Abstract
The aluminum (Al) cation Al3+ is highly rhizotoxic and is a major stress factor to plants on acid soils, which cover large areas of tropical and boreal regions. Many woody plant species are native to acid soils and are well adapted to high Al3+ conditions. In tropical regions, both woody Al accumulator and non-Al accumulator plants occur, whereas in boreal regions woody plants are non-Al accumulators. The mechanisms of these adaptations can be divided into those that facilitate the exclusion of Al3+ from root cells (exclusion mechanisms) and those that enable plants to tolerate Al3+ once it has entered the root and shoot symplast (internal tolerance mechanisms). The biochemical and molecular basis of these mechanisms have been intensively studied in several crop plants and the model plant Arabidopsis. In this review, we examine the current understanding of Al3+ exclusion and tolerance mechanisms from woody plants. In addition, we discuss the ecology of woody non-Al accumulator and Al accumulator plants, and present examples of Al3+ adaptations in woody plant populations. This paper complements previous reviews focusing on crop plants and provides insights into evolutionary processes operating in plant communities that are widespread on acid soils.
Keywords: acid soils, adaptation, aluminum, organic acids, tolerance, resistance, toxicity
INTRODUCTION
Aluminum (Al) is a prevalent constituent of most soils and is one of the major stresses to plants in acid soils. Most of the Al in soils is incorporated into aluminosilicates and other precipitated forms, which are harmless to plants. Under acid soil conditions, these minerals solubilize to a limited extent, and the toxic ion Al3+ is released into the soil solution (Kinraide, 1997). This form of Al is capable of inhibiting root growth and damaging cells at the root apex, which is the most sensitive part of the root to Al3+ (Ryan et al., 1993; Kochian, 1995). However, the mechanism underlying Al3+ toxicity is not clearly understood. Because Al3+ can interact with a number of extracellular and intracellular structures, many different mechanisms of Al3+ toxicity have been proposed. These mechanisms include modification of the cell wall, disruption of the plasma membrane and transport processes, interruption of signaling pathways, and Al3+ binding to the DNA (Kochian et al., 2005).
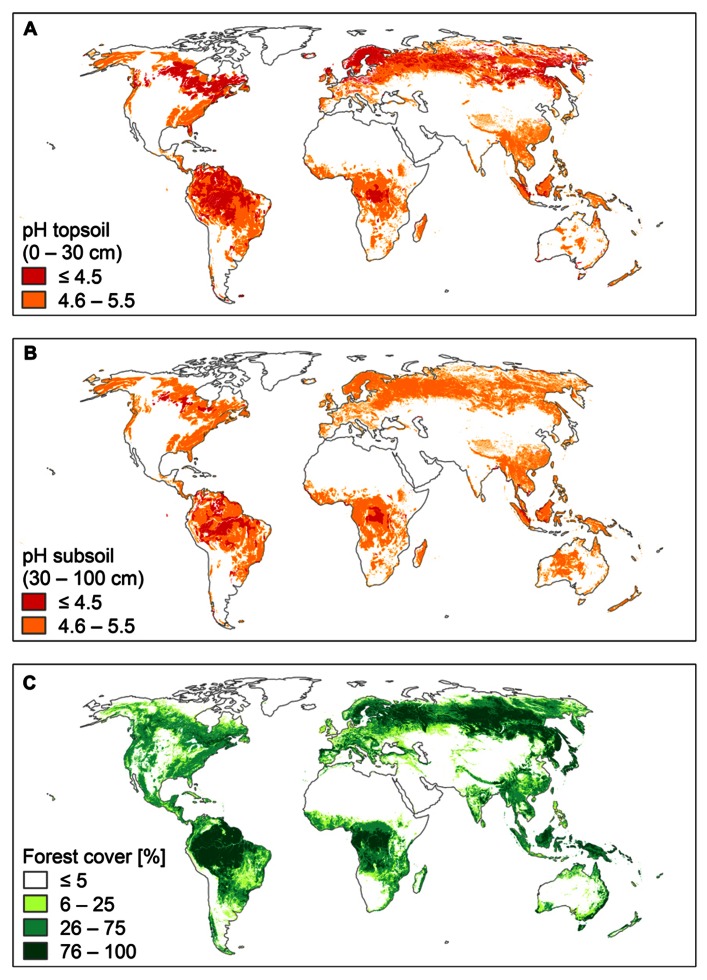
Al3+ toxicity is an important research topic, because many crop plants are susceptible in acid soils, and their growth and yield are limited by high Al3+ conditions. Less attention is paid to native plant communities, which tolerate acid soil conditions over large areas in different biomes. Acid soils occupy about 30% of the ice-free land area in the world and primarily occur in the humid tropics and the boreal region. Large parts of these soils (about 67%) are covered by forests and woodland (von Uexküll and Mutert, 1995; Figure 1). The biodiversity and high biomass production of both tropical and boreal forests suggest that their plants are not affected by Al3+ toxicity.
FIGURE 1.
World acid soils and world forests. (A) pH of topsoil (0–30 cm), (B) pH of subsoil (30–100 cm), and (C) forest cover. Soil pH is presented in two classes: pH ≤ 4.5 (strongly acid soils) and pH 4.6–5.5 (moderately acid soils). Data were retrieved from the Harmonized World Soil Data Base (FAO/IIASA/ISRIC/ISS-CAS/JRC, 2012). Forest cover is presented in four classes: ≤5, 6–25, 26–75, and 76–100%. Data were retrieved from the Food Insecurity, Poverty and Environment Global GIS Database (FGGD; FAO and IIASA, 2007).
Al3+ toxicity may occur in forests that are exposed to acid deposition derived from air pollutants (Cronan and Schofield, 1979; Ulrich et al., 1980; Larssen et al., 2006). On sensitive sites, acid deposition accelerates soil acidification and leads to increased Al3+ concentrations in the soil solution (Blaser et al., 1999; Fowler et al., 1999). Recently, it was found that soils, affected by acid deposition, showed signs of recovery due to the reduction in sulfate deposition (Stoddard et al., 1999; Evans et al., 2001). However, inputs of nitric acid and ammonia continue to alter the chemistry of forest soils and are likely to promote acidification (Graf Pannatier et al., 2011; Zang et al., 2011). Acid soils characteristically contain high amounts of Al3+ and low amounts of the base cations (BC) Ca2+, Mg2+, and K+, which are important plant nutrients. Since Al3+ and BC interact at the plasma membrane surface, it is not the soil Al3+ concentration alone that determines the plant responses to Al3+ exposure (Sverdrup and Warfvinge, 1993; Cronan and Grigal, 1995; Kinraide, 2003). A ratio of Ca2+/Al3+ or BC/Al3+ in the soil solution lower than 1 is widely used as an ecological indicator for potentially adverse effects of Al3+ stress and nutrient imbalance on tree growth. Alternative indicators are based on the Al and Ca concentrations in fine roots and provide information on the availability of toxic Al3+ in the soils (e.g., Brunner et al., 2004; Richter et al., 2007; Vanguelova et al., 2007).
Al3+ EXCLUSION AND Al3+ TOLERANCE MECHANISMS
The mechanisms conferring resistance to Al3+ have been the focus of intensive research in crop plants and in the model plant Arabidopsis. Many different mechanisms have been suggested, but for most of them, the supporting genetic and physiological evidence is not provided. Therefore, these mechanisms have to remain speculation or hypotheses until supporting data is provided. One exception is the Al3+-induced efflux of organic acids from roots, which has been demonstrated to be a major Al3+ resistance mechanism in several plant species (Delhaize et al., 1993, 2007).
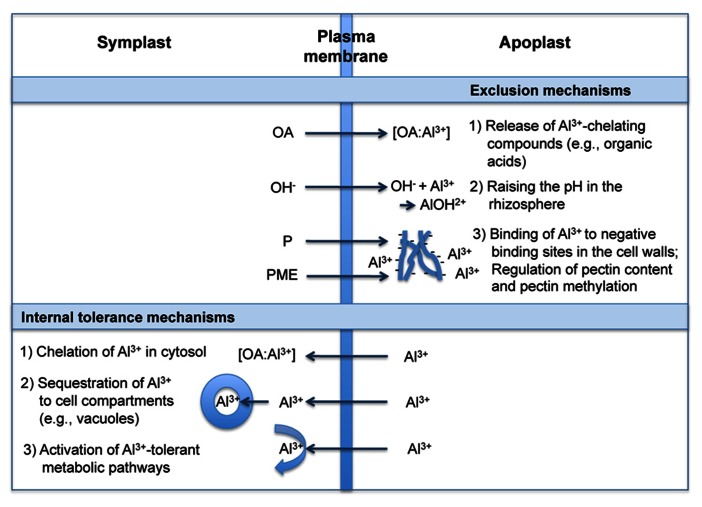
Following Ryan and Delhaize (2010) and Horst et al. (2010), the term “Al3+ resistance” is used here as a plant property that allows a plant to grow with little or no injury under elevated Al3+ conditions. The potential mechanisms conferring resistance to Al3+ can be broadly divided into those that exclude Al3+ from the root symplast (exclusion mechanisms) and those that enable plants to cope with Al3+ safely, once it enters the symplast (internal tolerance mechanisms; e.g., Kochian, 1995). Exclusion mechanisms depend on the release of ligands which chelate and detoxify Al3+ externally and limit its uptake in the cytosol. Tolerance mechanisms include those that chelate the Al3+ entering the root cells, with subsequent transport and sequestration into less sensitive parts of the plant and subcellular compartments. The physiology, biochemistry, and molecular biology of these mechanisms have been thoroughly discussed in several review articles (e.g., Jones and Ryan, 2003; Kochian et al., 2004; Ma, 2007; Poschenrieder et al., 2008; Horst et al., 2010; Ryan et al., 2011; Delhaize et al., 2012; Inostroza-Blancheteau et al., 2012). The proposed principles of these mechanisms are summarized in Figure 2.
