Rapid tumor cell proliferation frequently outpaces adequate vascularization, resulting in a limited cellular oxygen supply. Low cellular oxygen, ‘hypoxia' is often associated with necrotic tumor foci, but also with the selection of aggressive survival mutations. Interplay between tumor cells and the associated stroma (including endothelial cells) is critical in determining tumor progression.1 A rate-limiting ‘angiogenic switch' in response to hypoxia promotes tumor vascularization. The consequence is a partial restoration of oxygen to the growing tumor edge and this is repeated with tumor expansion.2 The molecular processes mediating these transitions are of potential therapeutic relevance. Two pathways that are central to the hypoxic response in cancers are regulated by (1) the major tumor suppressor p53; and (2) the crucial modulator of new vasculature, ‘neo-angiogenesis', vascular endothelial growth factor (VEGF). The study by Ghahremani et al.3 in CDD 2013 provides a new insight into the complex cross-talk between these two pathways under hypoxic conditions relevant to tumorigenesis. The consequences for tumor vascularization of disrupting these pathways, define the major novelty of this study, with important implications for p53-targeted anti-cancer therapy.
Ghahremani et al.3 demonstrated the novel finding that induction of VEGF transcription during acute hypoxia occurred in a p53-dependent manner. This surprising induction occurred following exposure to hypoxia for 4 h in non-transformed mouse embryo fibroblasts (MEFs). This paralleled a modest elevation of VEGF protein. In normal tissue subjected to hypoxic damage, the anticipated function of a rapid induction of VEGF would be to promote healing; as poor wound recovery is associated with low oxygen levels.4 This suggests a prosurvival function for VEGF in hypoxia, which could have catastrophic consequences if unrestrained. In the following chronic phase, measured at 24 h, mRNA levels of VEGF were lower relative to 4 h, but remained elevated above basal levels (Figure 1). The protein levels of VEGF were higher at 24 h than at 4 h.3 Additional incremental measures will be vital to identify the peak of VEGF expression. Importantly, the quenching of VEGF induction after 24 h of sustained hypoxia3 suggests that strict regulation of a potent stimulant of new vasculature is critical to healthy cell maintenance.
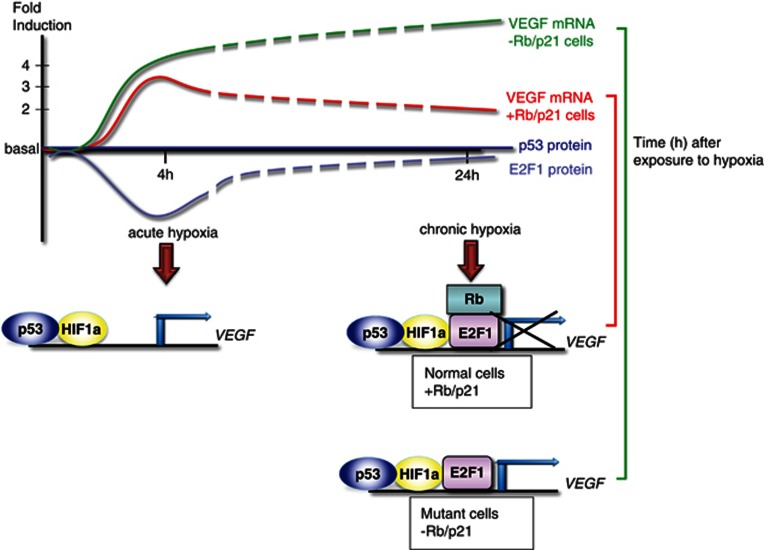
Figure 1.
