Abstract
Background
Studies exploring the relationship between prenatal vitamin D exposure and childhood asthma have yielded conflicting results. Higher vitamin D intake during pregnancy has been shown to lower the risk of childhood wheeze, yet a study of maternal late-pregnancy serum 25-hydroxyvitamin D suggested higher serum concentrations may be associated with increased childhood asthma.
Objective
To assess the relationship between mothers’ serum 25-hydroxyvitamin D status and asthma and wheeze phenotypes in their children at age 6 years. Secondly, to explore the relationship between maternal 25-hydroxyvitamin D status and objective measures of childhood atopy and lung function.
Methods
Serum 25-hydroxyvitamin D was measured at 34 weeks’ gestation in the mothers of 860 children born at term. Wheeze was classified as either transient or persistent/late using questionnaire data collated from 6, 12, 24 and 36 months and 6 years. At 6 years spirometry was performed and atopic status was determined by skin prick testing, exhaled nitric oxide was measured in 451 and bronchial hyperresponsiveness in 216 children.
Results
There were no significant associations between maternal late-pregnancy 25-hydroxyvitamin D status and either asthma or wheeze at age 6 years. Maternal vitamin D status was not associated with transient or persistent/late wheeze; no significant association was found between persistent/late wheeze when subdivided according to atopic status. No associations were found with skin sensitisation or lung function.
Conclusions
This study provides no evidence that exposure to higher concentrations of 25-hydroxyvitamin D in maternal serum during late pregnancy increases the risk of childhood asthma, wheeze or atopy.
Keywords: asthma epidemiology, asthma, paediatric asthma
INTRODUCTION
Vitamin D has multiple effects beyond those upon calcium metabolism and skeletal integrity. A role in asthma and atopic disease has been suggested as many immune cells possess vitamin D receptors[1] and genetic association has been demonstrated between variants in this receptor and asthma.[2] However, the relationship between vitamin D and asthma has proven controversial. Whilst Wjst and Dold proposed rising asthma prevalence to be a consequence of increased consumption of vitamin D-fortified foods,[3] Litonjua and Weiss argued, in contrast, that deficiency of vitamin D may be responsible.[4] Epidemiological support can be found for either viewpoint. A Southampton study found increased infantile eczema and childhood asthma in the children of mothers with higher late-pregnancy serum 25-hydroxyvitamin D.[5] However, this small study was not specifically designed to look at atopic outcomes. Conversely, several cohort studies have found lower maternal vitamin D intake during pregnancy to be associated with increased childhood wheeze risk.[6-9]
Serum 25-hydroxyvitamin D reflects total body vitamin D.[10] Most vitamin D is derived from photosynthesis in the skin.[10] Serum measures may more accurately characterise total exposure than estimated intake records which do not account for sun exposure. Maternal 25-hydroxyvitamin D status was measured in the Dutch KOALA study but was not found to be associated with lung function at age 6 years; however, asthma prevalence, wheezing phenotypes and atopy were not assessed.[11]
This is the first study to prospectively assess the relationship between maternal 25-hydroxyvitamin D status during pregnancy and childhood asthma, wheeze and atopy and the first to consider wheeze separately in atopic and non-atopic children. This is important as persistent wheeze with atopy differs in clinical presentation from non-atopic persistent wheeze and is likely to be of separate aetiology.[12] Data from a large population-based cohort were used to investigate whether higher maternal 25-hydroxyvitamin D status at 34 weeks’ gestation is associated with increased childhood asthma or wheeze risk. Objective measures of lung function and skin sensitisation were used to test the secondary hypotheses that maternal 25-hydroxyvitamin D status is associated with evidence of altered immune or respiratory development.
METHODS
Participants
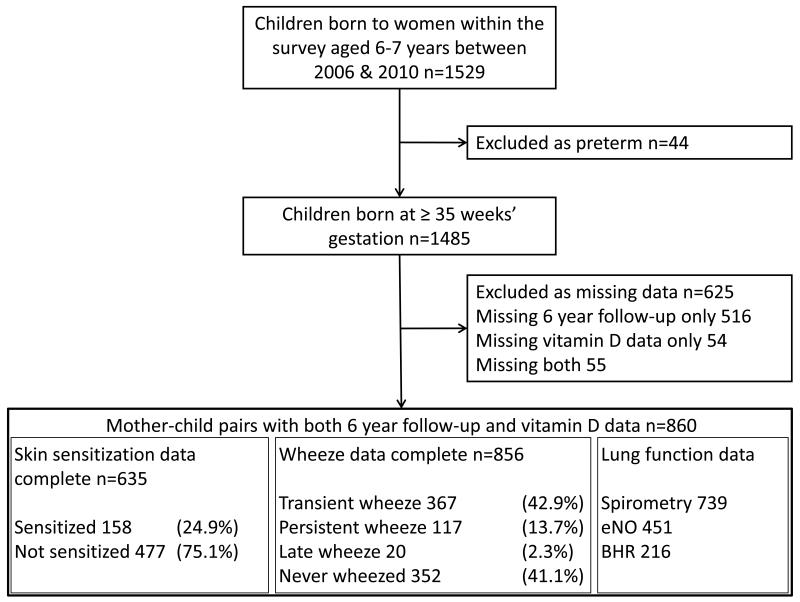
Participants were mother-child pairs from the Southampton Women’s Survey.[13] Women aged 20-34 years were recruited during 1998-2002; those who became pregnant were followed through pregnancy and their children visited at 6, 12, 24 and 36 months and 6 years. To exclude effects of prematurity, whilst maximising power, infants born < 35 weeks’ gestation were excluded. Children aged six years during 2006-2010 were invited for follow-up; 1485 mother-child pairs were eligible, maternal vitamin status and 6-year follow-up data were available for 860 pairs (Figure 1). Questionnaire data were collected during a home-visit, during this visit spirometry was also attempted. Due to resource limitations clinic-based bronchial provocation testing and exhaled nitric oxide measurements were limited to unselected subsets of participants. Parental consent was obtained and ethical approval was granted by the Southampton and South West Hampshire Local Research Ethics Committee (LREC Number 276/97, 307/97, 089/99 and 06/Q1702/104).