FIGURE 2.
Mechanisms of plant roots to deal with Al3+ (modified from Jones and Ryan, 2003; Inostroza-Blancheteau et al., 2012). OA, organic acids; P, pectin; PME, pectin methylesterase.
In this paper, we review the literature on proposed Al3+ resistance mechanisms of woody plants. We summarize information obtained from crops and Arabidopsis, and then review relevant results from similar studies in woody plants. In addition, we discuss the occurrence of Al accumulators and Al excluders in different forest biomes of the world.
Al3+ EXCLUSION
RELEASE OF SUBSTANCES THAT CHELATE AND DETOXIFY Al3+
The best-documented mechanism of Al3+ exclusion is the Al3+-activated efflux of organic acids from roots. Typical organic acids released by plants are citrate, malate, and oxalate. These organic acids are deprotonated anions at the pH found in the cytosol, and once transported out of the root, they chelate the toxic Al3+ in the rhizosphere, forming stable and non-toxic complexes. Citrate and malate are present in all plant cells because they are involved in the mitochondrial respiratory cycle. Oxalate is a common cellular constituent involved in Ca2+ regulation, ion balance, and metal detoxification (Franceschi and Nakata, 2005; Rahman and Kawamura, 2011). The three organic acid anions form complexes with Al3+ with the following order of strength: citrate > oxalate > malate (Libert and Franceschi, 1987; Jones and Ryan, 2003). Physiological and genetic evidence from several plant species shows that the Al3+-activated efflux of organic acid anions indeed confers resistance to Al3+. The most convincing support comes from genotypes within a species that show contrasting levels of Al3+ resistance. In wheat (Triticum aestivum), for example, a pair of near-isogenic lines that differ in resistance at a single locus was used to show that the Al3+-activated efflux of malate from roots was greater in the resistant genotype than in the sensitive genotype (Delhaize et al., 1993). The same wheat genotypes were used to clone the Al3+-activated malate transporter (TaALMT1) gene, the first Al3+ resistance gene isolated from plants (Sasaki et al., 2004). This gene encodes a plasma membrane-bound protein responsible for the efflux of malate from roots. Subsequently, TaALMT1-like genes were isolated from several additional plant species, including Arabidopsis and rape (Brassica napus; Hoekenga et al., 2006; Ligaba et al., 2006; see also Delhaize et al., 2007). Citrate efflux, on the other hand, was found to be mediated by members of another protein family, the multidrug and toxic compound extrusion (MATE) family (Furukawa et al., 2007; Magalhaes et al., 2007). The molecular background of oxalate exudation has not yet been identified.
Numerous studies, some of which are described here, have found that woody plants release organic acid anions following Al3+ exposure. In the model tree poplar (Populus), the Al3+-activated release of organic acid anions has been studied both at the physiological and molecular level. In young rooted cuttings of Populus tremula, Al3+ induces the release of citrate and oxalate (Qin et al., 2007). In a follow-up study with the same poplar clone, Grisel et al. (2010) identified a MATE gene with a 60% amino acid sequence identity to the AtMATE1 gene of Arabidopsis. The poplar gene is induced by Al3+ in both root and stem tissue, but not in the leaves, consistent with a function of this gene in the efflux of citrate from roots. In seedlings of two other poplar species, Populus tremuloides and Populus trichocarpa, Al3+ induced the exudation of citrate, malate, and oxalate from roots (Naik et al., 2009). In these species, organic acids accounted for 20–64% of the total C released upon Al3+ exposure (Naik et al., 2009). The minimal concentrations of Al3+ required to induce organic acid exudation in Populus tremula are between 50 and 100 μM Al3+ (Table 1; Qin et al., 2007). Using the same solution culture medium, similar threshold values were found in the two coniferous trees Cryptomeria japonica and Pinus thunbergii (Hirano et al., 2012; Table 1). Although these studies clearly demonstrate that Al3+ induces the release of organic acid anions from roots, direct physiological and genetic evidence for their role in Al3+ resistance has not been established. For example, it is not known whether the organic acid anions are released primarily from the root tip, which would be indicative for a role in Al3+ resistance.
Table 1.
Organic acid release from roots of Cryptomeria japonica (μmol g–1 day–1 FW), Pinus thunbergii (μmol g–1 day–1 FW), and Populus tremula (μmol g–1 DW) after exposition to Al3+ (according to Qin et al., 2007; Hirano et al., 2012).
| Tree species | Al3+ concen-tration (μM) | Citrate | Malate | Oxalate |
|---|---|---|---|---|
| Cryptomeria japonica | 0 | 0.08a | 0.10a | 0.14a |
| 100 | 0.39 | 0.14 | 0.49 | |
| 500 | 0.41 | 0.14 | 0.75 | |
| 1000 | 0.38 | 0.13 | 0.70 | |
| P valueb | ns | ns | * | |
| Pinus thunbergii | 0 | 0.03a | 0.03a | 0.47 |
| 100 | 0.10 | 0.03a | 1.08 | |
| 500 | 0.10 | 0.03a | 2.93 | |
| 1000 | 0.11 | 0.03a | 4.04 | |
| P value | * | ns | ** | |
| Populus tremula | 0 | 0.0a | 0.0a | 0.1 |
| 50 | 0.8 | 0.0a | 4.5 | |
| 100 | 1.9 | 0.0a | 3.9 | |
| 200 | 18.4 | 0.0a | 5.6 | |
| 500 | 20.5 | 0.0a | 25.7 | |
| 1000 | 20.3 | 0.0a | 18.8 | |
| P value | *** | ns | *** |
aBelow detection limit.
bANOVA: ***P < 0.001, **P < 0.01, *P < 0.05; ns, not significant.
Woody plant species vary considerably in the organic acid compounds they release in response to Al3+ exposure. Many species exude more than one organic acid anion, with various combinations of malate, oxalate, and succinate. In the broad-leaved deciduous and evergreen trees and shrubs assayed so far, citrate is the most common organic acid anion identified (Table 2 and references therein). Of the five coniferous tree species analyzed, three released oxalate, and the two remaining citrate and succinate, respectively (Table 2).
Table 2.
Al3+-activated release of organic acids from roots of woody plants.
| Plant species | Al3+-activated organic acids | Reference |
|---|---|---|
| Non-mycorrhizal roots; coniferous trees | ||
| Cryptomeria japonica | Citrate, oxalate | Hirano et al. (2012) |
| Picea abies | Oxalate | Heim et al. (2001) |
| Picea abies | – | Eldhuset et al. (2007) |
| Pinus sylvestris | Oxalate | Ahonen-Jonnarth et al. (2000) |
| Pinus thunbergii | Citrate, oxalate | Hirano et al. (2012) |
| Non-mycorrhizal roots; broad-leaved trees (deciduous) | ||
| Populus tremula | Citrate, oxalate | Qin et al. (2007) |
| Populus tremuloides | Citrate, malate, oxalate, succinate | Naik et al. (2009) |
| Populus trichocarpa | Citrate, malate, oxalate, succinate | Naik et al. (2009) |
| Non-mycorrhizal roots; broad-leaved trees and shrubs (evergreen) | ||
| Acacia auriculiformis | Citrate, oxalate | Nguyen et al. (2003) |
| Camellia sinensis | Oxalate | Morita et al. (2011) |
| Camellia sinensis | – | Ishikawa et al. (2000) |
| Cinnamomum camphora | Citrate | Osawa et al. (2011) |
| Citrus grandis | Citrate, malate | Yang et al. (2011) |
| Citrus junos | Citrate | Deng et al. (2009) |
| Citrus sinensis | Citrate, malate | Yang et al. (2011) |
| Eucalyptus camaldulensis | Citrate, oxalate | Tahara et al. (2008) |
| Eucalyptus camaldulensis | Citrate, oxalate | Nguyen et al. (2003) |
| Eucalyptus cloeziana | Citrate | Silva et al. (2004) |
| Eucalyptus dunnii | Citrate, malate, oxalate | Silva et al. (2004) |
| Eucalyptus globulus | Citrate, malate | Silva et al. (2004) |
| Eucalyptus grandis | Citrate | Silva et al. (2004) |
| Eucalyptus saligna | Citrate | Silva et al. (2004) |
| Eucalyptus urophylla | Citrate, malate, oxalate | Silva et al. (2004) |
| Melaleuca bracteata | Citrate | Tahara et al. (2008) |
| Melaleuca cajuputi | Citrate, malate | Tahara et al. (2008) |
| Melaleuca cajuputi | Citrate, oxalate | Nguyen et al. (2003) |
| Melaleuca leucadendra | Citrate | Nguyen et al. (2003) |
| Mycorrhizal roots | ||
| Picea abies | Succinate | Heim et al. (2003) |
| Picea abies | – | Eldhuset et al. (2007) |
| Pinus densiflora | Citrate | Tahara et al. (2005) |
| Pinus sylvestris | Oxalate | Ahonen-Jonnarth et al. (2000) |
The references are divided into studies dealing either with non-mycorrhizal roots or with mycorrhizal roots.
Little is known about other substances that may be released by roots to chelate Al3+. Proposed compounds include polypeptides, phenolic compounds, cyclic hydroxamates, and rhizodepositions in the form of mucilage (Jones and Ryan, 2003; Poschenrieder et al., 2008). In the tea plant, Camellia sinensis, Morita et al. (2011) observed beside of oxalate an increase of the release of caffeine, a phenolic compound, in response to Al3+ exposure. Phenolic compounds were also exuded in Al3+-treated Eucalyptus camaldulensis and two Melaleuca species (Nguyen et al., 2003).
