Abstract
Background:
Due to the high prevalence of overweight and obesity there is a need to identify cost-effective approaches for weight loss in primary care and community settings.
Objective:
We evaluated the cost effectiveness of two weight loss programmes of 1-year duration, either standard care (SC) as defined by national guidelines, or a commercial provider (Weight Watchers) (CP).
Design:
This analysis was based on a randomised controlled trial of 772 adults (87% female; age 47.4±12.9 years; body mass index 31.4±2.6 kg m−2) recruited by health professionals in primary care in Australia, United Kingdom and Germany. Both a health sector and societal perspective were adopted to calculate the cost per kilogram of weight loss and the ICER, expressed as the cost per quality adjusted life year (QALY).
Results:
The cost per kilogram of weight loss was USD122, 90 and 180 for the CP in Australia, the United Kingdom and Germany, respectively. For SC the cost was USD138, 151 and 133, respectively. From a health-sector perspective, the ICER for the CP relative to SC was USD18 266, 12 100 and 40 933 for Australia, the United Kingdom and Germany, respectively. Corresponding societal ICER figures were USD31 663, 24 996 and 51 571.
Conclusion:
The CP was a cost-effective approach from a health funder and societal perspective. Despite participants in the CP group attending two to three times more meetings than the SC group, the CP was still cost effective even including these added patient travel costs. This study indicates that it is cost effective for general practitioners (GPs) to refer overweight and obese patients to a CP, which may be better value than expending public funds on GP visits to manage this problem.
Keywords: cost effectiveness, commercial provider, standard care, weight loss
Introduction
The prevalence of overweight and obesity is placing a substantial burden on health-care resources, even in developed countries.1 Overweight and obesity accounts for 44% of the global burden of type 2 diabetes mellitus, 23% of ischaemic heart disease and 7–41% of certain cancers.2 Therefore obesity management programmes that are both efficacious and cost effective are needed. Policy makers are increasingly seeking evidence of the cost effectiveness of interventions. It is important to know whether it is more cost effective to support and fund programmes already in place or subsidise others (including extant commercial weight loss programmes).
A partnership between primary-care providers and commercial organisations may be a practical approach, whereby participants can benefit from early lifestyle intervention for weight management. Observational data3, 4 show that this approach has the potential to deliver weight management programmes at the necessary scale in a community setting and at potentially relatively low cost. Our recent 12-month randomised controlled trial (RCT) involving three countries (Australia, the United Kingdom and Germany) showed referral to a commercial weight-loss community intervention programme (Weight Watchers—commercial provider (CP)) produced greater weight loss compared with standard care (SC).5 Similar efficacy of this CP has been demonstrated in other RCTs.6, 7 However, the cost effectiveness of CPs over SC has not been estimated. We calculated this using data from the above trial.5 Previous estimates of the cost of the CP have been done, but were small scale and used limited data.8
Our aim was to evaluate the cost effectiveness of a CP compared with conventional SC for both weight loss and quality of life (QOL). A societal perspective was also adopted as we have previously reported that those attending the CP had more frequent visits,5 which may have contributed to the success of the CP.
Methods
Clinical trial
This cost-effectiveness analysis used data from an RCT whereby overweight and obese adults were randomised to receive 12-month access to a CP or SC by a primary-care provider in Australia, the United Kingdom and Germany. Participants were recruited by their general practitioners (GPs) and randomised to one of the two groups. A full list of inclusion and exclusion criteria, as well as a description of the two intervention groups are in the report of the primary findings from the study.5 All participants were aged ⩾18 years with a body mass index of 27–35 kg m−2, and had at least one risk factor for obesity-related disease. Participants randomised to the CP group received vouchers to attend a weekly community CP meeting. Those randomised to SC received weight-loss advice delivered by a GP/primary care professional at their local medical practice. The frequency of these SC visits was at the discretion of the GP and the participant. The frequency of such visits was recorded, with GP visits only being counted for SC. GPs and primary-care professionals were provided with and encouraged to use relevant national clinical guidelines for weight management.5
Health economic evaluation
A health-care funder and societal perspective was adopted for the cost-effectiveness evaluation and included direct health costs to the government or patient arising from costs of intervention delivery and costs to patients for participation.
Costing data
Commercial programme
The costing for the CP group was based on a monthly payment plan, which included unlimited access to meetings and online electronic web tools. The cost of the referral visit to the CP was also included in the costing.
Standard care
For SC, the cost applied was that of a consultation lasting 20 minutes or less with a GP. In the United Kingdom, all consults provided in the SC group were by a nurse.
Valuation of costs
The cost of the CP for each country was sourced directly from Weight Watchers International, Inc. (New York, NY, USA). Unit costs for all other intervention and conventional resources were obtained from the relevant governments/health-care authorities (Table 1). Costs were estimated for patient travel to attend either CP or SC consultations. This was based on the assumption that patients travelled within a 10-km radius to their intervention. The number of participant visits to either the CP or SC was recorded throughout the 1-year study.