Figure 1.
Maternal serum 25-hydroxyvitamin D
Maternal venous blood was sampled at 34 weeks’ gestation. To ensure stability samples were centrifuged, separated and stored at −80°C within 24 hours of sampling. 25-hydroxyvitamin D concentrations were measured by a Vitamin D External Quality Assessment Scheme compliant laboratory, with quality control samples in each batch. The radioimmunoassay used (DiaSorin, Minnesota, USA) had a coefficient of variability < 10%.
Maternal vitamin D intake
At 11 and 34 weeks’ gestation average frequencies of consumption over the preceding 3 months were recorded using a 100-item food frequency questionnaire (FFQ). The FFQ has been validated against 4-day food diaries and maternal micronutrient concentrations.[14] Nutrient intakes were calculated by multiplying frequency of consumption by nutrient content for each food or supplement. Early and late pregnancy intakes were averaged to provide an estimate of pregnancy intake. Energy adjustment can be used to correct for over or underestimation of intake in the FFQ, however, intake data were not automatically adjusted for total energy intake as supplementary intake vitamin D from supplements do not increase in line with energy intake.
Atopy
Skin prick testing was conducted at 1 and 3 years using cat, dog, house dust mite (Dermatophagoides pteronyssinus), grass pollens, egg and milk allergens (Hollister-Stier, Spokane, WA); at age 6, tree pollens (ALK Abelló Hørsholm, Denmark) were also tested. For validity a ≥ 3 mm positive and a 0 mm negative control response were required, atopy was defined as any allergen response ≥ 3 mm.
Airway inflammation
Exhaled nitric oxide (eNO) was measured online by trained research nurses according to ERS/ATS recommendations.[15] Measurements were recorded during controlled expiration at 50 ml/sec using a NIOX® chemiluminescence analyser (Aerocrine, Sweden). A mean value was calculated from three readings where possible. eNO data were normalised using an inverse square root transformation then standardised as a z-score. The sign of the values was reversed so that high untransformed eNO values gave rise to high standardised scores.
Childhood asthma and wheeze
Respiratory assessment was conducted by research nurses using questions from the ISAAC core questionnaire wheezing module.[16] Specifically, mothers were asked at each visit whether their child had experienced ‘episodes of chestiness associated with wheezing or whistling in his/her chest since they were last seen’ and at 6 years whether their child had ‘ever been diagnosed with asthma by a doctor’. The asthma outcome was refined to current asthma by including only 6-year-old children diagnosed with asthma who had experienced asthma symptoms or received asthma medication within the last year. The wheeze phenotypes were based upon those of the Tuscon Children’s Respiratory Study;[17] questionnaire data from 6, 12, 24 and 36 months and six years were combined to define:
Transient wheeze: wheeze at ages 6, 12, 24 or 36 months but no wheeze and no asthma treatment at 6 years.
Persistent wheeze: wheeze at ages 6, 12, 24 or 36 months and wheeze or asthma treatment at 6 years.
Late-onset wheeze: no wheeze at ages 6, 12, 24 or 36 months but wheeze or asthma treatment at 6 years.
The persistent and late-onset groups were combined because the late-onset group contained few children (Figure1). The persistent/late wheeze group was sub-classified according to atopic status determined by skin prick testing.
Lung function
Spirometry was performed according to ATS/ERS guidelines,[18] although without noseclips to avoid discomfort. Flow-volume loops were measured for all children by experienced research nurses using a portable Koko spirometer with incentive software (KoKo version 4; PDS Instrumentation; Louisville, USA). Absolute values of FEV1 and FVC were recorded with and without standardisation for height to explore whether any effect of maternal 25-hydroxyvitamin D status upon wheeze risk was mediated by an effect upon child’s height.
