Introduction
Femoral artery caliber or disease precludes vascular access in a significant minority of candidates for transcatheter aortic valve replacement or aortic endograft therapy.
We propose an alternative access route to the abdominal aorta for large-vessel introducer sheaths by direct puncture from the adjoining inferior vena cava (IVC). We reason that the veins are larger and more compliant than corresponding arteries, that venous decompression may avert hemorrhage in confined-space arterial perforation, and that acquired aorto-caval fistulas are not immediately life-threatening. We close the caval-aortic access tract using nitinol occluders. We also test intentional failure to close the caval-aortic access tract.
Methods and Results
14 swine underwent survival testing after transfemoral access. Aspirin and heparin achieved an activated clotting time of 250-300s to “maximize” risk of hemorrhage.
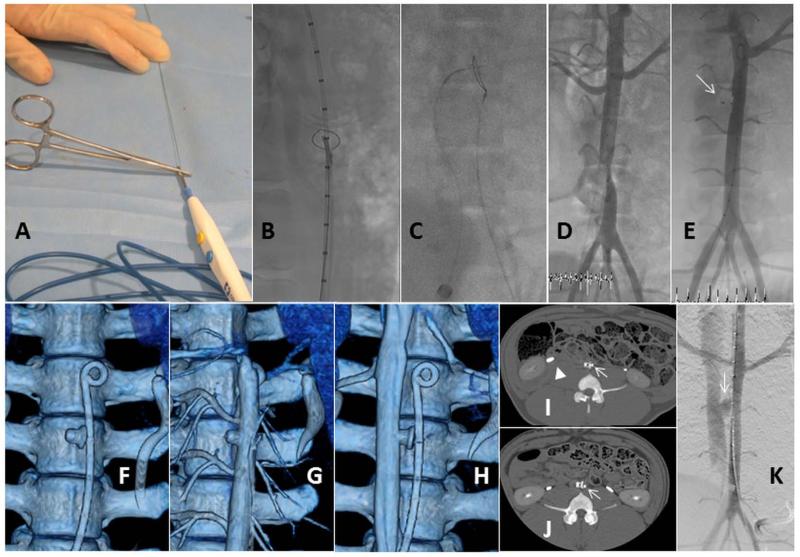
A 10mm snare was positioned in the abdominal aorta to serve as a target. From the IVC, a coaxial puncture system consisted of a stiff-tip 0.014” guidewire (Asahi ConfianzaPro 9 or equivalent) inside a Piggyback 0.035” wire convertor (Vascular Solutions) inside a curved 5Fr Cobra catheter. Under anteroposterior and lateral X-ray fluoroscopy the guidewire tip is directed at the snare target [Figure-A-C]. The guidewire was connected to an electrosurgery pencil using hemostats and energized for 1-2 seconds in “cutting mode” for crossing. The snare confirmed intraluminal position. After exchange for a stiff 0.035” guidewire, an 18Fr introducer sheath connects femoral vein to aorta [Figure-D]. RF-guidewire traversal was successful in all, requiring 1.2±0.6 puncture attempts and 2.0 (interquartile range 2.0, 3.8) minutes. There was no significant oximetric shunt, hemodynamic change, or hemoglobin fall.
Figure.
(A) Electrosurgery pencil is connected to the guidewire using hemostats. X-ray lateral (B) and anteroposterior (C) views of crossing from IVC to aorta. (D) 18Fr introducer sheath from femoral vein into aorta. (E) After tract closure with duct occluder (arrow). (F-H) Dynamic CTA of pre-contrast, aortic, and venous phases after closure without extravasation. Follow-up CTA after typical device (arrow) closure (I) shows the single retroperitoneal hematoma and iliopsoas effacement (arrowhead). (J) shows no blood or effacement. (K) An intentional unrepaired aorto-caval fistula (arrow) is fully decompressed into the IVC.
Afterwards the tract was closed with a nitinol occluder device [Figure-E]. The 0.014” guidewire was positioned across the tract as a “buddy” to simplify recrossing in case of inadvertent pull-through of the closure device. For the 18Fr sheath we selected a 8/6 Amplatzer Duct Occluder (St Jude) for 8 animals and a 6mm Memopart VSD occluder (Lepu) in 2. The aorto-caval tract was successfully closed in all 10 animals attempted [Figure-F-H]. In 3 this required a second pass using the “buddy wire.” All remained occluded after 1-4 weeks. There was no important hemorrhage and no aortic dissection on angiography, dynamic CTA or MRI at 1.5T [Figure-I]. One had a small retroperitoneal thrombus compressing the IVC, after an early-experience procedure [Figure-J]; it recovered uneventfully without abnormal clinical signs. At necropsy, all 10 occluder devices were partially (1 week) or completely (4 week) endothelialized, with surrounding fibrosis but no hematoma.