Table 1. CP and SC group costs and sources.
| Resource | Unit cost (local currency) | Unit cost (USD) | Average number of visits over 12 months | 12-month cost (USD) | Source |
|---|---|---|---|---|---|
| CP | |||||
| WW attendance | |||||
| Australia | $59.95 AUD per month | $60.55 | 33.0 | $726.60 | www.weightwatchers.com.au |
| United Kingdom | £12.95 GBP per 1st month then £19.99 per month | $21.24/1st month then $32.78 | 36.4 | $381.86 | www.weightwatchers.co.uk |
| Germany | €39 EUR per month | $55.38 | 23.1 | $664.56 | www.weightwatchers.de |
| Primary-care referral | |||||
| Australia | $34.90 per participant | $35.25 | 1.0 | $35.25 | MBS9—Item 23 |
| United Kingdom | £36 per participant | $59.04 | 1.0 | $59.04 | PSSRU10 |
| Germany | €19.17 per participanta | $27.22 | 1.0 | $27.22 | Kassenärztliche Bundesvereinigung11 |
| Medication | |||||
| Australia (Figure 1) | $445.70 for 12 months | $450.16 | $450.16 | PBS12 | |
| Patient travel | |||||
| Australia | $12.60 per round trip | $12.73 | 36.4 | $419.96 | ATO13 |
| United Kingdom | £5.00 per round trip | $8.20 | 36.4 | $298.48 | PSSRU,10 HMRC14, 15, 16 |
| Germany | €6.00 per round trip | $8.52 | 23.1 | $196.81 | |
| SC | |||||
| General Practitioner consult | |||||
| Australia | $34.90 | $35.25 | 10.7 | $377.16 | MBS9—Item 23 |
| United Kingdom (Nurse) | £12 | $19.68 | 13.3 | $261.74 | PSSRU10 |
| Germany | €19.17a | $27.22 | 11.3 | $307.60 | Kassenärztliche Bundesvereinigung11 |
| Dietetic/psychology consult | |||||
| Australia | $59.90 | $60.50 | 0.066 | $3.99 | MBS9—Items 10 954/10968 |
| United Kingdom | £57.5 | $94.30 | 0.066 | $6.22 | PSSRU10 |
| Germany | €60 | $85.20 | 0.066 | $5.62 | Schulungs-Gemeinschaft München Ost e.V17 |
| Medication | |||||
| Australia (Figure 1) | $500.60 for 12 months | $505.61 | $505.61 | PBS12 | |
| Patient travel | |||||
| Australia | $12.60 per round trip | $12.73 | 10.7 | $137.01 | ATO13 |
| United Kingdom | £5.00 per round trip | $8.20 | 13.3 | $109.60 | PSSRU,10 HMRC14, 15, 16 |
| Germany | €6.00 per round trip | $8.52 | 11.3 | $96.84 | |
Abbreviations: ABS, Australian Bureau of Statistics; ATO, Australian Taxation Office; AUD, Australian dollar; CP, commercial provider; EUR, Euro; GBP, Great Britain pound; MBS, Medicare Benefits Schedule; ONS, Office for National Statistics; PBS, Pharmaceutical Benefits Scheme; PSSRU UK, Personal Social Services Research Unit; SC, standard care. At the time of writing (early 2011), 1 AUD was 1.01 USD; 1 GBP was 1.64 USD; 1 EUR was 1.42 USD according to XE currency exchange (http://www.xe.com).
For Germany, a general GP visit was calculated as GPs are not able to charge a fee for weight-loss advice.
Opportunity costs of employment were not considered because participants could attend their intervention outside working hours, during their lunch break, or on weekends. Childcare costs were not considered as children of any age are welcome at the CP meetings and can accompany their parent to an SC visit.
Australia
In Australia, medical care is priced by the Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS). Professional attendances were considered as ‘Group A1—general practitioner attendances to which no other item applies (level B)'—MBS item 23.
United Kingdom
The cost of a GP referral and nurse consultation were calculated according to the Personal Social Services Research Unit (PSSRU).10
Germany
The costs of a primary-care visit were calculated from 2007–2008 data (later data not available) and indexed using the geometric mean of the increase of GP costs 2007–2008,11 according to the Working Group ‘Methods in Health Economic Evaluation'.18
Outcomes measured and statistical analyses
Weight loss and change in QOL were measured. While in Germany, bodyweight (in light clothes without shoes) was measured in GP practices with standard scales, in the other countries measurements were with a Tanita BC-418 segmental body composition analyser (Tanita Corporation of America, Arlington Heights, IL, USA). Participants were weighed on six occasions over 12 months (baseline, month 2, 4, 6, 9 and 12) and completed the Impact of Weight on Quality of Life Questionnaire-Lite (IWQOL-Lite)19, 20 on three occasions (baseline, months 6 and 12).
All participants who completed a baseline assessment were included in an intention to treat analysis using last-observation carried forward. A utility score was derived using the algorithm described by Brazier et al.21 A completers only analysis was performed to calculate mean weight loss and change in utility score between groups but was not used in the cost-effectiveness calculations.
Outcomes were analysed by linear regression with fixed effects for continuous normal data; intervention group (CP vs SC) and baseline measurement were used as the fixed effects.