Bronchial hyperresponsiveness (BHR) was measured by bronchial provocation challenge. Methacholine was administered using a dosimeter (Koko; PDS Instrumentation; Louisville, USA) and a compressed air-driven nebuliser (Sidestream®; Respironics, UK). Challenges were conducted according to ATS/ERS guidelines using incremental methacholine concentrations (0.06 mg/ml to 16 mg/ml).[19] Challenges were terminated following the 16 mg/ml dose or a 20% fall in FEV1. BHR was expressed as the inverse of the slope of the regression line through FEV1 drop and logged methacholine concentration: Log.slope=100/[regression slope of FEV1 drop and log10(cumulative methacholine dose) + 10] The constant removes negative values and the inverse transformation produces a normally distributed variable.[20] Lower inverse log.slope values indicate increased BHR.
Statistical methods
Poisson regression with robust variance was used to model relative risk for binary outcomes. This is more appropriate than logistic regression for common outcomes where odds ratios cannot be interpreted as relative risks and so are hard to interpret.[21] Transient and persistent/late wheeze phenotypes were mutually exclusive; children suffering one of these types of wheeze were not at risk of the other so relative risks were calculated by comparing children with transient or persistent/late wheeze to those who had never wheezed. Relative risks for persistent/late wheeze with atopy and persistent/late wheeze without atopy were calculated using non-atopic children who had never wheezed as the comparator group. Relationships between maternal 25-hydroxyvitamin D status and continuous outcome variables were explored using linear regression.
Potential confounders were identified a priori and tested for association with each respiratory outcome. Models were developed comprising all the confounding variables listed in Table 1 which were significantly associated with each outcome (p < 0.1). Birthweight and gestation were initially excluded from the multivariable models as they may lie on the causal pathway. Similarly, season and year of blood sampling were initially excluded from the multivariable models to preserve variation in the exposure variable which may drive an effect upon outcome. The analyses were repeated including these variables if they were significantly associated with the outcomes. 25-hydroxyvitamin D was analysed as a continuous variable to maximise power, however, the relationship between this exposure and each outcome was checked for linearity by fitting a quadratic term.
Table 1.
Comparison of SWS mother-child pairs with complete data with those lacking either maternal 25-hydroxyvitamin D or 6-year follow-up data but born in the same time period.
| Mother-child pairs in analysis (n= 860) |
Mother-child pairs with missing data (n= 625) |
P-value | ||
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age at child’s birth (mean (SD)) | 30.37 (3.81) | 29.63 (3.76) | <0.001 | |
| Primiparous (n (%)) | ||||
| No | 462 (53.72) | 369 (59.04) | 0.042 | |
| Yes | 398 (46.28) | 256 (40.96) | ||
| Qualifications (n (%)) | None | 14 (1.63) | 35 (5.61) | 0.001 |
| GCSE D-G | 84 (9.78) | 67 (10.74) | ||
| GCSE A*-C | 248 (28.87) | 179 (28.69) | ||
| A Level | 249 (28.99) | 186 (29.81) | ||
| HND | 64 (7.45) | 40 (6.41) | ||
| Degree | 200 (23.28) | 117 (18.75) | ||
| Parents’ social class (n (%)) | 1 | 88 (10.35) | 57 (12.26) | 0.031 |
| 2 | 425 (50.00) | 201 (43.23) | ||
| 3N | 234 (27.53) | 123 (26.45) | ||
| 3M | 67 (7.88) | 54 (11.61) | ||
| 4 | 34 (4.00) | 23 (4.95) | ||
| 5 | 2(0.24) | 7 (1.51) | ||
| Smoked in pregnancy (n (%)) | ||||
| No | 723 (85.46) | 469 (78.17) | <0.001 | |
| Yes | 123 (14.54) | 131 (21.83) | ||
| Maternal asthma (n (%)) | ||||
| No | 673 (78.9) | 468 (75.97) | 0.184 | |
| Yes | 180 (21.1) | 148 (24.03) | ||
| Maternal childhood eczema (n(%)) | ||||
| No | 703 (82.51) | 501 (81.33) | 0.561 | |
| Yes | 149 (17.49) | 115 (18.67) | ||
| Maternal rhinitis (n (%)) | ||||
| No | 494 (57.91) | 372 (60.39) | 0.341 | |
| Yes | 359 (42.09) | 244 (39.61) | ||
| Maternal atopy (n (%)) | ||||
| No | 406 (53.28) | 255 (56.67) | 0.253 | |
| Yes | 356 (46.72) | 195 (43.33) | ||
| Pre-pregnancy BMI, kg/m2 (median (IQR)) | 24.32 (22.01-27.53) | 24.05 (21.93-27.35) | 0.996 | |
| Height, cm (mean (SD)) | 163.50 (6.65) | 162.81 (6.01) | 0.041 | |
| Serum 25-hydroxyvitamin D,nmol/l (median (IQR)) | 59.00 (40.52-84.89) | 53.00 (38.47-79.19) | 0.027 | |
| Paternal characteristics | ||||
| Paternal asthma (n (%)) | ||||
| No | 697 (82.39) | 486 (80.46) | 0.351 | |
| Yes | 149 (17.61) | 118 (19.54) | ||