VEGF is subject to complex regulation in response to acute and chronic hypoxia (as previously defined21). After 4 h of hypoxia (acute phase), p53 and HIF-1α induce VEGF transcription leading to a significant induction of mRNA. After 24 h (chronic phase) VEGF mRNA levels drop, but remain well above basal levels. In parallel time, E2F1 protein levels decrease in the acute phase and then normalize with extended hypoxia, while p53 protein levels were not observed to change.3 A role for E2F1 and Rb in the repression of VEGF is proposed, where E2F1 in the absence of Rb induces VEGF expression8
The mechanisms that dictate the distinct VEGF expression in the acute and chronic hypoxic responses are of clinical relevance. The elevated expression of VEGF associated with the acute response was unexpectedly attributed to the concerted binding of p53 and hypoxia-inducible factor 1α (HIF-1α, a major regulator of the hypoxic response) to a specific promoter region of VEGF3 (Figure 1). A provocative question then is, how is this induction switched to repression upon prolonged exposure to hypoxia?
In a previous study of primary cells,5 comparable chronic hypoxia provoked growth arrest, accompanied by HIF-1α activation. HIF1α was found to induce p21 expression and hypophosphorylation of the retinoblastoma protein (pRb), in a p53-independent manner.5 In contrast to this and other studies (reviewed in Sermeus and Michiels6), Ghahremani et al.3 did not observe an elevation in p53 and p21 protein levels under chronic hypoxia, the reason for which remains unclear. Further, it would be important to examine whether the cellular fate is also different. Ghahremani et al.3 found an inverse correlation between elevated levels of E2F1 and decreased VEGF transcription in MEFs in response to chronic hypoxia. This is consistent with the finding in ischemic mice, where downregulation of VEGF required the concerted action of p53 with E2F1.7 The presence of pRb was shown to be critical for this repression.8 In line with these findings, Ghahremani et al.3 showed that in the absence of pRb/p107 or p21, VEGF levels were accumulated unimpeded in a p53/E2F1-dependent manner.3 In Figure 1, we propose a simplistic model integrating the current literature that may offer some clues to the hypoxic regulation of VEGF in healthy cells. This does not exclude additional regulators, in particular the involvement of Mdm2, by mechanisms which are yet to be explored.3
The consequences of VEGF disregulation is a major focus of the Ghahremani's study.3 Tumor vascularization was more extensive when human colon cancer cells (HCT116) were co-injected with p21-null MEFs than with wt MEFs. This increased vascularization was associated with elevated VEGF mRNA levels.3 This suggests that induction of VEGF in the context of compromised p21 in non-transformed cells is a vital dictator of tumor angiogenesis.
A direct impact of tumor cells on angiogenesis was also demonstrated.3 Engrafted retinoblastoma cells, lacking an intact Rb pathway, were unexpectedly identified to exhibit a positive association between p53, elevated VEGF protein levels and increased tumor vascularization. Relevant to an influence of the tumor on the stroma, the Rb-E2F1 and Mdm2-p53 pathways are defective in the majority, if not all human tumors,9 and p21 depletion has also been reported in gastric10 and colorectal cancers.11 Identification of mutations associated with the stroma, in comparison, is extremely controversial, however methylation of key targets may be important for activation of the stromal fibroblasts (reviewed in Shimoda et al.12). The work of Ghahremani et al.3 emphasizes the critical importance of VEGF to dictate angiogenesis and indicates the need to consider the tumor microenvironment in its entirety when designing therapy.
Cancer therapy through reactivation of the function of the major tumor suppressor p53 is an earnest campaign that may either be achieved through suppressing its negative regulators or correcting mutated structure (reviewed in Lane et al.13). Pertinently, mutant p53 itself has also been associated with elevated VEGF levels (through induction of EGR1).14 The relevance of this for tumorigenesis is that p53 is mutated in at least 50% of all cancers. Whether it is altered also in stromal cells is highly controversial (reviewed in Addadi et al.15). The studies by Ghahremani et al.3 suggest that reactivating p53 in a context where VEGF levels are elevated potentially has diabolical consequences for tumor progression.