Cost-effectiveness analysis
The cost-effectiveness analysis was performed for each country separately, incorporating differences of the cost of the commercial intervention, medical consultation fee structure, salary structures and other costing data.22 An incremental cost-effectiveness ratio (ICER) was calculated giving the net costs of CP relative to SC. The ICER represents the additional expenditure required to generate an additional unit of benefit, and was expressed as the cost per QALY. QALYs were calculated from the IWQOL-Lite results. A preference based single index was estimated for each country21 to provide country specific ICERs. This formula was used to calculate cost per QALY.
 |
An analysis was also performed to calculate the cost per kilogram of weight loss.
Sensitivity analysis
One-way sensitivity analyses were performed for the Australian site to include medication costs. An average costing for each subject was calculated in 3-month time periods so an annual cost could be estimated. Drug costs were obtained from the 2011 PBS pricing index—‘dispensed price for maximum quantity' and units were based on the subject's self-reported data. All medications and dosages prescribed were collected at each of the individual subject's visits. The difference in total medication costs between CP and SC, plus the costs of implementing the intervention, divided by the difference in QALYs, gave an additional cost-effectiveness ratio.
A further sensitivity analysis was performed including referral to allied health professionals in the SC group. An assumption was made that a proportion of GPs in the SC group referred their patients to a dietitian and/or psychologist for specific advice. A 1.1% referral rate for six consults over a 1-year time period has been applied, which was the probability of referral to an allied health professional during a GP visit in Australia in 2009–2010.23
Scenario analysis
As the CP costs sourced in Table 1 reflect commercial pricing decisions and are financial costs, the programme costs were re-evaluated according to opportunity costs. The CP is identical across countries and the cost to deliver the intervention is similar. The cost-effectiveness consequences of reducing programme costs in all countries to the equivalent of the Weight Watchers NHS referral scheme (GBP 45 for 12 sessions4) was examined. This is based on an attendance of 36 CP sessions per year (GBP 135–12 session cost multiplied by 3). The Weight Watchers NHS referral scheme was used as it is an existing system.
Results
Clinical trial results
Baseline characteristics
Baseline characteristics of participants have been reported previously.5 The mean (s.d.) age of subjects was 47.4 (12.9) years, the mean body mass index was 31.4 (2.6) kg m−2, and 87% were females; 6.5% had type 2 diabetes.
Weight loss
Both treatment groups lost weight but mean 12-month weight loss was significantly greater for CP than SC in all three countries (CP: −5.1±0.3 kg vs SC: −2.3±0.2 kg; P<0.0001).5 Table 2 shows the mean weight loss per participant and country.
Table 2. Mean weight loss (kg) between baseline and month 12 per participant, treatment group and country (using LOCF, and completers only).
| CP | Mean | s.d. | SC | s.d. | N | P | ||
|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Coefficientb | ||||||
| Australia | ||||||||
| LOCF | −6.21 | 6.53 | 120 | −2.72 | 4.93 | 123 | −3.49 | <0.0001 |
| CCa | −7.75 | 6.48 | 78 | −4.20 | 5.11 | 63 | −3.55 | 0.001 |
| United Kingdom | ||||||||
| LOCF | −4.91 | 5.10 | 91 | −1.73 | 3.13 | 87 | −3.18 | <0.0001 |
| CCa | −7.52 | 5.28 | 27 | −4.46 | 3.97 | 17 | −3.06 | 0.047 |
| Germany | ||||||||
| LOCF | −3.84 | 6.58 | 107 | −2.31 | 4.35 | 131 | −1.53 | 0.033 |
| CCa | −5.40 | 7.01 | 71 | −2.71 | 5.00 | 88 | −2.69 | 0.005 |
Abbreviations: CP, commercial provider; LOCF, last-observation carried forward; SC, standard care.
Complete case.
Coefficient is the change in weight (kg) for CP compared with SC.
Quality of life
Seven hundred seventy-two participants commenced the study. However, only 744 participants completed an IWQOL-Lite questionnaire at the initial baseline visit. Of this latter group, 12 participants were excluded owing to incomplete questionnaires. A further 73 participants were excluded owing to missing data when the algorithm was applied for conversion to utility scores, leaving 659 participants who could be included in the intention to treat analysis. Four hundred forty-four participants completed the 12-month study. There was a significantly greater change in utility for CP than SC in Australia and the United Kingdom but not in Germany. A 0.021 and 0.015 improvement in utility score for the CP relative to SC was found in Australia and the United Kingdom, respectively, and a 0.009 improvement in utility score for the CP relative to SC was evident in Germany. When analysing completers only, there was a significantly greater change in utility for the CP than SC in Australia and Germany but not in the United Kingdom (owing to the small sample size) (Table 3). From a pooled analysis of all three countries the greater change in utility for CP than SC remained significant (results not shown).