| Paternal childhood eczema (n(%)) | ||||
| No | 739 (88.29) | 531 (88.06) | 0.893 | |
| Yes | 98 (11.71) | 72 (11.94) | ||
| Paternal rhinitis (n (%)) | ||||
| No | 558 (66.59) | 400 (66.12) | 0.852 | |
| Yes | 280 (33.41) | 205 (33.88) | ||
| Child’s characteristics | ||||
| Gender (n (%)) | ||||
| Male | 445 (51.74) | 333 (53.45) | 0.516 | |
| Female | 415 (48.26) | 290 (46.55) | ||
| Birth weight, kg (mean (SD)) | 3483.63 (494.52) | 3467.09 (498.12) | 0.529 | |
| Gestational age, weeks (median (IQR)) | 40 .14 (39.14-41.00) | 40.14 (39.14-41.00) | 0.914 | |
| Months of breastfeeding | None | 132 (15.70) | 131 (23.69) | <0.001 |
| (n (%)) | < 1 | 168 (19.98) | 116 (20.98) | |
| 1 - 3 | 157 (18.67) | 122 (22.06) | ||
| 4 – 6 | 156 (18.55) | 72 (13.02) | ||
| 7 – 11 | 139 (16.53) | 81 (14.65) | ||
| 12 or more | 89 (10.58) | 31 (5.61) | ||
| Age of introduction solid food, weeks (median (IQR)) | 17.38 (15.04-17.67) | 17.38 (15.04-17.38) | 0.282 | |
| Mother smoked during child’s infancy (n (%)) | ||||
| No | 707 (82.88) | 439 (76.88) | 0.005 | |
| Yes | 146 (17.12) | 132 (23.12) | ||
| Cats/dogs in home during child’s infancy (n (%)) | ||||
| No | 432 (50.47) | 295 (53.25) | 0.307 | |
| Yes | 424 (49.53) | 259 (46.75) | ||
| Age at testing, years (median(IQR)) | 6.46 (6.34-6.61) | 6.44 (6.35-6.60) | 0.797 | |
Binary outcomes were compared by χ2 test, categorical outcomes by a χ2 test for trend, and continuous variables using t-tests, after transformation where appropriate, or a ranksum test.
As the analyses were designed a priori to test a limited number of hypotheses and not all the tests were independent, use of a Bonferroni correction was considered over-conservative.[22] As a compromise we focused on results with P-values ≤ 0.025 and considered consistency of the findings in our interpretation.
RESULTS
Participants
Participant mothers were broadly similar in terms of asthma, atopy and allergic disorders to those mothers for whom maternal 25-hydroxyvitamin D status or follow-up data were incomplete. Participant mothers were slightly older, taller, less likely to smoke in pregnancy, more likely to be primiparous and of higher educational attainment and social class than those with incomplete data. Participant children were less likely to be exposed to environmental tobacco smoke and more likely to have been breastfed than those with incomplete data (Table 1). Similarly, those children contributing skin prick, spirometry, eNO or BHR data were broadly similar to those that did not.
The median, (IQR) maternal 25-hydroxyvitamin D concentration was 59.0 nmol/l, (40.5-84.9 nmol/l). The highest serum 25-hydroxyvitamin D value was 203 nmol/l, 29% of women had values greater than 80 nmol/l and 10% of women had serum levels greater than 110 nmol/l. Serum 25-hydroxyvitamin D concentrations were slightly lower in those women lost to follow-up (53.0, (38.5-79.2) nmol/l), probably reflecting socioeconomic and associated lifestyle factors. Eighty seven children (10.1%) had current doctor-diagnosed asthma, whilst 504 (58.9%) had experienced wheeze at or before six years of age. A total of 137 (16.0 %) children were assigned to the persistent/late wheeze phenotype; of these 51.1 % were atopic and 48.9 % non-atopic (Table 2). Technically acceptable measures of FEV1, BHR and eNO were available from 739, 216 and 451 children respectively (Figure 1).
Table 2.
Distribution of child participants between outcome groups.
| Wheeze outcome | N (%) |
| Current doctor-diagnosed asthma (aged 6 years) | 87/860 (10.1) |
| Current wheeze in the last 12 months (aged 6 years) | 117/860 (13.6) |
| Ever wheezed at or before 6 years | 504/856 (58.9) |
| Never wheezed | 352/856 (41.8) |
| Transient wheeze (before 3 years not after) | 367/856 (42.0) |
| Persistent/late wheeze (beyond or after 3 years) | 137/856 (16.0) |
| Atopic persistent/late wheeze | 46/632 (7.3) |
| Non-atopic persistent/late wheeze | 48/632 (7.6) |
| Atopic outcome | |
| Skin sensitisation | 158/635 (24.9) |
Asthma and wheeze
There was no significant association between maternal 25-hydroxyvitamin D status at 34 weeks’ gestation and current asthma; neither was 25-hydroxyvitamin D status associated with wheeze at or before 6 years of age nor current wheeze in the 12 months preceding the 6-year assessment (Table 3). There were no significant associations with the transient or persistent/late wheeze phenotypes and subdividing the persistent/late phenotype by atopic status did not reveal any significant associations (Table 3). There was no evidence for a non-linear relationship between maternal 25-hydroxyvitamin D and any wheeze phenotype (data not shown).