RAISING THE pH IN THE RHIZOSPHERE
According to Kochian et al. (2005), only one study to date has unequivocally demonstrated that raising the pH in the rhizosphere can protect plants from Al3+. For two distinct classes of Al3+-tolerant Arabidopsis mutants, it was shown that Al3+ resistance is mediated by the exclusion of Al3+ from the root either by exudation of malate and citrate (Larsen et al., 1998) or by H+ influx at the root apex (Degenhardt et al., 1998). The H+ influx resulted in an increase in the rhizosphere pH, and subsequently in a significant decrease in the Al3+ activity around the root tip. However, there is currently no evidence to support that this mechanism operates in Arabidopsis ecotypes. Whether the roots of woody plants make use of such a mechanism remains elusive.
MODIFICATION OF Al3+ BINDING SITES IN THE CELL WALL OF ROOT CELLS
The cell wall of root cells has been suggested to be a site of both Al3+ toxicity and Al3+ exclusion (Horst et al., 2010). It has been determined that up to 90% of the Al3+ absorbed by roots can be localized to the apoplast (Kochian, 1995). The primary site of Al3+ binding is probably the pectin matrix, which is largely composed of homopolymers of galacturonic acid (Mohnen, 2008; Horst et al., 2010). Al3+ is known to bind far more strongly to pectin than Ca2+, whose binding to the cell wall is required for proper cell wall functioning (Franco et al., 2004). It has been proposed that Al3+ binds to the cell wall through a replacement of Ca2+, making the cell wall more rigid, and thus reducing its extensibility which is required for normal cell elongation (Tabuchi and Matsumoto, 2001).
Several studies have suggested that the pectin content and the degree of pectin methylation are important determinants of the amount of Al3+ that can bind to the cell wall of root cells. In maize (Zea mays), rice (Oryza sativa), and common bean (Phaseolus vulgaris), differences in the pectin content and/or the degree of pectin methylation were linked with Al3+ sensitivity/resistance (Eticha et al., 2005; Yang et al., 2008; Rangel et al., 2009). Al3+-resistant lines of all three plant species were found to have a higher degree of pectin methylation, and a lower cell wall Al content when compared to Al3+-sensitive lines, supporting a role for pectin methylation in Al3+ exclusion. A modulating role for the degree of pectin methylation is further supported by the finding that the expression of pectin methylesterase (PME), the enzyme responsible for the demethylation of pectin, was lower in Al3+-resistant lines than in Al3+-sensitive lines (Maron et al., 2008; Yang et al., 2008).
Current evidence, based on X-ray microanalyses, indicates that the apoplast is a major site of Al accumulation also in woody plants. In Al3+-treated seedlings of the conifer Picea abies, Al was found in both epidermal and cortical cells of the root tip (Heim et al., 1999). In both cell types, more than 88% of the total Al localized to the cell wall. In addition, it was observed that the amount of Ca in the cell wall of both cell types was much lower in Al3+-treated seedlings than in control plants, suggesting that Al3+ replaced Ca2+ at the exchange sites of the cell wall. These findings are further substantiated by results of a study conducted in Picea abies and Populus tremula, cultivated in a model ecosystem for 3 years (Brunner et al., 2008). In Picea abies, Al accumulated continuously over time in the cell wall of root epidermal cells, whereas in Populus tremula, Al accumulated in the cell wall of both root epidermal and cortical cells. In both species, Al did not accumulate intracellularly (Table 3).
Table 3.
Al accumulation in fine roots of Picea abies and Populus tremula [Al concentrations of bulk material; Al net counts of compartments using , energy-dispersive X-ray spectroscopy (EDX)-analyses] after growth in weakly acidic soil (pH 6.5) with different length of exposition time (according to Brunner et al., 2008).
| Tree species | Time (year) | Al concentration (mg g–1) | Al counts in epidermal cells | Al counts in cortical cells | ||
|---|---|---|---|---|---|---|
| Cell wall | Intracellular | Cell wall | Intracellular | |||
| Picea abies | 0.5 | 2.12 | 213 | 83 | 126 | 94 |
| 1.5 | 8.23 | 284 | 73 | 122 | 72 | |
| 2.5 | 9.50 | 355 | 101 | 140 | 80 | |
| P valuea | ** | *** | ns | ns | ns | |
| Populus tremula | 0.5 | 1.81 | 168 | 67 | 96 | 65 |
| 1.5 | 16.40 | 359 | 50 | 152 | 51 | |
| 2.5 | 5.36 | 338 | 57 | 163 | 63 | |
| P value | –b | *** | ns | ** | ns | |
aRepeated measures ANOVA: ***P < 0.001, **P < 0.01, *P < 0.05, ns, not significant.
bNo statistical analysis possible because of missing replicates.
In root cells of woody plants, little evidence exists about the relationship between cell wall polysaccharides and Al3+ sensitivity/resistance. In a set of poplar clones, representing several interspecific crosses, it was found that the Al content of the root symplast was higher in Al3+-resistant clones than in Al3+-sensitive clones (Smith et al., 2011). The Al content of the root symplast, on the other hand, was lower in Al3+-resistant clones than in Al3+-sensitive clones. This pattern of cellular Al distribution suggests that the cell wall of root cells prevented Al3+ from entering the root symplast. Additional parameters investigated were pectin and callose, the latter of which is a widely used indicator of early Al3+ toxicity symptoms (Hirano et al., 2004, 2012; Kochian et al., 2005). Treatment with Al3+ increased pectin and callose levels in all clones, but more prominently in Al3+-sensitive clones. A clear conclusion about the impact of pectin could not be drawn because the degree of pectin methylation was not assessed.
Al3+ TOLERANCE
CHELATION OF Al3+ WITH ORGANIC SUBSTANCES IN THE CYTOSOL
Organic acid anions and phenolic compounds have also been implicated in internal Al3+ tolerance. Once Al3+ enters the cell, the concentration of free Al3+ cations in the cytosol will be very low, but even at these concentrations, Al3+ remains a hazard. The very high affinity of Al3+ for oxygen ligands allows it to compete with other ions for metabolically important sites despite a large disparity in their concentrations (Jones and Ryan, 2003).
Indeed, studies of several woody plant species demonstrate that intracellular Al3+ is chelated by organic acid anions. In the small shrub Melastoma malabathricum, upon entering the root, Al3+ binds to citrate, and the Al–citrate complex itself is transported from the root to the shoot (Watanabe and Osaki, 2001). In the leaves, the Al-citrate complex is transformed into Al–oxalate 1:1, 1:2, and 1:3 complexes. The former two complexes are potentially toxic to the plant. A similar transformation of Al–organic acid complexes is described for the tea plant. Upon entering the root cell, Al3+ binds to oxalate and then is transported from the root to the shoot in the form of Al–citrate and Al–malate complexes (Morita et al., 2004, 2008). In a comparison of several Eucalyptus species, the concentration of root tip malate was found to correlate positively with the degree of Al3+ resistance in the presence of Al3+ (Silva et al., 2004). In contrast, in the poplar clones of the above-mentioned study, the concentrations of symplastic citrate and formate correlated closely with Al3+ sensitivity (Smith et al., 2011).
Besides organic acids, there are other complex forming compounds, e.g., phenolic substances, that bind Al3+ in the cytosol. For example, in the tea plant, Al–catechin complexes were described (Nagata et al., 1992). In the sepals of the small shrub Hydrangea macrophylla, Al3+ is bound to both 3-caffeoylquinic acid and delphinidin 3-glucoside, where Al3+ is thought to play a role in stabilizing the two organic compounds, and thus causing the color to change from red to blue (Ma et al., 2001). In the root apices of the camphor tree (Cinnamomum camphora), an accumulation of proanthocyanidin, which is composed of flavan-3-ols (e.g., catechin), has been demonstrated (Osawa et al., 2011). An increase in root phenolics has been observed by Ofei-Manu et al. (2001) in a series of woody plants upon Al3+ exposure, including Camellia sinensis, Cryptomeria japonica, E. viminalis, Gleditsia triacanthos, Picea abies, Pinus densiflora, Pinus thunbergii, Populus tremuloides, Robinia pseudoacacia, and Rhus succedanea.
SEQUESTRATION OF Al3+ TO METABOLICALLY LESS SENSITIVE COMPARTMENTS
The uptake and storage of high Al3+ concentrations in aerial parts of the plant is a trait common to many plant species of tropical regions, where the ability to cope with Al3+ stress is a strong prerequisite for survival (Ryan and Delhaize, 2010). Plants that accumulate >1 mg g-1 DW Al are considered Al-hyperaccumulators (Jansen et al., 2002). Plant families with woody Al-hyperaccumulators storing very large amounts of Al (>10 mg g-1 DW) in their leaves include Melastomataceae, Rubiaceae, and Theaceae (Matsumoto et al., 1976; Watanabe et al., 1997; Masunaga et al., 1998; Jansen et al., 2003; Olivares et al., 2010; Gonzalez-Santana et al., 2012).
A typical example of a woody plant capable of accumulating large amounts of Al (>15 mg g-1) in its leaves is the tree species Richeria grandis from the Venezuelan cloud forest. Using X-ray microanalysis, Cuenca et al. (1991) showed that Al is stored extracellularly in the cell wall of mature leaves. Further extracellular cell wall storage of Al was demonstrated in the woody Al-hyperaccumulators Camellia sinensis, Conostegia xalapensis, Faramea marginata, and Melastoma malabathricum. The cell wall of the epidermal and the mesophyll cells of the leaves were the main sites for Al accumulation (Watanabe and Osaki, 2001; Britez et al., 2002; Tolra et al., 2011; Gonzalez-Santana et al., 2012). Interestingly, the chloroplasts of Qualea grandiflora and Callisthene major, two Al-hyperaccumulating woody plant species from the Vochysiaceae family, which grow in the Brazilian Cerrado, have been suggested as a primary compartment for Al sequestration (De Andrade et al., 2011). Al3+ can also be sequestered into cells specialized for storage functions, e.g., idioblasts containing Ca-oxalate crystals have been considered as a location for Al3+ detoxification in the leaves of Corchorus olitorius (Mazen, 2004).