Table 3. Change in utility scores (quality of life) between baseline and month 12 for CP compared with SC (using LOCF, and completers only).
| Analysis by country | Coefficient (to 3 decimal places)a | 95% Confidence Interval | P | N | |
|---|---|---|---|---|---|
| LOCF | |||||
| Australia | 0.021 | 0.006 | 0.037 | 0.006 | 243 |
| United Kingdom | 0.015 | 0.001 | 0.028 | 0.033 | 178 |
| Germany | 0.009 | -0.006 | 0.024 | 0.222 | 238 |
| Completers Only | |||||
| Australia | 0.023 | 0.003 | 0.044 | 0.027 | 141 |
| United Kingdom | 0.020 | -0.025 | 0.064 | 0.382 | 44 |
| Germany | 0.021 | 0.001 | 0.040 | 0.039 | 159 |
Abbreviations: CP, commercial provider; LOCF, last-observation carried forward; SC, standard care.
Coefficient is the change in utility score for CP compared with SC.
Intervention costs
Australia
The average number of visits for each participant attending the CP or their GP was 33.0 and 10.7, respectively. Unit costs were summed for each resource measurement to obtain a total cost for each intervention (Table 1). The annual cost per patient was AUD 754 and AUD 373 for CP and SC, respectively. When including patient travel, the annual cost was AUD 1170 and AUD 508 for the respective programmes.
United Kingdom
The average number of visits for each patient attending the CP or their GP was 36.4 and 13.3, respectively. The annual cost per patient was GBP 269 and GBP 160 for CP and SC, respectively. Including patient travel, costs were GBP 451 and GBP 226 for the respective programmes.
Germany
The average number of visits for each patient attending the CP or their GP was 23.1 and 11.3, respectively. The annual cost per patient was EUR 487 and EUR 217 for CP and SC, respectively. Including patient travel, costs were EUR 626 and EUR 284 for the respective programmes.
Cost effectiveness
Australia
The cost per kilogram of weight loss was AUD 121 and AUD 137 for the CP and SC, respectively. The ICER for the CP relative to SC was AUD 18 085 (Table 4).
Table 4. Costs per kilogram of weight loss per group and country and ICERs (cost per QALY) for the CP compared with SC from both a health-care funder and societal perspective.
| Country |
Cost per kilogram of weight loss—excluding time and travel |
Cost per kilogram of weight loss—including time and travel |
Excluding travel |
Including travel |
||||
|---|---|---|---|---|---|---|---|---|
| WW | SC | WW | SC | ICER | 95% CI | ICER | 95% CI | |
| Australia (AUD) | $121 | $137 | $188 | $187 | $18 085 | $10 239, $68 041 | $31 349 | $17 723, $115 290 |
| (USD) | $122 | $138 | $190 | $189 | $18 266 | $10 342, $68 721 | $31 663 | $17 900, $116 443 |
| United Kingdom (GBP) | £55 | £92 | £92 | £131 | £7378 | £3851, £42 698 | £15 242 | £7986, £89 286 |
| (USD) | $90 | $151 | $151 | $215 | $12 100 | $6316, $70 025 | $24 996 | $13 098, $146 429 |
| Germany (EUR) | €127 | €94 | €163 | €127 | €28 826 | €10 890, Dominated | €36 318 | €13 561 Dominated |
| (USD) | $180 | $133 | $231 | $180 | $40 933 | $15 464, Dominated | $51 571 | $19 257, Dominated |
Abbreviations: AUD, Australian dollar; EUR, Euro; GBP, Great Britain pound; ICER, incremental cost-effectiveness ratio; SC, standard care. At the time of writing (early 2011), 1 AUD was 1.01 USD; 1 GBP was 1.64 USD; 1 EUR was 1.42 USD according to XE currency exchange (http://www.xe.com).
United Kingdom
The cost per kilogram of weight loss was GBP 55 and GBP 92 for the CP and SC, respectively. The ICER for the CP relative to SC was GBP 7378 (Table 4).
Germany
The cost per kilogram of weight loss was EUR 127 and EUR 94 for the CP and SC, respectively. The ICER for the CP relative to SC was EUR 28 826 (Table 4).
Sensitivity analysis
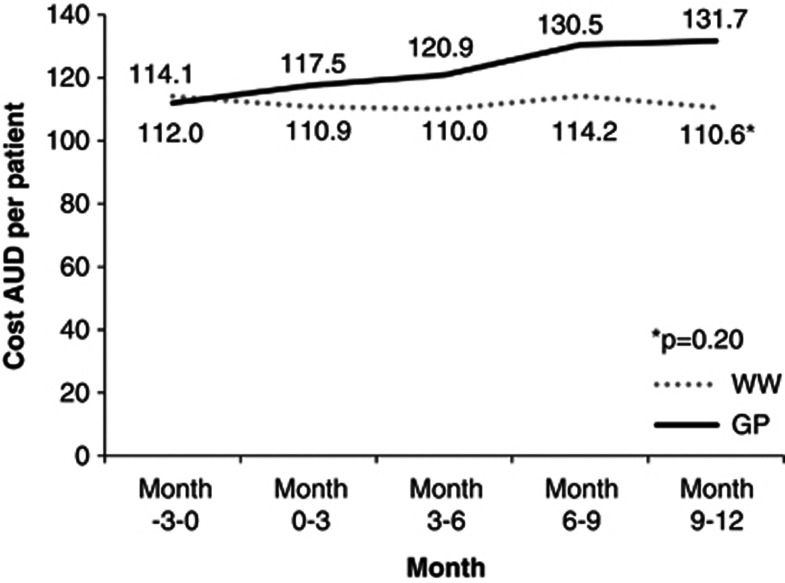
While not reaching statistical significance, the average medication cost per patient increased during the 12-month study period for the SC group (Figure 1). Medication costs remained the same for the CP group. The mean number of prescriptions was 1.9 per patient. These were for hypertension, cardiovascular disease, dyslipidemia, diabetes mellitus, thyroid disease, reflux, arthritis and musculoskeletal disease, and other conditions. No outliers who may have had the capacity to overwhelm any trends were evident. The highest costs were associated with proton pump inhibitors, cardiovascular disease and dyslipidemia.