Table 3.
Relationship between maternal late-pregnancy maternal 25-hydroxyvitamin D status and offspring asthma and wheeze at age 6 years
| Univariable Model | Final model | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | n | RR | (95% CI) | P-value | n | |
| Current doctor-diagnosed asthma at 6 years | 0.97 | (0.91, 1.04) | 0.36 | 860 | 0.98 | (0.92, 1.04) | 0.56 | 836 |
| Current wheeze at 6 years | 0.98 | (0.92, 1.03) | 0.40 | 860 | 0.99 | (0.94, 1.05) | 0.76 | 833 |
| Any wheeze at or before 6 years | 1.00 | (0.98, 1.01) | 0.61 | 856 | 1.00 | (0.98, 1.02) | 0.95 | 823 |
| Transient wheeze | 1.00 | (0.98, 1.02) | 0.85 | 719 | 1.00 | (0.98, 1.02) | 0.89 | 707 |
| Persistent/late wheeze | 0.98 | (0.93, 1.03) | 0.37 | 489 | 0.98 | (0.94, 1.03) | 0.49 | 475 |
| Persistent/late wheeze with atopy | 0.90 | (0.81, 0.99) | 0.03 | 257 | 0.91 | (0.84, 0.99) | 0.04 | 251 |
| Persistent/late wheeze without atopy | 0.99 | (0.91, 1.06) | 0.73 | 259 | 1.01 | (0.94, 1.09) | 0.73 | 253 |
Data presented as change in relative risk per 10 nmol/l change in maternal serum 25-hydroxyvitamin D status at 34 weeks’ pregnancy.
Adjusted models: asthma - maternal education, maternal asthma, paternal asthma and paternal rhinitis; wheeze at age 6 - maternal education, maternal asthma, paternal rhinitis, pets in the home during the child’s infancy; wheeze at or before 6 years - maternal BMI, child’s gender, maternal education, maternal asthma, paternal asthma, maternal rhinitis; transient wheeze - maternal BMI, child’s gender, mother’s parity, maternal asthma, maternal rhinitis; Persistent/late - maternal education, smoking during pregnancy, maternal asthma, paternal asthma, maternal rhinitis; Persistent/late wheeze with atopy-child’s gender, maternal asthma, paternal asthma, paternal rhinitis, pets in the home during infancy; Persistent/late wheeze without atopy-maternal age, smoking during pregnancy, maternal asthma, paternal asthma, pets in the home during infancy.
Atopy and eNO
Maternal 25-hydroxyvitamin D status at 34 weeks’ gestation was not associated with skin sensitisation at 1, 3 or 6 years or with exhaled nitric oxide at age 6 years (Table 4). There was no evidence for a non-linear relationship between maternal 25-hydroxyvitamin D and either skin sensitisation or eNO (data not shown).
Table 4.
Relationship between maternal late-pregnancy maternal 25-hydroxyvitamin D status and offspring atopy and airway inflammation
| Univariable Model | Final model | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | n | RR | (95% CI) | P-value | n | |
| Atopy age 1 year | 0.94 | (0.88, 1.01) | 0.08 | 773 | 0.96 | (0.90, 1.03) | 0.24 | 685 |
| Atopy age 3 years | 0.99 | (0.94, 1.05) | 0.81 | 676 | 0.99 | (0.94, 1.04) | 0.58 | 661 |
| Atopy age 6 years | 0.97 | (0.93, 1.02) | 0.26 | 635 | 0.99 | (0.95, 1.04) | 0.71 | 545 |
| beta | (95% CI) | P-value | n | beta | (95% CI) | P-value | n | |
| Exhaled nitric oxide | −0.014 | (−0.044,0.016) | 0.36 | 451 | −0.0204 | (−0.050,0.009) | 0.18 | 434 |
Data presented as change in relative risk per 10 nmol/l change in maternal serum 25-hydroxyvitamin D status at 34 weeks’ pregnancy.
Adjusted models: atopy age 1 year - child’s gender, parents’ social class, maternal atopy; atopy age 3 years - child’s gender, exposure to smoke in infancy, maternal eczema, atopy age 6 years - child’s age at testing, child’s gender, parents social class, maternal asthma, paternal rhinitis, maternal atopy; exhaled nitric oxide - child’s age at testing, maternal asthma, paternal rhinitis, maternal height.
Lung function
Maternal 25-hydroxyvitamin D at 34 weeks’ gestation was not associated with either absolute or standardised values of FEV1 or FVC at 6 years. Maternal 25-hydroxyvitamin D status was not associated with BHR (Table 5). There was no evidence for a non-linear relationship between maternal 25-hydroxyvitamin D and any measure of lung function (data not shown).
Table 5.