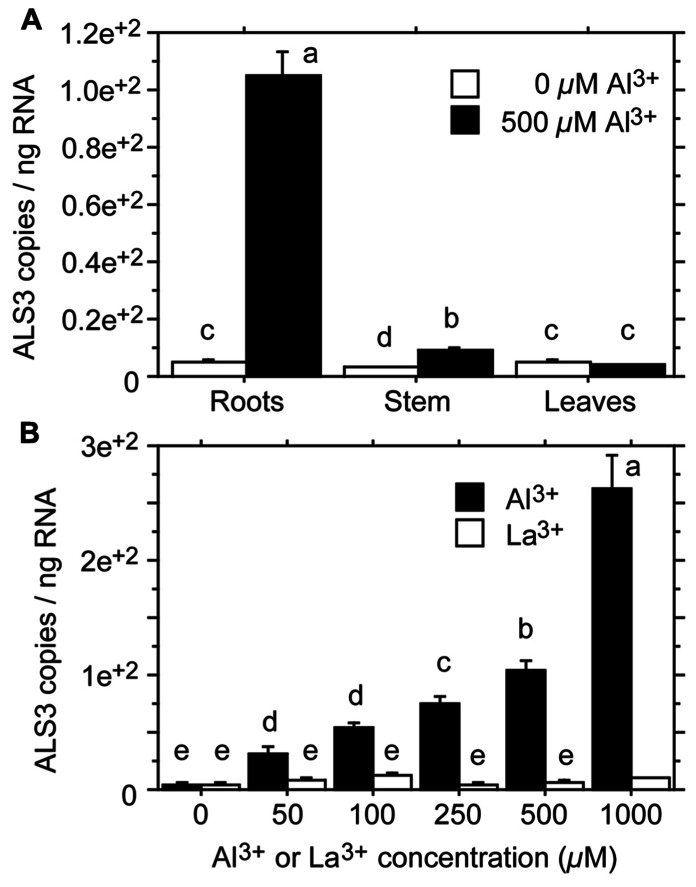
Transport of Al from the root to the shoot is likely to involve complexes of Al with organic acids (see above). Little is known about the transport of Al across the plasma membrane and further sequestration into subcellular compartments. Transport across membranes requires transport proteins. Candidates for such proteins have been identified in the Al3+-sensitive mutants als1 and als3 of Arabidopsis. Both mutants are mutated in genes encoding proteins that belong to the ATP-binding cassette (ABC) transporter superfamily. ALS1 (Al3+-sensitive) is a half type ABC transporter, whereas ALS3 is an ABC transporter-like protein, lacking the ABC domain. The functions and substrates of ALS1 and ALS3 are not known, but the mutant phenotypes, the subcellular localization of the proteins, and tissue-specific gene expression have led to the assumption that the two proteins sequester and transport Al3+ to overcome Al3+ toxicity. ALS1 is likely to be involved in the intracellular transport of Al3+ to vacuoles of root tip cells and cells of the plant vasculature (Larsen et al., 2007). ALS3 is mainly localized to the plasma membrane of root cortex cells and phloem cells throughout the plant, suggesting that it mediates the intercellular redistribution of accumulated Al away from sensitive tissues (Larsen et al., 2005). In Populus tremula, Grisel et al. (2010) identified an ALS3-like gene with a 79% amino acid sequence identity with the Arabidopsis ALS3 gene. The poplar gene was found to be expressed in the root, stem, and leaves, and was strongly induced by Al3+ in the root (44-fold; Figure 3A). In addition, the poplar gene was inducible by Al3+, but not by La3+ (lanthanum; Figure 3B), consistent with the finding that the Arabidopsis mutant als3 is not affected by La3+ (Larsen et al., 1997).
FIGURE 3.
Expression levels of ALS3 in tissues of Populus tremula treated with Al3+ for 2 days. (A) Expression levels in root, stem and leaf tissue after treatment with 0 or 500 μM Al3+. (B) Expression levels in the root tissue after treatment with 0, 50, 100, 250, 500, or 1000 μM Al3+ or La3+. Transcript levels were quantified by absolute qRT-PCR. Different letters indicate significant differences between treatments and elements: ANOVA; P < 0.05 (redrawn from Grisel et al., 2010).
ACTIVATION OF METABOLIC PATHWAYS TO OVERCOME THE TOXIC EFFECTS OF Al3+
Moderate Al3+ concentrations are not fatal, and roots may at least partially recover (see also Matsumoto and Motoda, 2012). This is well documented in Populus tremula treated with either no Al3+ or increasing concentrations of Al3+ up to 1,000 μM. Two phases of root growth could be distinguished: a rapid Al3+-induced growth inhibition (within 6 h at Al3+ concentrations > 250 μM) and a subsequent phase of growth recovery (within 2 days at Al3+ concentrations í 500 μM; Grisel et al., 2010). The root growth of plants treated with 1,000 μM Al3+ further decreased. This pattern of root growth recovery may reflect the success of the roots in activating metabolic pathways to overcome the toxic effects of Al3+ and/or Al3+ resistance mechanisms. Matsumoto and Motoda (2013) suggested that the recovery of roots exposed to Al3+ is associated with the reduction of Al3+-induced oxidative stress. In Populus tremula, two genes of the oxidative stress pathway (a peroxidase gene and an alternative oxidase gene) were strongly induced upon Al3+ exposition after 6 h (>8-fold), while their expression decreased to control levels after 2 days (Grisel et al., 2010).
Two additional genes of Populus tremula that may play a role in root growth recovery encode CorA-like Mg2+ transporters (Grisel et al., 2010). These genes were induced up to fivefold by Al3+. The activity of a homologous CorA-like Mg2+ transporter from Arabidopsis was shown to be blocked by micromolar concentrations of Al3+, when expressed in bacteria (Li et al., 2001). In addition, the same CorA-like Mg2+ transporter alleviated Al3+ toxicity when overexpressed in planta (Deng et al., 2006). Mg2+ has been reported to be able to alleviate Al3+ toxicity in a number of crop plants (see reviews of Bose et al., 2011; Chen and Ma, 2013). Various mechanisms have been put forward to explain how Mg2+ can alleviate Al3+ toxicity. These mechanisms include increased ionic strength of the solutions, reduction in Al3+ saturation at the apoplastic exchange sites, and decreased Al3+ activity at the root cell plasma membrane surface (Bose et al., 2011). However, the identified Mg2+ transporters indicate that, besides of electrostatic interactions, biochemical processes may be involved in the rescue of Al3+ toxicity.
ADAPTATIONS
The tropical forests and the forests of boreal and temperate regions have evolved on geological timescales under very different conditions. The forests of boreal and temperate regions were repeatedly affected by the Pleistocene glaciations, while the impact of the Pleistocene climatic fluctuations was certainly much less severe in tropical regions. Most tropical forests have not been disturbed for hundred thousands of years, and thus typically grow on highly weathered soils, which are strongly acidic, both in the topsoil and the subsoil (Figures 1A,B).
Accumulating evidence shows that in tropical regions both Al excluders and Al accumulators occur. Examples of woody species that are strong excluders are Melaleuca cajuputi, Acacia mangium, and Leucaena leucocephala (Osaki et al., 1997), which are all capable of exuding organic acid anions from their roots (see also Table 2). Examples of woody species that store high amounts of Al in leaves are Melastoma malabathricum and H. macrophylla. Other woody species, such as Vaccinium macrocarpon, store high amounts of Al in their roots (Osaki et al., 1997). Most Al-hyperaccumulator plants are shrub-type broad-leaved woody plants.
Phylogenetic analyses indicate that Al hyperaccumulation is a trait that has arisen a number of times, and this trait is scattered over more than 20 orders across about 45 families belonging to magnoliids (e.g., Laurales), eudicots (e.g., Proteales), rosids (e.g., Malpighiales, Myrtales), and asterids (e.g., Gentianales, Ericales; Table 4; Jansen et al., 2002). To date, Al accumulation in tall trees has only been found in a few tree species of the Euphorbiaceae (Osawa et al., 2013). The predominance of Al accumulators within non-flowering plants suggests that Al accumulation evolved early in the evolution of land-plants and is probably a primitive characteristic associated with survival in ancient Al3+-rich environments (Jansen et al., 2002).
Table 4.
Families of woody plants with strong and/or numerous Al-hyperaccumulators (according to Jansen et al., 2002).
| Clade | Order | Family |
|---|---|---|
| Magnoliids | Laurales | Lauraceae, Monimiaceae, Siparunaceae |
| Eudicots | Proteales | Proteaceae |
| Eurosids I | Malpighiales | Euphorbiaceae |
| Eurosids II | Myrtales | Crypteroniaceae, Melastomataceae, Vochysiaceae |
| Asterids | Ericales | Diapensiaceae, Symplocaceae, Ternstroemiacieae, Theaceae |
| Euasterids I | Gentianales | Rubiaceae |
An analysis of the variation in foliar Al and macronutrient concentrations in a global dataset of plant species in a phylogenetic framework showed that the frequency distribution of foliar Al concentration in tropical regions clearly had two peaks (“bimodal”), whereas in temperate regions it showed only one peak (“unimodal”; Metali et al., 2012). This conclusion supports the hypothesis that Al accumulators and non-Al accumulators exist as distinct, but overlapping, groups of species. The estimated threshold value of foliar Al concentrations that distinguishes Al accumulators from non-Al accumulators varies geographically. The foliar threshold of tropical plants is higher (2.3–3.9 mg g-1) than that of temperate plants (1.1 mg g-1; Metali et al., 2012). Among angiosperm species, there was a significant phylogenetic signal in foliar Al concentrations, substantiating results of previous studies, suggesting a greater prevalence of Al accumulators in some families than in others (e.g., Jansen et al., 2002, 2003). A phylogenetical signal may also arise when related species occupy relatively similar habitats that differentially influence nutrient uptake and accumulation (Thompson et al., 1997). For example, Schreeg et al. (2010) have provided evidence for significant associations between high soil Al and Mn concentrations and the distributions of trees in the Vochysiaceae and Myrtaceae on a 50-ha plot in a semideciduous moist forest in Panama.