Figure 1.
Mean medication cost in AUD per patient by group for each 3-month time period.
When medications were included, the annual cost per patient was AUD 1200 and AUD 874 for the CP and SC, respectively. With patient travel, programme costs were AUD 1616 and AUD 1009 for respective interventions. Including medication costs resulted in a decrease in the ICER for the CP relative to SC to AUD 15 522.
Including the costs associated with referral to a health professional decreased the ICER to AUD 17 901, GBP 7117, and EUR 28 403.
Scenario analysis
If programme costs in Australia and Germany are equated to the UK Weight Watchers NHS referral scheme, the ICER for the CP relative to SC becomes dominant (more health benefit at a lower cost) in both countries. In the United Kingdom, the ICER is lowered to GBP 655. The cost per kilogram of weight loss for the CP becomes AUD 41, GBP 35 and EUR 46.
Discussion
This analysis shows the CP is a cost-effective option, over 1 year, for weight management in persons within a body mass index range of 27–35 kg m−2. While the SC group also achieved a significant weight loss over this period in all three countries, the trial results may not reflect routine practice. Therefore this CP may be an effective programme for primary-care providers to refer patients. The efficacy of the CP may be in part attributable to the shared care approach as the subject was initially referred by their GP.24
In Australia and the United Kingdom, the cost per kilogram of weight loss was lower for the CP than SC. In Germany, the cost per kilogram of weight loss was lower for SC. It is difficult to compare these data with other weight loss studies because of different costing methodologies used. Previous lifestyle intervention studies did not include indirect costs such as patient travel and vary in terms of duration of outcomes, reporting costs from USD 6125 to USD 133 per kilogram of weight loss,26 and USD 6.4027 to USD 48 per pound lost.28 In contrast there is data of a programme with only AUD 7.30 per kilogram of weight loss.29 When we adjusted for the CP financial costs based on commercial pricing decisions, and costed according to economic prices, the cost per kilogram of weight loss was lower for the CP compared with SC in all three countries.
As the cost per kilogram of weight loss is not commonly used by decision makers, a cost per QALY gained was also calculated. As an example of health resources used by this demographic group, medication usage was estimated for the Australian sample. When medications and their usage were included, the cost per QALY was lowered. Regardless of this, and using the commonly accepted threshold of <$50 000 per QALY,30, 31 the CP proved to be cost effective from both a health sector and societal perspective in Australia.
In the United Kingdom, the ICER level that the National Institute of Health and Clinical Excellence has adopted is GBP 20–30 000 per QALY gained.32 Using this standard our results show the CP in the United Kingdom is a cost-effective programme from both health care and societal perspectives.
In Germany, obesity is not considered a disease in the health-care system. Therefore, obesity treatment is usually not undertaken in primary care. Comparing with cost-effective thresholds for pharmaceutical and surgical interventions in Europe (<€50 000),33 the CP is again cost effective when both perspectives were adopted.
The cost disparity of the CP and SC between countries had a large effect on the outcome, indicating that the results (cost per kilogram of weight loss and cost per QALY gained) are sensitive to the costs of the programme. In the United Kingdom, the cost to attend the CP was the lowest. When the CP costs were lowered to this value, the ICER for the CP relative to SC becomes dominant (more health benefit at a lower cost) in both Australia and Germany. Therefore the CP generated a better health outcome and cost less than SC. In the United Kingdom, this lowers the ICER to GBP 655.
It is possible that the SC group would have higher rates of referral to allied health due to greater contact with their GP. While attempting to compensate for such associated allied health costs in the SC group by applying a 1.1% referral rate,23 the only data available relate to the general population rather than specifically for an overweight and obese population, for which the referral rate is likely to be higher. Including costs associated with referral to allied health decreased the ICER in each country. As the CP subjects were not allowed to participant in other weight-loss programmes or seek other assistance for weight loss, these associated costs were not included for the CP group.
The cost of this early prevention approach targeting overweight and obese adults is low compared with the cost associated with the burden of type 2 diabetes. The average annual cost per person with type 2 diabetes in 2003 was AUD 536034 but prevention and intervention costs do vary considerably.35 As shown by previous lifestyle interventions, the degree of weight loss in the current study reduces the risk of type 2 diabetes in those at high risk.36 Despite weight regain being common, a so-called ‘metabolic memory effect' has been shown in Diabetes Treatment and Prevention Outcome Studies36, 37 and it could be anticipated that such metabolic benefit would occur with the weight loss achieved in this trial. Therefore, adopting cost-effective weight management programmes as demonstrated in this study, and an intervention that has the capacity to be large scale (CP), may be an effective way to reduce the prevalence of obesity associated diseases such as type 2 diabetes and their costs.