Relationship between maternal late-pregnancy maternal 25-hydroxyvitamin D status and offspring lung function at 6 years
| Univariable Model | Final Model | |||||||
|---|---|---|---|---|---|---|---|---|
| beta | (95% CI) | P-value | n | beta | (95% CI) | P-value | n | |
| FEV1 absolute | −0.0007 | (−0.0054,0.0039) | 0.76 | 739 | −0.0001 | (−0.0046,0.0043) | 0.95 | 731 |
| FEV1 z-score | 0.012 | (−0.0078,0.033) | 0.23 | 739 | 0.011 | (−0.0091,0.031) | 0.28 | 739 |
| FVC absolute | −0.001 | (−0.0068,0.0045) | 0.69 | 739 | −0.0001 | (−0.0054,0.0052) | 0.96 | 730 |
| FVC z-score | 0.013 | (−0.010,0.036) | 0.27 | 739 | 0.012 | (−0.011,0.035) | 0.31 | 739 |
| BHR slope | −0.084 | (−0.194, −0.025) | 0.13 | 216 | −0.102 | (−0.211, −0.008) | 0.07 | 208 |
Data presented as change in relative risk per 10 nmol/l change in maternal serum 25-hydroxyvitamin D status at 34 weeks’ pregnancy.
Adjusted models: FEV1 absolute - child’s age at testing, child’s gender, parity, maternal BMI, maternal height; FEV1 z-score - child’s gender; FVC absolute - child’s age at testing, child’s gender, age at introduction of solid foods, maternal BMI, maternal height, FVC z-score - child’s gender; BHR - maternal age, smoking in pregnancy, paternal eczema, age at introduction of solid foods.
Alternative multivariable models
Birthweight was not associated with any outcome and was therefore not considered a confounder. Gestation, however, was associated with all wheeze and skin sensitisation outcomes except current asthma and persistent/late wheeze with atopy; the absence of association between maternal 25-hydroxyvitamin D and these variables remained when gestation was included in the multivariable models. Birthweight was associated with absolute measures of FEV1 and FVC, but including birthweight in the multivariable models did not reveal any associations between maternal 25-hydroxyvitamin D and these measures (Tables E1-3). When adjustments were made for season and year of blood sampling the absence of any association between maternal 25-hydroxyvitamin D and childhood wheeze, atopy or lung function variables was unchanged (Tables E4-6).
Maternal vitamin D intake
The median (IQR) average total daily maternal vitamin D intake was 4.2 mcg/day (3.0 - 6.7 mcg/day). During early pregnancy 39% of women took supplements containing vitamin D, whilst during late pregnancy, 22% supplemented with vitamin D. For women taking supplements, median supplementary-intake (IQR) was 4.1 mcg/day (1.5-8.3 mcg/day) in early pregnancy and 5.8 mcg (2.5-12.2 mcg/day) vitamin D /day, in late pregnancy. Only 11.5% of women achieved an average intake of 10 mcg/day, currently recommended by the department of health.[23] Correlation coefficients for early, late and average intake with status at 34 weeks’ gestation were 0.25, 0.33 and 0.33, respectively. Neither total nor food-derived maternal intake was associated with asthma, or any wheeze outcome (Tables E7 & E10). No significant associations were found between intake and skin sensitisation, (Tables E8 & E11) or any measure of lung function (Tables E9 & E12). A statistically significant positive association was found between food-derived vitamin D and eNO (Table E5) but the lack of a similar association with total intake (Table E2) suggests that this is not a clinically robust association. There was no evidence for a significant association between total maternal vitamin D intake and any outcome when, for consistency with previous birth cohort analyses, the results were energy-adjusted and analysed as a categorical variable according to quartile of vitamin D intake (Tables E13-15).
DISCUSSION
This study found no evidence that higher maternal serum 25-hydroxyvitamin D in late pregnancy is associated with an increased risk of asthma or atopy. There were no significant associations between maternal 25-hydroxyvitamin D status at 34 weeks’ gestation and asthma, transient or persistent/late wheeze, skin sensitisation at 1, 3 or 6 years or eNO, FEV1, FVC or BHR at age 6 years.
It has been suggested that serum 25-hydroxyvitamin D levels in pregnancy should be above 80 nmol/l, 29% of the women in this study had serum 25-hydroxyvitamin D concentrations above this level. Both supplementation and 25-hydroxyvitamin D levels were higher in the current study than in our previous cohort where a positive association was found between maternal 25-hydroxyvitamin D status and childhood eczema and asthma.[5] Whilst a high prevalence of supplementation might reduce the ability of the present study to detect any effect of dietary insufficiency, failure to confirm a harmful effect of high 25-hydroxyvitamin D status cannot be attributed to lower exposure; it is more likely that the earlier study was underpowered to assess specific clinical outcomes.