Compared to soils of tropical regions, soils of boreal and temperate regions are generally younger, although large areas, such as Siberia and Beringia, were ice-free during the Last Glacial Maximum (26,500–18,000 year before present; Clark et al., 2009). However, soils of these ice-free areas were severely affected by cold climate and permafrost, strongly limiting soil chemical and soil biological processes. As a consequence, soils in boreal and temperate regions are generally less weathered and thus less acidic than tropical soils, particularly in the subsoil (Figure 1B). In contrast, topsoils of the boreal regions are highly acid due to the strong acidifying effect of the coniferous litter during the incomplete decomposition process and the formation of humic acids (Schachtschabel et al., 1992).
Aluminum concentrations measured in roots from temperate or boreal woody species suggest, that the immobilization of Al3+ in the cell wall is most likely an important adaptation. Al concentrations in the fine roots of common temperate and boreal woody species usually exceed the limit for being hyperaccumulators (>1 mg g-1). For example fine roots of Abies alba, Castanea sativa, Fagus sylvatica, Picea abies, Pinus cembra, and Pinus montana all have values of 1–10 mg g-1 DW (Zysset et al., 1996; Brunner et al., 2002; Genenger et al., 2003; Hirano et al., 2006; Richter et al., 2011; see also Table 3). All these woody species are ectomycorrhizal, suggesting that ectomycorrhizal structures, such as fungal mantle and Hartig net, further contribute to the accumulation of Al in roots by immobilizing Al in the cell wall of the fungal hyphae (Brunner and Frey, 2000; Heim et al., 2003) or in the fungal vacuoles (Martin et al., 1994). Ectomycorrhizas in temperate and boreal regions have, we assume, the function not only to take up water and nutrients but also to immobilize toxic Al3+. Because ectomycorrhizal trees and ericoid-mycorrhizal Ericales dominate in temperate and boreal regions, we propose that this mechanism to immobilize Al3+ is a major mechanism for excluding Al3+ from the roots of woody plants in these regions, a suggestion first proposed by Jansen et al. (2002). Moreover, mycorrhizal fungi may well contribute significantly to the cycling of Al in forest ecosystem because high concentrations of Al can be found in their fruiting bodies (Smits and Hoffland, 2009). An additional adaptation of tree roots from temperate or boreal forests to acid soils is the observation that the roots have a shorter lifespan, which means that the turnover rate of roots exposed to elevated Al3+ concentrations is higher (Godbold et al., 2003; Leuschner et al., 2004; Richter et al., 2013). Reasons for a shorter lifespan could be that Al accumulation has reached its saturation point faster compared to roots from non-acid soils, causing the root to die earlier.
Some woody plants in temperate regions have not evolved strategies to deal with high levels of Al3+ and thus are not adapted to acid soils. Examples are the two species Fraxinus excelsior and Acer pseudoplatanus, which both are not ectomycorrhizal. They grow mainly on forest sites with high pH and high base saturation, and are unlikely to occur on very acid soils (Weber-Blaschke et al., 2002). In a recent study, Walthert et al. (2013) showed that Fraxinus excelsior and Acer pseudoplatanus respond much more sensitively to soil properties than Fagus sylvatica does. The soil properties limiting their distribution are Al in the case of Fraxinus excelsior, and Al together with nutrient availability (C/N ratio) in the case of Acer pseudoplatanus. On the contrary, no soil-induced distribution limits were detectable for Fagus sylvatica (Walthert et al., 2013).
Differences in Al3+ resistance not only exist among different tree species but also among local populations of the same species. Kidd and Proctor (2000) assessed the level of Al3+ resistance in four populations of Betula pendula growing on soils that differ in soil acidity and levels of Al3+. Using a solution culture system which simulates natural soil solutions, the authors found that seedlings originating from populations from acid mineral soils (pH 4.3, Al3+ concentration 21.1 mg l-1), had a significant higher level of Al3+ resistance (measured with the root elongation rate) compared to seedlings from populations from non-acid soils (pH > 4.8, Al3+ < 5.3 mg l-1). A similar study was performed by Wilkins and Hodson (1989) who analyzed two populations of Picea abies growing on an acid mineral soil and a calcareous soil, respectively. The growth of seedlings from the acid soil was slightly stimulated by the Al3+ treatment, whereas the growth of that from the calcareous soil was greatly reduced. The results of these studies suggest that high Al3+ soil conditions are a significant force for population divergence due to strong selective pressure associated with adaptation.
Further evidence for population adaptation to acid soil conditions comes from a study of Pinus contorta growing along a steep gradient of soil acidity at the northern coast of California (Eckert et al., 2012). In this area, five well-developed marine terraces exist, whose soils represent a chronosequence ranging from fertile soils close to the coast to podzolic soils with low pH and high Al3+ concentrations about 5 km inland (Westman, 1975). Pinus contorta is one of three conifers that have colonized and diversified on the extreme podzolic soils. Shore pine (Pinus contorta ssp. contorta) is found along the lower terraces, while Bolander pine (Pinus contorta ssp. bolanderi), which has a pigmy growth habit, is endemic to the upper marine terraces. Using a candidate gene approach, Eckert et al. (2012) investigated the molecular basis for the colonization of the podzolic soils by Pinus contorta populations. Patterns of nucleotide diversity were analyzed in genes that are related to growth or response to several soil parameters, such as Al3+, BC, and phosphate. The majority of the 21 genes analyzed did not or only weakly deviate from neutrality in patterns of nucleotide diversity. However, two of the genes carried clear signatures of positive selection: one was a putative homolog of the Arabidopsis ALS3 gene and the other an inorganic phosphate transporter gene. The ALS3 gene was characterized by a derived non-synonymous mutation, which is extremely rare in populations of the lower terraces and almost or completely fixed in those of the higher terraces. The phosphate transporter gene included two highly divergent haplotypes, whose frequency differed among lower and higher terraces. The results of this study shed light on some of the genetic components underlying adaptation to local soil conditions along this unique environmental gradient.
CONCLUSION
Current evidence indicates that woody plants native to acid soils have evolved various strategies to overcome Al3+ stress. These strategies include exclusion of Al3+ from the root tip, probably through the release of organic acid anions. The formation of ectomycorrhizal structures, which are capable of accumulating Al in the cell wall of hyphal cells, may also be regarded as an exclusion strategy, reducing the degree of contact of root cells to Al3+. Internal strategies rely on the transport and sequestration of Al in aerial parts of the plant, where the Al may be stored in the cell wall of different leaf tissues, or in subcellular compartments, like chloroplasts or possibly vacuoles. Some woody plant species may also store high levels of Al in the cell wall of root cells. The biochemical and molecular mechanisms underlying these strategies, however, remain largely to be determined. The few studies performed so far in woody plants provide hints in which direction research may focus. With the exception of poplar, forest trees are generally not amenable to genetic engineering for testing the impact of candidate genes. Similarly, progenies of controlled crosses, which are valuable for testing segregation of specific traits and genes, exist only for a few tree species. An alternative approach would involve the identification of genes that play a role in adaptation through association of genetic variation with particular soil Al3+ conditions. This has already been initiated and has provided insights into the mechanisms of population adaptation to Al3+-rich environments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the reviewers for giving valuable comments on the manuscript. And we thank Silvia Dingwall for correcting and improving the English text. Some of the forest sites cited in this review paper belong to the Long-Term Forest Ecosystem Research Programme LWF of Switzerland.