While a key strength of this analysis allows us to draw on the results of a RCT to perform a within-trial cost, it also acts as a limitation as the cost-effectiveness analysis is restricted to a 1-year time period. Importantly, the QALY metric is not all that applicable over a 1-year time horizon; however, it has been used in this study for demonstration purposes so that results can be compared with cost-effectiveness thresholds.
Another key strength of this study was the use of the societal perspective to take into consideration all intervention costs. This follows the recommended approach for cost-effectiveness analyses.38 However, as the available data from the RCT did not enable us to capture several cost-offsets including reduced rates of obesity-related disease, frequency of hospitalisations averted or reduction in sick leave, the true cost effectiveness of the intervention programme may have been underestimated.
While it was assumed that no patient required time off from work to attend the CP or GP consultations, this information was not specifically captured. A large benefit of the CP and GP consults is that they are available out of work hours, at lunchtimes and on weekends, minimising the opportunity costs of employment. An assumption may also be made that a percentage of patients are commenced on other weight loss assistance therapies by their GP in a SC real life setting, but such treatments were not included in our analysis. The limitations of the trial design meant that other costs such as food products endorsed by the CP and out of pocked expenses for the SC group (for example, allied health professional consultations) were ignored.
For the Australian sensitivity analysis medications were grouped together and it was not possible to determine whether there was a change in costs relating to obesity or disease-specific drugs. However, as reported by the Counterweight Programme in the United Kingdom, the range of prescribing areas being affected by obesity was greater than expected.39 An increased prescription rate was found for categories such as gastrointestinal, infections, skin conditions, antihistamines, hypnotics, and drugs used in the treatment of nausea and vertigo. Most clinicians would not typically associate these with obesity.39 Therefore, there is a wide range of clinical conditions for which obesity is a contributory factor.
In Australia, despite the GP-treated (SC) group showing an increase in mean medication prescription over the 12-month period, it did not reach significance. This may reflect better control of risk factors by GPs as SC patients were required to see their primary-care professional on a more regular basis. However, despite this increase in prescriptions, they had no better control of risk factors for obesity-related conditions at the end of the 12-month study. Patients in the CP group had significantly greater total to HDL cholesterol ratio than in the SC group and serum insulin was lower, and weaker evidence existed of improvements in glucose, and HDL and LDL cholesterol. Small reductions in blood pressure were noted for both treatment groups.5 All these improvements may be related to, or influenced by, degree of weight loss. As the patients were overweight or obese with one or more additional risk factors for obesity-related disease, a slightly higher average number of medication scripts per person may be expected, and consequently a higher mean medication cost per patient. Results from a random Australian community sample of 3015 respondents with a mean age of 45.3 years found that 1411 (46.8%) were taking one or more prescribed medications.40 In this trial, it was 1.9 prescribed medications per patient.
Missing data were dealt with from an intention to treat perspective using last-observation carried forward. Despite a mixed model or multiple imputation approach now being commonly used to deal with missing data,41 the last-observation carried forward method was adopted as it was more conservative than the mixed model results previously reported in an interim analysis for weight loss of this RCT.42 This would imply a higher cost per kilogram of weight loss for the CP relative to SC.
The models by which IWQOL-Lite values are transformed into utilities has its weaknesses, and such a mapping exercise is second preference compared with the direct use of the EuroQoL Europe or the AQoL Australia, for example. But, the IWQOL-Lite is an obesity-specific measure of QOL sensitive to obesity-related QOL effects, and it performs well in terms of conventional psychometric criteria in obese populations.19, 43, 44 Despite the concerns with this mapping approach, it is possible to produce a robust model for predicting SF-6D index values from the IWQOL-Lite.21
It has been argued that there is too much reliance on health professionals such as GPs to treat overweight and obesity.45 This is in part because GPs do not have additional or alternative resources to manage this issue beyond a standard GP consultation. The ability to be able to refer to cost-effective commercial programmes may assist. However, due to the out of pocket cost it may still be beyond the financial reach of a substantial proportion of the population, particularly those who need it most.46 Thus, governments could consider funding cost-effective commercial programmes in preference to GP visits for managing overweight and obesity. In Australia, for example, the addition of items to Medicare and private health insurance, providing financial incentives to better treatment, could help support individuals in changing their lifestyle behaviours.45
This study provides data from three different countries and consistent results. The cost per kilogram of weight loss was lower for the CP versus SC in Australia and the United Kingdom. When adjusting for the CP financial costs based on commercial pricing decisions, and costing according to economic prices, the cost per kilogram of weight loss was lower for the CP compared with SC in all three countries. The CP is cost effective when assessed by the commonly accepted threshold of a cost <$50 000 per QALY. Importantly, despite participants in the CP group attending on average three meetings per month in the United Kingdom and Australia, and two meetings per month in Germany, compared with only one appointment per month for the SC group, the CP remained cost effective when including these added patient travel costs.