Vitamin D supplementation during pregnancy is known to benefit calcium metabolism and bone health[24] and many believe may protect against cardiovascular, autoimmune, and malignant disease via ‘fetal imprinting’.[25] The National Institute for Health and Clinical Excellence[26] has suggested that pregnant women may wish to consider vitamin D supplementation. Recently a randomised controlled-trial has demonstrated that daily supplementation with 4000 IU vitamin D can increase maternal serum 25-hydroxyvitamin D concentration without adverse events.[27] However, few adequately powered studies have considered the effects of increased maternal 25-hydroxyvitamin D upon relevant clinical outcomes.[28]
Relationship between maternal late-pregnancy serum 25-hydroxyvitamin D status and childhood asthma and wheeze
This study found no evidence that higher late-pregnancy maternal serum 25-hydroxyvitamin D is associated with increased asthma. Although an inverse relationship between energy-adjusted maternal vitamin D intake and asthma at age 5 was found in a Finnish cohort,[6] this was significant only for food-derived not total intake. Associations with food derived intake only may be vulnerable to confounding by other nutrients present in vitamin D-rich food and socioeconomic factor or they may arise as a result of multiple comparisons. The majority of studies reporting inverse associations between early vitamin D exposure and adverse respiratory outcomes, reported associations with wheeze but not asthma. Many of these studies relied upon maternal intake data[6-9] and relatively short follow-up.[7-8]
Vitamin D intake studies are vulnerable to confounding by socio-economic and lifestyle factors and by the effects of other nutrients found in vitamin D-containing foods. Such confounding has been suggested to explain the absence of an association between 25-hydroxyvitamin D status and lung function in adults with chronic obstructive pulmonary disease, despite an association with vitamin D intake; absence of association between vitamin D receptor genotype and lung function strengthened this argument.[30] Studies with short follow-up are obliged to use childhood wheeze as an outcome in the absence of a reliable asthma diagnosis in young children. Follow-up to 5 years was conducted in a New Zealand cohort and an inverse association found between cord blood 25-hydroxyvitamin D and wheeze, but again no association was found with asthma.[29] An inverse association was also found between cord blood 25-hydroxyvitamin D status and respiratory infections in early infancy, leading these authors to conclude that beneficial effects of vitamin D upon innate immunity may indirectly reduce wheeze risk in early childhood. The present study did not address the issue of childhood infection.
Relationship between maternal late-pregnancy serum 25-hydroxyvitamin D status and immune and respiratory development
Whilst it remains possible that vitamin D exerts an affect upon immune predisposition to wheeze with infection, rather than altered atopic immunity, an alternative explanation for the lack of demonstrable association between early vitamin D exposure and asthma could be the existence of different asthma phenotypes. Subdividing the persistent/late wheeze phenotype by atopic status did not reveal any association with maternal vitamin D status, although it remains possible that the phenotypes used in this study were excessively heterogeneous and that early vitamin D exposure may have differential effects upon late-onset compared with persistent wheeze, for example.
Animal studies suggest vitamin D may promote a proallergic Th2 phenotype.[31] Similarly, epidemiological evidence suggests high early life exposure to vitamin D supplementation[32] or high maternal 25-hydroxyvitamin D serum levels might predispose to allergic disorders.[5] No previous prospective epidemiological study, however, has investigated the relationship between maternal 25-hydroxyvitamin D status and objective measures of atopy. In this respect, the null findings in this study are reassuring: higher serum 25-hydroxyvitamin D concentrations in late pregnancy do not appear to increase skin sensitisation at 1, 3 or 6 years or eosinophilic airways inflammation at 6 years.
Early vitamin D exposure has been shown to alter the volume dependence of lung mechanics in an animal model, suggestive of altered tissue structure.[33] Altered development affecting lung structure and airway calibre would also be consistent with the results of maternal intake studies. However, this study confirms, in a larger cohort with more extensive characterisation of lung function including BHR, the findings of the KOALA study;[11] there was no evidence of a clinically significant alteration of lung function according to maternal late-pregnancy 25-hydroxyvitamin D status.
As the repeatability of the serum 25-hydroxyvitamin D levels is relatively low, there is a significant chance that a single serum measurement will lead to misclassification of exposure. As this misclassification is random, this may bias studies, such as this, based upon single serum samples toward the null or no effect. Furthermore, epidemiological studies are limited in their ability to discriminate causal from closely linked factors; this study cannot exclude the existence of a relationship between vitamin D exposure and wheeze or atopy which is hidden by an opposing relationship between incompletely controlled for seasonal and other factors upon these outcomes. Another feature of the study design which may have limited the likelihood of identifying an association between maternal 25-hydroxyvitamin D status and childhood wheeze outcomes is the use of frequent prospective questionnaires; this may have set too low a threshold to reflect significant pathology.
Whilst the present study did not have complete follow-up and those followed up differed from those who were not in terms of several socioeconomic variables, this should not alter the main conclusions unless the nature of any relationship between maternal 25-hydroxyvitamin D status and wheeze or atopic outcomes differed according to socioeconomic status or if the relationship were non-linear. We have no evidence to support either assertion. The null results in this study may have arisen as a consequence of measuring 25-hydroxyvitamin D status in late pregnancy only, however, whilst much respiratory development, particularly that of the airways, occurs early in pregnancy,[34] significant maturation of the immune system is believed to occur in late pregnancy.[35] Furthermore, the null result was supported by analyses based upon intake data which covered both the first and second trimesters.
In summary, neither higher late-pregnancy maternal 25-hydroxyvitamin D status nor high vitamin D intake during pregnancy was significantly associated with asthma or any wheeze phenotype. Moreover there was no evidence that early exposure to higher concentrations of 25-hydroxyvitamin D had a deleterious effect upon either lung function or atopic sensitisation. Together, these findings suggest the risk posed by vitamin D supplementation in terms of asthma and atopic diseases may not be a concern.