REFERENCES
- Ahonen-Jonnarth U., van Hees P. A. W., Lundström U. S., Finlay R. D. (2000). Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and metal concentrations. New Phytol. 146 557–567 10.1046/j.1469-8137.2000.00653.x [DOI] [Google Scholar]
- Blaser P., Zysset M., Zimmermann S., Luster J. (1999). Soil acidification in southern Switzerland between 1987 and 1997: a case study based on the critical load concept. Environ. Sci. Technol. 33 2383–2389 10.1021/es9808144 [DOI] [Google Scholar]
- Bose J., Babourina O., Rengel Z. (2011). Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 62 2251–2264 10.1093/jxb/erq456 [DOI] [PubMed] [Google Scholar]
- Britez R. M., Watanabe T., Jansen S., Reissmann C. B., Osaki M. (2002). The relationship between aluminum and silicon accumulations in leaves of Faramea marginata (Rubiaceae). New Phytol. 156 437–444 10.1046/j.1469-8137.2002.00531.x [DOI] [PubMed] [Google Scholar]
- Brunner I., Brodbeck S., Walthert L. (2002). Fine root chemistry, starch concentration, and ‘vitality’ of subalpine conifer forests in relation to soil pH. For. Ecol. Manag. 165 75–84 10.1016/S0378-1127(01)00633-8 [DOI] [Google Scholar]
- Brunner I., Frey B. (2000). Detection and localization of aluminum and heavy metals in ectomycorrhizal Norway spruce seedlings. Environ. Pollut. 108 121–128 10.1016/S0269-7491(99)00248-1 [DOI] [PubMed] [Google Scholar]
- Brunner I., Luster J., Günthardt-Goerg M. S., Frey B. (2008). Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environ. Pollut. 152 559–568 10.1016/j.envpol.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Brunner I., Zimmermann S., Zingg A., Blaser P. (2004). Wood-ash recycling affects forest soil and tree fine-root chemistry and reverses soil acidification. Plant Soil 267 61–71 10.1007/s11104-005-4291-z [DOI] [Google Scholar]
- Chen Z. C., Ma J. F. (2013). Magnesium transporters and their role in Al tolerance in plants. Plant Soil. 10.1007/s11104-012-1433-y. [DOI] [Google Scholar]
- Clark P. U., Dyke A. S., Shakun J. D., Carlson A. E., Clark J., Wohlfarth B., et al. (2009). The last glacial maximum. Science 325 710–714 10.1126/science.1172873 [DOI] [PubMed] [Google Scholar]
- Cronan C. S., Grigal D. F. (1995). Use of calcium/aluminum ratios as indicators of stress in forest ecosystems. J. Environ. Qual. 24 209–226 10.2134/jeq1995.00472425002400020002x [DOI] [Google Scholar]
- Cronan C. S., Schofield C. L. (1979). Aluminum leaching response to acid precipitation: effects on high-elevation watersheds in the Northeast. Science 204 304–306 10.1126/science.204.4390.304 [DOI] [PubMed] [Google Scholar]
- Cuenca G., Herrera R., Merida T. (1991). Distribution of aluminum in accumulator plants by X-ray microanalysis in Richeria grandis Vahl leaves from a cloud forest in Venezuela. Plant Cell Environ. 14 437–441 10.1111/j.1365-3040.1991.tb00954.x [DOI] [Google Scholar]
- De Andrade L. R. M., Gomes Barros L. M., Echevarria G. F., do Amaral L. I. V., Cotta M. G., Rossatto D. R., et al. (2011). Al-hyperaccumulator Vochysiaceae from the Brazilian Cerrado store aluminum in their chloroplasts without apparent damage. Environ. Exp. Bot. 70 37–42 10.1016/j.envexpbot.2010.05.013 [DOI] [Google Scholar]
- Degenhardt J., Larsen P. B., Howell S. H., Kochian L. V. (1998). Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 117 19–27 10.1104/pp.117.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E., Gruber B. D., Ryan P. R. (2007). The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 581 2255–2262 10.1016/j.febslet.2007.03.057 [DOI] [PubMed] [Google Scholar]
- Delhaize E., Ma J. F., Ryan P. R. (2012). Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 17 341–348 10.1016/j.tplants.2012.02.008 [DOI] [PubMed] [Google Scholar]
- Delhaize E., Ryan P. R., Randall P. J. (1993). Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 103 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Luo K., Li Z., Yang Y., Hu N., Wu Y. (2009). Overexpression of Citrus junos mitochondrial citrate synthase gene in Nicotiana benthamiana confers aluminium tolerance. Planta 230 355–365 10.1007/s00425-009-0945-z [DOI] [PubMed] [Google Scholar]
- Deng W., Luo K., Li D., Zheng X., Wei X., Smith W., et al. (2006). Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J. Exp. Bot. 57 4235–4243 10.1093/jxb/erl201 [DOI] [PubMed] [Google Scholar]
- Eckert A. J., Shahi H., Datwyler S. L., Neale D. B. (2012). Spatially variable natural selection and the divergence between parapatric subspecies of lodgepole pine (Pinus contorta, Pinaceae). Am. J. Bot. 99 1323–1334 10.3732/ajb.1200055 [DOI] [PubMed] [Google Scholar]
- Eldhuset T. D., Swensen B., Wickstrøm T., Wollebæk G. (2007). Organic acids in root exudates from Picea abies seedlings influenced by mycorrhiza and aluminium. J. Plant Nutr. Soil Sci. 170 645–648 10.1002/jpln.200700005 [DOI] [Google Scholar]
- Eticha D., Stass A., Horst W. J. (2005). Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ. 28 1410–1420 10.1111/j.1365-3040.2005.01375.x [DOI] [Google Scholar]
- Evans C. D., Cullen J. M., Alewell C., Kopácek J., Marchetto A., Moldan F., et al. (2001). Recovery from acidification in European surface waters. Hydrol. Earth Syst. Sci. 5 283–297 10.5194/hess-5-283-2001 [DOI] [Google Scholar]
- FAO and IIASA. (2007) Mapping Biophysical Factors That Influence Agricultural Production and Rural Vulnerability. Rome: FAO and IIASA [Google Scholar]
- FAO/IIASA/ISRIC/ISS-CAS/JRC. (2012). Harmonized World Soil Database version 1.2. Rome: FAO/Laxenburg: IIASA [Google Scholar]
- Fowler D., Cape J. N., Coyle M., Flechard C., Kuylenstierna J., Hicks K., et al. (1999). The global exposure of forests to air pollution. Water Air Soil Pollut. 116 5–32 10.1023/A:1005249231882 [DOI] [Google Scholar]
- Franceschi V. R., Nakata P. A. (2005). Calcium oxalate in plants: formation and function. Annu. Rev. Plant Biol. 56 41–71 10.1146/annurev.arplant.56.032604.144106 [DOI] [PubMed] [Google Scholar]
- Franco C. R., Chagas A. P., Jorge R. A. (2004). Ion-exchange equilibria with aluminum pectinates. Colloids Surf. A Physicochem. Eng. Asp. 204 183–192 10.1016/S0927-7757(01)01134-7 [DOI] [Google Scholar]
- Furukawa J., Yamaji N., Wang H., Mitani N., Murata Y., Sato K., et al. (2007). An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 48 1081–1091 10.1093/pcp/pcm091 [DOI] [PubMed] [Google Scholar]
- Genenger M., Zimmermann S., Hallenbarter D., Landolt W., Frossard E., Brunner I. (2003). Fine root growth and element concentrations of Norway spruce as affected by wood ash and liquid fertilisation. Plant Soil 255 253–264 10.1023/A:1026118101339 [DOI] [Google Scholar]
- Godbold D. L., Fritz H. W., Jentschke G., Meesenburg H., Rademacher P. (2003). Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity. Tree Physiol. 23 915–921 10.1093/treephys/23.13.915 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Santana I. H., Marquez-Guzman J., Cram-Heydrich S., Cruz-Ortega R. (2012). Conostegia xalapensis (Melastomataceae): an aluminum accumulator plant. Physiol. Plant. 144 134–145 10.1111/j.1399-3054.2011.01527.x [DOI] [PubMed] [Google Scholar]
- Graf Pannatier E., Thimonier A., Schmitt M., Walthert L., Waldner P.(2011). A decade of monitoring at Swiss Long-Term Forest Ecosystem Research (LWF) sites: can we observe trends in atmospheric acid deposition and in soil solution acidity? Environ. Monit. Assess. 174 3–30 10.1007/s10661-010-1754-3 [DOI] [PubMed] [Google Scholar]
- Grisel N., Zoller S., Künzli-Gontarczyk M., Lampart T., Münsterkotter M., Brunner I., et al. (2010). Transcriptome responses to aluminum stress in roots of aspen (Populus tremula). BMC Plant Biol. 10:185 10.1186/1471-2229-10-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim A., Brunner I., Frey B., Frossard E., Luster J. (2001). Root exudation, organic acids, and element distribution in roots of Norway spruce seedlings treated with aluminium in hydroponics. J. Plant Nutr. Soil Sci. 164 519–526 10.1002/1522-2624(200110)164:5 [DOI] [Google Scholar]
- Heim A., Brunner I., Frossard E., Luster J. (2003). Aluminum effects on Picea abies at low solution concentrations. Soil Sci. Soc. Am. J. 67 895–898 10.2136/sssaj2003.0895 [DOI] [Google Scholar]
- Heim A., Luster J., Brunner I., Frey B., Frossard E. (1999). Effects of aluminium treatment on Norway spruce roots: aluminium binding forms, element distribution, and release of organic acids. Plant Soil 216 103–116 10.1023/A:1004728122261 [DOI] [Google Scholar]
- Hirano Y., Frey B., Brunner I. (2012). Contrasting reactions of roots of two coniferous tree species to aluminum stress. Environ. Exp. Bot. 77 2–18 10.1016/j.envexpbot.2011.10.007 [DOI] [Google Scholar]
- Hirano Y., Graf Pannatier E., Zimmermann S., Brunner I. (2004). Induction of callose in roots of Norway spruce seedlings after short-term exposure to Al. Tree Physiol. 24 1270–1283 10.1093/treephys/24.11.1279 [DOI] [PubMed] [Google Scholar]
