required to explore the question whether a therapeutic window for the use of TRAIL receptor agonists may exist in clinical settings. Ultimately, the goal is the targeted induction of cell death by TRAIL specifically in tumor cells, while sparing normal cells.
At present, TRAIL receptor agonists including soluble recombinant TRAIL ligand or fully human TRAIL receptor antibodies are under evaluation in clinical trials for the treatment of cancer.6 This highlights the relevance and actuality of research aiming at a better understanding of the full range of biological activities, including activation of survival pathways that can be engaged by TRAIL receptors in malignant and non-malignant cells.
The author declares no conflict of interest.
References
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- Roder C, Trauzold A, Kalthoff H. Impact of death receptor signaling on the malignancy of pancreatic ductal adenocarcinoma. Eur J Cell Biol. 2011;90:450–455. doi: 10.1016/j.ejcb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Azijli K, Weyhenmeyer B, Peters GJ, de Jong S, Kruyt FA.Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family Cell Death Differ 2013. e-pub ahead of print 12 April 2013. [DOI] [PMC free article] [PubMed]
- Yang A, Wilson NS, Ashkenazi A. Proapoptotic DR4 and DR5 signaling in cancer cells: toward clinical translation. Curr Opin Cell Biol. 2010;22:837–844. doi: 10.1016/j.ceb.2010.08.001. [DOI] [PubMed] [Google Scholar]