Acknowledgments
We acknowledge the assistance of Namson Lau, Crystal Lee, Colman Taylor, Toby Gould and Hannah Verry who provided critical analysis of the draft paper; James Gerofi for his mathematical expertise; Stephan Jacob for his guidance and assistance relating to the German health care system; and to the rest of the team who contributed to the acquisition of data and recruitment of study participants (Annie Simpson, Suzanne Pearson, Louise Aston, Julia Stoll), and study design (Ulrike Amann-Gassner, Adrian Mander).
Author contributions
The author's responsibilities were as followsNRF: acquisition of data, analysis and interpretation of data, and writing of the paper. SC, DS: critical review and writing of the paper. ADO, RS: analysis of data. CH, SBW, RH: health economic contribution for German specific data and critical review of the paper; ALA: health economic contribution for UK specific data and critical review of the paper. SAJ, HH: study design and conception, obtained funding and critical review of the paper. IDC: study design and conception, obtained funding and writing the paper. All authors read and approved the final paper.
Trial registration
This study is registered with the International Standard Register of Clinical Trials, ISRCTN 85485463.
Role of the funding source
This study was investigator-initiated but funded by Weight Watchers International through a grant to the Medical Research Council (United Kingdom). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
NRF and IDC have received research grants for other clinical trials funded by Sanofi-Aventis, Allergan, Eli Lilly and Novo Nordisk. IDC was a board member for the SCOUT trial, and currently for the EXSCEL trial, and has received payment for lectures from iNova Pharmaceuticals, Eisai Pharmaceuticals, Pfizer Australia and Servier Laboratories (Australia). SAJ has received research grants for other clinical trials from Sanofi-Aventis and Coca Cola, and is a member of the Tanita Medical Advisory Board and receives a fee for nutrition articles and lectures for Rosemary Conley Enterprises. HH is on the Advisory Board for Weight Watchers International and has received payment for lectures from Sara Lee, Novartis, Sanofi-Aventis and Bristol-Myers Squibb.
References
- WHO Obesity: preventing and managing the global epidemic. Report of a WHO consultation.: World Health Organisation. 2000. [PubMed]
- WHO Global health risks: mortality and burden of disease attributable to selected major risks World Health Organization; 2009. Contract No.: ISBN 978 92 4 156387 1. [Google Scholar]
- Lavin JH, Avery A, Whitehead SM, Rees E, Parsons J, Bagnall T, et al. Feasibility and benefits of implementing a Slimming on Referral service in primary care using a commercial weight management partner. Public Health. 2006;120:872–881. doi: 10.1016/j.puhe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Ahern AL, Olson AD, Aston LM, Jebb SA. Weight Watchers on prescription: An observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health. 2011;11:434. doi: 10.1186/1471-2458-11-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;8:61344–61345. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, et al. Weight loss with self-help compared with a structured commercial program—a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. A randomised controlled trial to compare a range of commercial or primary care led weight reduction programmes with a minimal intervention control for weight loss in obesity: the Lighten Up trial. BMJ. 2011;343:d6500. doi: 10.1136/bmj.d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobiac L, Vos T, Veerman L. Cost-effectiveness of Weight Watchers and the Lighten Up to a Healthy Lifestyle program. Aust NZJ Public Health. 2010;34:240–247. doi: 10.1111/j.1753-6405.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- DoHA Medicare Benefits Schedule Book. Department of Health and Ageing, Australian Government; 2010 (cited 18 January 2011); Available from: http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-201011 .
- PSSRU Unit Costs of Health and Social Care. Personal Social Services Research Unit, United Kingdom; 2010 (20 February cited 2011); available from: http://www.pssru.ac.uk .
- Kassenärztliche Bundesvereinigung (The National Association of Statutory Health Insurance Physicians) Grunddaten zur vertragsärztlichen Versorgung 2009, Berlin 2010 Contract No.: Tabelle II.1.
- DoHA Pharmaceutical Benefits Scheme. Department of Health and Ageing, Australian Government; 2011 (cited 02 February 2011); available from: http://www.pbs.gov.au/pbs/home .
- ATO Claiming a deduction for car expenses using the cents per kilometre method. Canberra: Australian Taxation Office, Australian Government; 2009–10 (cited 25 February 2011); available from: http://www.ato.gov.au/individuals/content.asp?doc=/content/33874.htm .
- HMRC Travel—mileage and fuel allowances. HM Revenue & Customs UK; 2010/11 (cited 17th September 2011); available from: http://www.hmrc.gov.uk/rates/travel.htm .
- Leitsatz zum Urteil des Zweiten Senats vom 9. Dezember 2008(2008)
- Suche nach Arzt oder Psychotherapeut (database on the internet)2011. Available from http://arztsuche.kvb.de/cargo/app/erweiterteSuche.htm?apvOnly=true .
- Schulungs-Gemeinschaft München Ost e.V. Individuelle Ernährungsberatung Munich: Schulungs-Gemeinschaft München Ost e.V.,2010(updated 05/2011; cited 2011); available from: http://www.schulung-muenchen-ost.de/11.html .