- Hirano Y., Walthert L., Brunner I. (2006). Callose in root apices of European chestnut seedlings; a physiological indicator of aluminium stress. Tree Physiol. 26 431–440 10.1093/treephys/26.4.431 [DOI] [PubMed] [Google Scholar]
- Hoekenga O. A., Maron L. G., Piñeros M. A., Cançado G. M., Shaff J., Kobayashi Y., et al. (2006). AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103 9738–9743 10.1073/pnas.0602868103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst W. J., Wang Y., Eticha D. (2010). The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann. Bot. 106 185–197 10.1093/aob/mcq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza-Blancheteau C., Rengel Z., Alberdi M., de la Luz Mora M., Aquea F., Arce-Johnson P., et al. (2012). Molecular and physiological strategies to increase aluminum resistance in plants. Mol. Biol. Rep. 39 2069–2079 10.1007/s11033-011-0954-4 [DOI] [PubMed] [Google Scholar]
- Ishikawa S., Wagatsuma T., Sasaki R., Ofei-Manu P. (2000). Comparison of the amount of citric and malic acids in Al media of seven plant species and two cultivars each in five plant species. Soil Sci. Plant Nutr. 46 751–758 10.1080/00380768.2000.10409141 [DOI] [Google Scholar]
- Jansen S., Broadley M. R., Robbrecht E., Smets E. (2002). Aluminum hyperaccumulation in Angiosperms: a review of its phylogenetic significance. Bot. Rev. 68 235–269 10.1663/0006-8101(2002)0682.0.CO;2 [DOI] [Google Scholar]
- Jansen S., Watanabe T., Dessein S., Smets E., Robbrecht E. (2003). A comparative study of metal levels in leaves of some Al-accumulating Rubiaceae. Ann. Bot. 91 657–663 10.1093/aob/mcg071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. L., Ryan P. R. (2003). “Aluminum toxicity,” in Encyclopedia of Applied Plant Science eds Thomas B., Murphy D., Murray B. G. (London: Elsevier Academic Press; ) 656–664 [Google Scholar]
- Kidd P. S., Proctor J. (2000). Effects of aluminium on the growth and mineral composition of Betula pendula Roth. J. Exp. Bot. 51 1057–1066 10.1093/jexbot/51.347.1057 [DOI] [PubMed] [Google Scholar]
- Kinraide T. B. (1997). Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminium. J. Exp. Bot. 48 1115–1124 10.1093/jxb/48.5.1115 [DOI] [Google Scholar]
- Kinraide T. B. (2003). Toxicity factors in acidic forest soils: attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Eur. J. Soil Sci. 54 323–333 10.1046/j.1365-2389.2003.00538.x [DOI] [Google Scholar]
- Kochian L. V. (1995). Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 46 237–260 10.1146/annurev.pp.46.060195.001321 [DOI] [Google Scholar]
- Kochian L. V., Hoekenga O. A., Pineros M. A. (2004). How do crop plants tolerate acid soils? – mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 55 459–493 . 10.1146/annurev.arplant.55.031903.141655 [DOI] [PubMed] [Google Scholar]
- Kochian L. V., Pineros M. A., Hoekenga O. A. (2005). The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274 175–195 10.1007/s11104-004-1158-7 [DOI] [Google Scholar]
- Larsen P. B., Cancel J., Rounds M., Ochoa V. (2007). Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 225 1447–1458 10.1007/s00425-006-0452-4 [DOI] [PubMed] [Google Scholar]
- Larsen P. B., Degenhardt J., Tai C. Y., Stenzler L. M., Howell S. H., Kochian L. V. (1998). Aluminum-resistant Arabidopsis mutants that exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiol. 117 9–18 10.1104/pp.117.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. B., Geisler M. J. B., Jones C. A., Williams K. M., Cancel J. D. (2005). ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 41 353–363 10.1111/j.1365-313X.2004.02306.x [DOI] [PubMed] [Google Scholar]
- Larsen P. B., Kochian L. V., Howell S. H. (1997). Al inhibits both shoot development and root growth in als3, an Al-sensitive Arabidopsis mutant. Plant Physiol. 114 1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larssen T., Lydersen E., Tang D., He Y., Gao J., Liu H., et al. (2006). Acid rain in China. Environ. Sci. Technol. 40 418–425 10.1021/es0626133 [DOI] [PubMed] [Google Scholar]
- Leuschner C., Hertel D., Schmid I., Koch O., Muhs A., Hölscher D. (2004). Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility. Plant Soil 258 43–56 10.1023/B:PLSO.0000016508.20173.80 [DOI] [Google Scholar]
- Li L., Tutone A. F., Drummond R. S. M., Gardner R. C., Luan S. (2001). A novel family of magnesium transport genes in Arabidopsis. Plant Cell 13 2761–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert B., Franceschi V. R. (1987). Oxalate in crop plants. J. Agric. Food Chem. 35 926–938 10.1021/jf00078a019 [DOI] [Google Scholar]
- Ligaba A., Katsuhura M., Ryan P. R., Shibasaka M., Matsumoto H. (2006). The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 142 1294–1303 10.1104/pp.106.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. F. (2007). Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 264 225–252 10.1016/S0074-7696(07)64005-4 [DOI] [PubMed] [Google Scholar]
- Ma J. F., Ryan P. R., Delhaize E. (2001). Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 6 273–278 10.1016/S1360-1385(01)01961-6 [DOI] [PubMed] [Google Scholar]
- Magalhaes J. V., Liu J., Guimarães C. T., Lana U. G. P., Alves V. M. C., Wang Y. H., et al. (2007). A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 39 1156–1161 10.1038/ng2074 [DOI] [PubMed] [Google Scholar]
- Maron L. G., Kirst M., Mao C., Milner M. J., Menossi M., Kochian L. V. (2008). Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol. 179 116–128 10.1111/j.1469-8137.2008.02440.x [DOI] [PubMed] [Google Scholar]
- Martin F., Rubini P., Cote R., Kottke I. (1994). Aluminum polyphosphate complexes in the mycorrhizal basidiomycete Laccaria bicolor: a 27Al-nuclear magnetic resonance study. Planta 194 241–246 10.1007/BF01101683 [DOI] [Google Scholar]
- Masunaga T., Kubota D., Hotta M., Wakatsuki T. (1998). Mineral composition of leaves and bark in aluminium accumulators in a tropical rain forest in Indonesia. Soil Sci. Plant Nutr. 44 347–358 10.1080/00380768.1998.10414456 [DOI] [Google Scholar]
- Matsumoto H., Hirasawa E., Morimura S., Takahashi E. (1976). Localization of aluminium in tea leaves. Plant Cell Physiol. 17 627–631 10.1007/s10265-010-0344-3 [DOI] [Google Scholar]
- Matsumoto H., Motoda H. (2012). Aluminum toxicity recovery processes in root apices. Possible association with oxidative stress. Plant Sci. 185–186 1–8 10.1016/j.plantsci.2011.07.019 [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Motoda H. (2013). Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil. 363 399–410 10.1007/s11104-012-1396-z. [DOI] [Google Scholar]
- Mazen A. M. A. (2004). Calcium oxalate deposits in leaves of Corchorus olitorius as related to accumulation of toxic metals. Russ. J. Plant Physiol. 51 281–285 10.1023/B:RUPP.0000019226.03536.21 [DOI] [Google Scholar]
- Metali F., Salim K. A., Burslem D. F. R. P. (2012). Evidence of foliar aluminium accumulation in local, regional and global datasets of wild plants. New Phytol. 193 637–649 10.1111/j.1469-8137.2011.03965.x [DOI] [PubMed] [Google Scholar]
- Mohnen D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11 266–277 10.1016/j.pbi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Morita A., Horie H., Fujii Y., Takatsu S., Watanabe H., Yagi A., et al. (2004). Chemical forms of aluminum in xylem sap of tea plants (Camellia sinensis L.). Phytochemistry 65 2775–2780 10.1016/j.phytochem.2004.08.043 [DOI] [PubMed] [Google Scholar]
- Morita A., Yanagisawa O., Maeda S., Hiradate S. (2008). Mechanism for the detoxification of aluminum in roots of tea plant (Camellia sinensis (L.) Kuntze). Phytochemistry 69 147–153 10.1016/j.phytochem.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Morita A., Yanagisawa O., Maeda S., Takatsu S., Ikka T. (2011). Tea plant (Camellia sinensis L.) roots secrete oxalic acid and caffeine into medium containing aluminium. Soil Sci. Plant Nutr. 57 796–802 10.1080/00380768.2011.629176 [DOI] [Google Scholar]
- Nagata T., Hayatsu M., Kosuge N. (1992). Identification of aluminium forms in tea leaves by 27Al NMR. Phytochemistry 31 1215–1218 10.1016/0031-9422(92)80263-E [DOI] [Google Scholar]
- Naik D., Smith E., Cumming J. R. (2009). Rhizosphere carbon deposition, oxidative stress and nutritional changes in two poplar species exposed to aluminum. Tree Physiol. 29 423–436 10.1093/treephys/tpn035 [DOI] [PubMed] [Google Scholar]
- Nguyen N. T., Nakabayashi K., Thompson J., Fujita K. (2003). Role of exudation of organic acids and phosphate in aluminum tolerance of four tropical woody species. Tree Physiol. 23 1041–1050 10.1093/treephys/23.15.1041 [DOI] [PubMed] [Google Scholar]
- Ofei-Manu P., Wagatsuma T., Ishikawa S., Twaraya K. (2001). The plasma membrane strength of the root-tip cells and root phenolic-compounds are correlated with Al tolerance in several common woody plants. Soil Sci. Plant Nutr. 47 359–375 10.1080/00380768.2001.10408399 [DOI] [Google Scholar]
- Olivares E., Colonnello G., Pena E., Rodriguez L. (2010). Aluminum accumulation in nineteen Melastomataceae species from three contrasting plant formations in acid soils. J. Plant. Nutr. Soil Sci. 183 453–460 10.1002/jpln.200900152 [DOI] [Google Scholar]