Another alarming implication of these studies3 is its challenge of the dogma that p53 function is always beneficial in the struggle against cancer. This finding adds to a recent report in breast cancer that treatment was less effective in the context of functional p53.16 Specifically in the current study, under hypoxia, elevated VEGF levels were associated with increased p53 levels (although the fate of these cells in vitro was not described), and retinoblastomas were more extensively vascularized in the presence of p53.3 These findings suggest that reactivation of p53 in a context of elevated VEGF levels would promote vascularization of human tumors, which would be anticipated to offer a dangerous tumor expansion capacity.3
Despite enormous initial rational expectations that VEGF inhibition would curb primary cancers and thwart metastasis and invasion (championed by Folkman17), the bitter facts are that more aggressive metastatic tumors frequently stem from these therapies (review in Coleman and Ratcliffe18). Apparently logical expectations of benefit from the co-administration of p53 activators, together with VEGF inhibitors, should be treated with caution. Lessons from the retinoblastoma model may be pertinent,3 where p53 activation only in the context of dual attack on an additional distinct pathway (i.e., the topoisomerase I inhibitor) was effective.19 Together these studies indicate the growing realization that effective cancer therapies will best result from individually tailored drug combinations, rationally designed on the basis of the individual cancer molecular profile and tested in an appropriate model.20
Acknowledgments
The work in the authors lab is supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (NHMRC #1026988, #1026999 and 1049179), by a grant from the CASS Foundation, the Victorian Cancer Agency (CAPTIV), PCF, Cancer Council Victoria and by the VESKI award. YH is NHMRC Senior Research Fellow (NHMRC #628426).
The authors declare no conflict of interest.
References
- Pietras K, Ostman A. Exp Cell Res. 2010. pp. 1324–1331. [DOI] [PubMed]
- Bergers G, Benjamin LE. Nat Rev Cancer. 2003. pp. 401–410. [DOI] [PubMed]
- Farhang Ghahremani, et al. Cell Death Differ 2013. e-pub ahead of print 1 March 2013; doi: 10.1038/cdd.2013.12 [DOI]
- Schugart RC, Friedman A, Zhao R, et al. Proc Natl Acad Sc USA. 2008. pp. 2628–2633. [DOI] [PMC free article] [PubMed]
- Goda N, et al. Mol Cell Biol. 2003. pp. 359–369. [DOI] [PMC free article] [PubMed]
- Sermeus A, Michiels C. Cell Death Dis. 2011. p. e164. [DOI] [PMC free article] [PubMed]
- Qin G, et al. Proc Natl Acad Sci USA. 2006. pp. 11015–11020. [DOI] [PMC free article] [PubMed]
- Pillai S, Kovacs M, Chellappan S. Cancer Res. 2010. pp. 4931–4940. [DOI] [PMC free article] [PubMed]
- Polager S, Ginsberg D. Nat Rev Cancer. 2009. pp. 738–748. [DOI] [PubMed]
- Ogawa M, et al. Br J Cancer. 1997. pp. 1617–1620. [DOI] [PMC free article] [PubMed]
- Ogino S, et al. Cancer Epidemiol Biomarkers Prev. 2009. pp. 2513–2521. [DOI] [PMC free article] [PubMed]
- Shimoda M, Mellody KT, Orimo A. Semin Cell Dev Biol. 2010. pp. 19–25. [DOI] [PMC free article] [PubMed]
- Lane DP, Cheok CF, Lain S. Cold Spring Harbor Perspect Biol. 2010. p. a001222. [DOI] [PMC free article] [PubMed]
- Weisz L, et al. Cancer Res. 2004. pp. 8318–8327. [DOI] [PubMed]
- Addadi Y, et al. Cancer Res. 2010. pp. 9650–9658. [DOI] [PMC free article] [PubMed]
- Jackson JG, Lozano G.Oncogene 2013. e-pub ahead of print 14 January 2013; doi: 10.1038/onc.2013.610 [DOI]
- Folkman J. N Engl J Med. 1971. pp. 1182–1186. [DOI] [PubMed]
- Coleman ML, Ratcliffe PJ. Nat Med. 2009. pp. 491–493. [DOI] [PubMed]
- Laurie NA, et al. Nature. 2006. pp. 61–66. [DOI] [PubMed]
- Chen Z, et al. Nature. 2012. pp. 613–617. [DOI] [PMC free article] [PubMed]
- Pires IM, et al. Cancer Res. 2010. pp. 925–935. [DOI] [PMC free article] [PubMed]