Supplementary Material
Acknowledgements
The research was supported by infrastructure provided by the Southampton NIHR Respiratory Biomedical Research Unit and Nutrition, Diet and Lifestyle Biomedical Research Centre. Clinical investigations were conducted in Southampton Wellcome Trust Clinical Research Facility. We would also like to gratefully acknowledge the expertise of Professor Ramasamyiyer Swaminathan and Dr Sasala Wickramasinghe for providing 25-hydroxyvitamin D radioimmunoassays in the Chemical Pathology department at St. Thomas’ Hospital, London.
Funding: This work within the Southampton Women’s Survey has been funded by the Medical Research Council, University of Southampton, British Heart Foundation, and the Food Standards Agency (contract no N05071). The research is supported by infrastructure provided by the Southampton NIHR Respiratory Biomedical Research Units and Nutrition, Diet and Lifestyle Biomedical Research Centre. Dr KC Pike was supported by a grant from the British Lung Foundation.
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Group and co-owners or contracting owning societies (where published by the BMJ Group on their behalf), and its Licensees to permit this article (if accepted) to be published in Thorax and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) none of the authors have support from any company for the submitted work; (2) KCP, HMI, SMR, JSAL, CC, NCH, KMG & GR have no relationships with companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) KCP, HMI, SMR, JSAL, CC, NCH, KMG & GR have no non-financial interests that may be relevant to the submitted work.
REFERENCES
- 1.Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 2.Poon AH, Laprise C, Lemire M, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170(9):967–73. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 3.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54(7):757–9. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 4.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Gale CR, Robinson SM, Harvey NC, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erkkola M, Kaila M, Nwaru BI, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39(6):875–82. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 7.Camargo CA, Jr., Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 9.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–9. doi: 10.1093/ajcn/85.3.853. 2010;35(6):1228-34. [DOI] [PubMed] [Google Scholar]
- 10.Holick M. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Current Opinion in Endocrinology, Diabetes and Obesity. 2002;9:87–98. [Google Scholar]
- 11.Cremers E, Thijs C, Penders J, et al. Maternal and child’s vitamin D supplement use and vitamin D level in relation to childhood lung function: the KOALA Birth Cohort Study. Thorax. 2011;66(6):474–80. doi: 10.1136/thx.2010.151985. [DOI] [PubMed] [Google Scholar]
- 12.Davies DE, Wicks J, Powell RM, et al. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111(2):215–25. doi: 10.1067/mai.2003.128. quiz 26. [DOI] [PubMed] [Google Scholar]
- 13.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35(1):42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson S, Godfrey K, Osmond C, et al. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur J Clin Nutr. 1996;50(5):302–8. [PubMed] [Google Scholar]
- 15.ATS/ERS recommendations for standardised procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. European Respiratory Journal. 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 16.Asher MI, Keil U, Anderson HR, Beasley R, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161(1):309–29. doi: 10.1164/ajrccm.161.1.ats11-99. Epub 2000/01/05. [DOI] [PubMed] [Google Scholar]
- 20.Chinn S, Arossa WA, Jarvis DL, et al. Variation in nebulizer aerosol output and weight output from the Mefar dosimeter: implications for multicentre studies. Eur Respir J. 1997;10(2):452–6. doi: 10.1183/09031936.97.10020452. [DOI] [PubMed] [Google Scholar]
- 21.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC medical research methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland M. An introduction to statistics. Oxford University Press; Oxford: 2000. [Google Scholar]
- 23.Department of Health [Accessed April 2012];Nutrition for pregnancy and the early years:what to eat for pregnancy. www.dh.gov.uk/en/Publichealth/Nutrition/Nutritionpregnancyearlyyears/DH_127622.
- 24.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 25.Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmunity reviews. 2010;9(11):709–15. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence . Antenatal care: routine care for healthy pregnant women Clinical Guideline CG62. Mar, 2008. [Google Scholar]
- 27.Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams SA. Vitamin D supplementation during pregnancy. J Bone Miner Res. 2011;26(10):2338–40. doi: 10.1002/jbmr.498. [DOI] [PubMed] [Google Scholar]
- 29.Camargo CA, Jr., Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 30.Shaheen SO, Jameson KA, Robinson SM, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66(8):692–8. doi: 10.1136/thx.2010.155234. [DOI] [PubMed] [Google Scholar]
- 31.Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J Allergy Clin Immunol. 2000;106(5):981–5. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 32.Hypponen E, Sovio U, Wjst M, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 33.Zosky GR, Berry LJ, Elliot JG, et al. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183(10):1336–43. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 34.Bucher U, Reid L. Development of the intrasegmental bronchial tree: the pattern of branching and development of cartilage at various stages of intra-uterine life. Thorax. 1961;16:207–18. doi: 10.1136/thx.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clapp DW. Developmental regulation of the immune system. Seminars in perinatology. 2006;30(2):69–72. doi: 10.1053/j.semperi.2006.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.