- Osaki M., Watanabe T., Tadano T. (1997). Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci. Plant Nutr. 43 551–563 10.1080/00380768.1997.10414782 [DOI] [Google Scholar]
- Osawa H., Endo I., Hara Y., Matsushima Y., Tange T. (2011). Transient proliferation of proanthocyanidin-accumulating cells on the epidermal apex contributes to highly aluminum-resistant root elongation in camphor tree. Plant Physiol. 155 433–446 10.1104/pp.110.166967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H., Ikeda S., Tange T. (2013). The rapid accumulation of aluminum is ubiquitous in both the evergreen and deciduous leaves of Theaceae and Ternstroemiaceae plant over a wide pH range in acidic soils. Plant Soil 363 49–59 10.1007/s11104-012-1285-5 [DOI] [Google Scholar]
- Poschenrieder C., Gunsé B., Corrales I., Barceló J. (2008). A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 400 356–368 10.1016/j.scitotenv.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Qin R., Hirano Y., Brunner I. (2007). Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. Tree Physiol. 27 313–320 10.1093/treephys/27.2.313 [DOI] [PubMed] [Google Scholar]
- Rahman M. M., Kawamura O. (2011). Oxalate accumulation in forage plants: some agronomic, climatic and genetic aspects. Asian-australas. J. Anim. Sci. 24 439–448 10.5713/ajas.2011.10208 [DOI] [Google Scholar]
- Rangel A. F., Rao I. M., Horst W. J. (2009). Intracellular distribution and binding state of aluminium in root apices of two common bean (Phaseolus vulgaris) genotypes in relation to Al toxicity. Physiol. Plant. 135 162–173 10.1111/j.1399-3054.2008.01183.x [DOI] [PubMed] [Google Scholar]
- Richter A. K., Hajdas I., Frossard E., Brunner I. (2013). Soil acidity affects fine root turnover of European beech. Plant Biosyst. 147 50–59 10.1080/11263504.2012.742471 [DOI] [Google Scholar]
- Richter A. K., Hirano Y., Luster J., Frossard E., Brunner I. (2011). Soil base saturation affects root growth of European beech seedlings. J. Plant Nutr. Soil Sci. 174 408–419 10.1002/jpln.200900351 [DOI] [Google Scholar]
- Richter A. K., Walthert L., Frossard E., Brunner I. (2007). Does low soil base saturation affect the vitality of fine roots of European beech? Plant Soil 298 69–79 10.1007/s11104-007-9338-x [DOI] [Google Scholar]
- Ryan P. R., Delhaize E. (2010). The convergent evolution of aluminium resistance in plants exploits a convenient currency. Funct. Plant Biol. 37 275–284 10.1071/FP09261 [DOI] [Google Scholar]
- Ryan P. R., DiTomaso J. M., Kochian L. V. (1993). Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J. Exp. Bot. 44 437–446 10.1093/jxb/44.2.437 [DOI] [Google Scholar]
- Ryan P. R., Tyerman S. D., Sasaki T., Furuichi T., Yamamoto Y., Zhang W. H., et al. (2011). The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J. Exp. Bot. 62 9–20 10.1093/jxb/erq272 [DOI] [PubMed] [Google Scholar]
- Sasaki T., Yamamoto Y., Ezaki B., Katsuhara M., Ahn S. J., Ryan P. R., et al. (2004). A wheat gene encoding an aluminum-activated malate transporter. Plant J. 37 645–653 10.1111/j.1365-313X.2003.01991.x [DOI] [PubMed] [Google Scholar]
- Schachtschabel P., Blume H. P., Brümmer G., Hartge K. H., Schwertmann U. (1992). Lehrbuch der Bodenkunde, 13 Auflage. Stuttgart: Ferdinand Enke Verlag [Google Scholar]
- Schreeg L. A., Kress W. J., Erickson D. L., Swenson N. G. (2010). Phylogenetic analysis of local-scale tree soil associations in a lowland moist tropical forest. PLoS ONE 5:e13685 10.1371/journal.pone.0013685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I. R., Novais R. F., Jham G. N., Barros N. F., Gebrim F. O., Nunes F. N., et al. (2004). Responses of eucalypt species to aluminum: the possible involvement of low molecular weight organic acids in the Al tolerance mechanism. Tree Physiol. 24 1267–1277 10.1093/treephys/24.11.1267 [DOI] [PubMed] [Google Scholar]
- Smith E., Naik D., Cumming J. R. (2011). Genotypic variation in aluminum resistance, cellular aluminum fractions, callose and pectin formation and organic acid accumulation in roots of Populus hybrids. Environ. Exp. Bot. 72 182–193 10.1016/j.envexpbot.2011.03.003 [DOI] [Google Scholar]
- Smits M. M., Hoffland E. (2009). Possible role of ectomycorrhizal fungi in cycling of aluminium in podzols. Soil Biol. Biochem. 41 491–497 10.1016/j.soilbio.2008.11.023 [DOI] [Google Scholar]
- Stoddard J. L., Jeffries D. S., Lükeville A., Clair T. A., Dillon P. J., Driscoll C. T., et al. (1999). Regional trends in aquatic recovery from acidification in North America and Europe. Nature 401 575–578 10.1038/44114 [DOI] [Google Scholar]
- Sverdrup H., Warfvinge P. (1993). The Effect of Soil Acidification on the Growth of Trees, Grass, and Herbs as Expressed by the (Ca + Mg + K)/Al Ratio Vol. 2, Report in Ecology and Environmental Engineering. Lund: Lund University [Google Scholar]
- Tabuchi A., Matsumoto H. (2001). Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol. Plant. 112 353–358 10.1034/j.1399-3054.2001.1120308.x [DOI] [PubMed] [Google Scholar]
- Tahara K., Norisada M., Tange T., Yagi H., Kojima K. (2005). Ectomycorrhizal association enhances Al tolerance by inducing citrate secretion in Pinus densiflora. Soil Sci. Plant Nutr. 51 397–403 10.1111/j.1747-0765.2005.tb00045.x [DOI] [Google Scholar]
- Tahara K., Norisada M., Yamanoshita T., Kojima K. (2008). Role of binding ligands in aluminum resistance of Eucalyptus camaldulensis and Melaleuca cajuputi. Plant Soil 302 175–187 10.1007/s11104-007-9464-5 [DOI] [Google Scholar]
- Thompson K., Parkinson J. A., Band S. R., Spencer R. E. (1997). A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytol. 136 679–689 10.1046/j.1469-8137.1997.00787.x [DOI] [PubMed] [Google Scholar]
- Tolra R., Vogel-Mikus K., Hajiboland R., Kump P., Pongrac P., Kaulich B., et al. (2011). Localization of aluminium in tea (Camellia sinensis) leaves using low energy X-ray fluorescence spectro-microscopy. J. Plant Res. 124 165–172 10.1007/s10265-010-0344-3 [DOI] [PubMed] [Google Scholar]
- Ulrich B., Mayer R., Khanna P. K. (1980). Chemical changes due to acid precipitation in a Loess-derived soil in Central Europe. Soil Sci. 130 193–199 10.1097/00010694-198010000-00005 [DOI] [Google Scholar]
- Vanguelova E., Hirano Y., Eldhuset T. D., Sas-Paszt L., Bakker M. R., Püttsepp U., et al. (2007). Tree fine root Ca/Al molar ratio – indicator of Al and acidity stress. Plant Biosyst. 141 460–480 10.1080/11263500701626192 [DOI] [Google Scholar]
- von Uexküll H. R., Mutert E. (1995). Global extent, development and economic impact of acid soils. Plant Soil 171 1–15 10.1007/BF00009558 [DOI] [Google Scholar]
- Walthert L., Graf Pannatier E., Meier E. S. (2013). Shortage of nutrients and excess of toxic elements in soils limit the distribution of soil-sensitive tree species in temperate forests. For. Ecol. Manag. 297 94–107 10.1016/j.foreco.2013.02.008 [DOI] [Google Scholar]
- Watanabe T., Osaki M. (2001). Influence of aluminum and phosphorus on growth and xylem sap composition in Melastoma malabathricum L. Plant Soil 237 63–70 10.1023/A:1013395814958 [DOI] [Google Scholar]
- Watanabe T., Osaki M., Tadano T. (1997). Aluminum-induced growth stimulation in relation to calcium, magnesium and silicate nutrition in Melastoma malabathricum L. Soil Sci. Plant Nutr. 44 655–666 10.1080/00380768.1998.10414489 [DOI] [Google Scholar]
- Weber-Blaschke G., Claus M., Rehfuess K. E. (2002). Growth and nutrition of ash (Fraxinus excelsior L.) and sycamore (Acer pseudoplatanus L.) on soils of different base saturation in pot experiments. For. Ecol. Manag. 167 43–56 10.1016/S0378-1127(01)00698-3 [DOI] [Google Scholar]
- Westman W. E. (1975). Edaphic climax pattern of the pygmy forest region of California. Ecol. Monogr. 45 109–135 10.2307/1942403 [DOI] [Google Scholar]
- Wilkins D. A., Hodson M. J. (1989). The effects of aluminium and Paxillus involutus Fr. on the growth of Norway spruce [Picea abies (L.) Karst.]. New Phytol. 113 225–232 10.1111/j.1469-8137.1989.tb04709.x [DOI] [PubMed] [Google Scholar]
- Yang J. L., Li Y. Y., Zhang Y. J., Zhang S. S., Wu Y. R., Wu P., et al. (2008). Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 146 602–611 10.1104/pp.107.111989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. T., Jiang H. X., Tang N., Chen L. S. (2011). Mechanisms of aluminum-tolerance in two species of citrus: secretion of organic acid anions and immobilization of aluminum by phosphorus in roots. Plant Sci. 189 521–530 10.1016/j.plantsci.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Zang U., Lamersdorf N., Borken W. (2011). Response of the fine root system in a Norway spruce stand to 13 years of reduced atmospheric nitrogen and acidity input. Plant Soil 339 435–445 10.1007/s11104-010-0598-5 [DOI] [Google Scholar]
- Zysset M., Brunner I., Frey B., Blaser P. (1996). Response of European chestnut to varying calcium/aluminum ratios. J. Environ. Qual. 25 702–708 10.2134/jeq1996.00472425002500040009x [DOI] [Google Scholar]