We tested intentional failure to close the 18Fr tract in 4 animals to simulate worst-case failure. This created a pulmonic/systemic shunt of 1.3 ± 0.4 that was 1.2 ±0.4 after one week. Immediate angiography and CT showed aortocaval flow through an irregularly-contoured access tract but no retention of extravascular contrast and no retroperitoneal hematoma [Figure-J]. Animals showed no evident clinical sequelae. One week later each fistula was crossed easily with a 0.035” guidewire and closed with a nitinol occluder. Immediate necropsy showed only a small decompressed pseudoaneurysm surrounding the occluder.
Discussion
We tested preclinical entry into the abdominal aorta via the femoral vein and IVC, followed by exit and closure without serious complications. The caval-aortic tract was created by energizing a coronary guidewire with an electrosurgery unit. The tract was closed using a ductus arteriosus nitinol occluder. Only 1/10 animals had any retroperitoneal hematoma, which in this case was small and well tolerated. Even when the fistula was intentionally left open, “catastrophic failure” was well tolerated acutely and subacutely, and remained simple to close. We believe this simple and rapid new technique may provide percutaneous options for valve and aortic procedures in patients otherwise deemed ineligible because of small-caliber, tortuous, calcific, or otherwise obstructed iliac arteries.
Caval-aortic access has several appeals. First, femoral veins are more compliant than arteries and routinely allow large cannulas 10mm or wider. Second, large arteriovenous fistulas, such as aortocaval fistulas arising from aneurysm or trauma (1) may be morbid but are not immediately life-threatening (2). Conversely, such fistulas avoid hemorrhage by decompressing high-flow arterial ruptures into the venous system. Third, the IVC abuts the infrarenal aorta with no interposed critical structures. Fourth, we used a device marketed to occlude a congenital arteriovenous communication, and which has been used in postoperative and congenital aortocaval fistula repair (3). Our application of radiofrequency energy through a guidewire is inspired by “facilitated” transseptal puncture by atrial transseptal needle connected to an electrosurgery generator (4).
Intentional catastrophic failure to close the caval-aortic access tract is tolerated acutely and subacutely, and nevertheless the tract is easily recrossed and closed afterwards.
Limitations include differences between human and swine. Patients may prove susceptible to major complications such as aortic dissection, aortic thrombosis, retroperitoneal hemorrhage, caval thrombosis and venous thromboembolism, lymphatic injury, or device migration or embolization.
Conclusions
Large therapeutic devices can be introduced into the aorta via the IVC in a simple catheter procedure, and the fistula closed uneventfully despite full anticoagulation. Intentional failure to close the caval-aortic access tract is well-tolerated and easily redressed. Human testing is warranted.
Footnotes
Competing interests
No medical device manufacturer contributed to this project.
Sources of Funding:
Supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (Z01-HL005062-08 and Z01-HL006040-01 to RJL).
Contributor Information
Majdi Halabi, Cardiovascular and Pulmonary Branch, Division of Intramural Research, National Heart Lung and Blood Institute, Bethesda, MD.
Kanishka Ratnayaka, Cardiovascular and Pulmonary Branch, Division of Intramural Research, National Heart Lung and Blood Institute, Bethesda, MD; Children’s National Medical Center, Washington, DC.
Anthony Z. Faranesh, Cardiovascular and Pulmonary Branch, Division of Intramural Research, National Heart Lung and Blood Institute, Bethesda, MD.
Marcus Y. Chen, Cardiovascular and Pulmonary Branch, Division of Intramural Research, National Heart Lung and Blood Institute, Bethesda, MD.
William H. Schenke, Cardiovascular and Pulmonary Branch, Division of Intramural Research, National Heart Lung and Blood Institute, Bethesda, MD.
Robert J. Lederman, Cardiovascular and Pulmonary Branch, Division of Intramural Research, National Heart Lung and Blood Institute, Bethesda, MD.
References
- 1.Antoniou GA, Koutsias S, Karathanos C, Sfyroeras GS, Vretzakis G, Giannoukas AD. Endovascular stent-graft repair of major abdominal arteriovenous fistula: a systematic review. J Endovasc Ther. 2009;16:514–23. doi: 10.1583/09-2725.1. [DOI] [PubMed] [Google Scholar]
- 2.Davis PM, Gloviczki P, Cherry KJ, Jr., et al. Aorto-caval and ilio-iliac arteriovenous fistulae. Am J Surg. 1998;176:115–8. doi: 10.1016/s0002-9610(98)00166-4. [DOI] [PubMed] [Google Scholar]
- 3.Godart F, Haulon S, Houmany M, et al. Transcatheter closure of aortocaval fistula with the amplatzer duct occluder. J Endovasc Ther. 2005;12:134–7. doi: 10.1583/04-1332.1. [DOI] [PubMed] [Google Scholar]
- 4.Bidart C, Vaseghi M, Cesario DA, et al. Radiofrequency current delivery via transseptal needle to facilitate septal puncture. Heart Rhythm. 2007;4:1573–6. doi: 10.1016/j.hrthm.2007.07.008. [DOI] [PubMed] [Google Scholar]