- Krauth C, Hessel F, Hansmeier T, Wasem J, Seitz R, Schweikert B. Empirical standard costs for health economic evaluation in Germany—a proposal by the working group methods in health economic evaluation [in German] Gesundheitswesen. 2005;67:736–746. doi: 10.1055/s-2005-858698. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- Mueller A, Holzapfel C, Hauner H, Crosby RD, Engel SG, Muhlhans B, et al. Psychometric Evaluation of the German Version of the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) Questionnaire. Exp Clin Endocrin Diabetes. 2011;119:69–74. doi: 10.1055/s-0030-1261922. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Kolotkin RL, Crosby RD, Williams GR. Estimating a preference-based single index for the Impact of Weight on Quality of Life-Lite (IWQOL-lite) instrument from the SF-6D. Value Health. 2004;7:490–498. doi: 10.1111/j.1524-4733.2004.74012.x. [DOI] [PubMed] [Google Scholar]
- Carter R, Moodie M, Markwick A, Magnus A, Vos T, Swinburn B, et al. Assessing Cost-Effectiveness in Obesity (ACE-Obesity): an overview of the ACE approach, economic methods and cost results. BMC Public Health. 2009;9:419. doi: 10.1186/1471-2458-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt H, Miller GC, Charles J, Henderson J, Bayram C, Pan Y, et al. General practice activity in AustraliaBEACH Bettering the Evaluation And Care of Health 2009-102009-10
- Richman RM, Webster P, Salgo AR, Mira M, Steinbeck KS, Caterson ID. A Shared Care approach in obesity management: he general practitioner and a hospital based service. Int J Obes. 1996;20:413–419. [PubMed] [Google Scholar]
- Gustafson A, Khavjou O, Stearns SC, Keyserling TC, Gizlice Z, Lindsley S, et al. Cost-effectiveness of a behavioral weight loss intervention for low-income women: The Weight-Wise Program. Prev Med. 2009;49:390–395. doi: 10.1016/j.ypmed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Sherwood NE, Jeffery RW, Pronk NP, Boucher JL, Hanson A, Boyle R, et al. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. Int J Obes. 2006;30:1565–1573. doi: 10.1038/sj.ijo.0803295. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Stunkard AJ, McKeon PE. Weight reduction at the work site: a promise partially fulfilled. Am J Psychiatry. 1985;142:47–52. doi: 10.1176/ajp.142.1.47. [DOI] [PubMed] [Google Scholar]
- Katz DL, Chan W, Gonzalez M, Larson D, Nawaz H, Abdulrahman M, et al. Technical skills for weight loss: Preliminary data from a randomized trial. Prev Med. 2002;34:608–615. doi: 10.1006/pmed.2002.1025. [DOI] [PubMed] [Google Scholar]
- Pritchard DA, Hyndman J, Taba F. Nutritional counselling in general practice: a cost effective analysis. J Epidemiol Community Health. 1999;53:311–316. doi: 10.1136/jech.53.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization—tentative guidelines for using clinical and economic evaulations. Can Med Assoc J. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- Salem L, Jensen CC, Flum DR. Are bariatric surgical outcomes worth their cost? A systematic review. J Am Coll Surg. 2005;200:270–278. doi: 10.1016/j.jamcollsurg.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Appleby J, Devlin N, Parkin D, Buxton M, Chalkidou K. Searching for cost effectiveness thresholds in the NHS. Health Policy. 2009;91:239–245. doi: 10.1016/j.healthpol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Neovius M, Narbro K. Cost-effectiveness of pharmacological anti-obesity treatments: a systematic review. Int J Obes. 2008;32:1752–1763. doi: 10.1038/ijo.2008.189. [DOI] [PubMed] [Google Scholar]
- Colagiuri S, Colagiuri R, Conway B, Grainger D, Davey P.DiabCo$t Australia: assessing the burden of Type 2 Diabetes in Australia, Diabetes Australia. Canberra December,2003
- Li R, Zhang P, Barker LE, Chowdhury FM, Zhang XP. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33:1872–1894. doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- Bray GA, Chatellier A, Duncan C, Greenway FL, Levy E, Ryan DH, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- Gibbs H, Broom J, Brown F, Laws R, Reckless J, Noble P, et al. The impact of obesity on drug prescribing in primary care. Br J Gen Pract. 2005;55:743–749. [PMC free article] [PubMed] [Google Scholar]
- Goldney RD, Fisher LJ. Use of prescribed medications in a South Australian community sample. Med J Aust. 2005;183:251–253. doi: 10.5694/j.1326-5377.2005.tb07030.x. [DOI] [PubMed] [Google Scholar]
- Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS ONE. 2009;4:e6624. doi: 10.1371/journal.pone.0006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AD, Jackson D, Seaman SR, Jebb SA, Mander AP.Missing data in weight loss randomised controlled trialsIn Astrup A, (ed).18th European Congress on Obesity (ECO 2011) Obesity Reviews: Instanbul, Turkey; 2011p106 [Google Scholar]
- Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–571. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese sub-groups. Obes Res. 2002;10:748–756. doi: 10.1038/oby.2002.102. [DOI] [PubMed] [Google Scholar]
- Kouris-Blazos A, Wahlqvist ML. Health economics of weight management: evidence and cost. Asia Pac J Clin Nutr. 2007;16:329–338. [PubMed] [Google Scholar]
- Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]