Abstract
We face a profound and emerging public health problem in the form of acute and chronic brain dysfunction. This affects both young and elderly intensive care unit (ICU) survivors and is altering the landscape of society. Two-thirds of ICU patients develop delirium, and this is associated with longer stays, increased costs and excess mortality. In addition, over one-half of ICU survivors suffer a dementia-like illness that impacts their physical and cognitive functional abilities and which appears to be related to the duration of their ICU delirium. A new paradigm of how Intensivists handle the brain is required. We propose a 3-step approach to address this emerging epidemic, which includes Screening, Prevention, and Restoration of brain function (SPR).
Screening combines risk factor identification and delirium assessment using validated instruments. Prevention of acute and chronic brain dysfunction requires implementation of a core model of care that combines evidence-based practices: awakening and breathing coordination with target -based sedation, delirium monitoring, and exercise / early mobility (ABCDE). Restoration introduces strategies of ongoing screening and treatment for ICU survivors at high risk of ongoing brain dysfunction. This practical system applying many evidence-based concepts, incorporates personalized medicine, systems based practice, and continuing research and development towards improving acute and chronic cognitive outcomes.
Keywords: Delirium, Intensive Care Unit, Risk Factors, Primary Prevention, Secondary Prevention, Tertiary Prevention, Quality Improvement, ICU-acquired Weakness, Sedation, Diagnosis, Treatment
Introduction
Over recent decades, intensive care unit (ICU) utilization has dramatically increased.(1) Changing ICU demographics account for a substantial share of this growth, with elderly patients currently accounting for up to 50% of all ICU admissions(2) and more than half of ICU days.(3) During this same period, ICU mortality is decreasing,(4) representing an achievement of the latest advances in critical care. Unfortunately, an emerging epidemic of acute and chronic brain dysfunction among ICU survivors threatens to overshadow this very success.(5,6) Whereas many of the successes have occurred at the “front end” of critical care (e.g., low tidal volume ventilation in acute respiratory distress syndrome(7)), this epidemic represents the impact of critical illness that persists after the acute illness and well beyond ICU and hospital discharge, i.e., at the “back-end” of critical care. At present, ICU-associated cognitive impairment is quietly altering the landscape of society and, if left untreated, will be a growing public health threat. This emerging epidemic demands that the ICU community develop and implement strategies targeted towards preservation of brain function, both within and beyond the ICU.
Background
ICU delirium is an acute brain dysfunction that is characterized by sudden, fluctuating changes in consciousness and cognition that develop over a brief time period.(8) The condition may be present on admission or may develop during the ICU stay, due to the critical illness itself or as a complication of medical treatment. ICU delirium is commonly characterized as hyperactive (agitation and emotional ability) or hypoactive (apathy and diminished responsiveness), the latter being more common.(9,10) The predominance of hypoactive features leads to under-recognition of ongoing acute cognitive dyfunction when validated assessment tools are not utilized, with up to 75% of cases overlooked.(11,12) In contrast, when measured with sensitive delirium assessment tools, ICU delirium is found to develop in about two-thirds of ICU patients, especially when mechanically ventilated.(13–17) The high prevalence of ICU delirium is particularly striking when one considers the independent influence of delirium upon a number of important outcomes. ICU delirium is associated with longer hospital stays,(18,19) billions of dollars in additional costs,(20) and substantial increases in mortality. The latter was first observed in a prospective cohort study of mechanically ventilated patients,(21) which demonstrated a 3-fold increase in mortality at six months for patients who experienced ICU delirium compared with those who did not (FIGURE 1). In addition, each additional day spent in delirium was associated with a 10% increased hazard of death (HR, 1.1; 95% confidence interval; 1.0–1.3). A subsequent study demonstrated similar findings, with a 10% increased hazard of death (HR 1.10; 95% confidence interval; 1.02–1.18) up to one year after critical illness with each additional day of ICU delirium.(22) These findings were independent of age, severity of illness, co-morbidities, coma, and exposure to psychoactive medications.
Figure 1. Delirium is Independently Associated with 6-month Mortality.
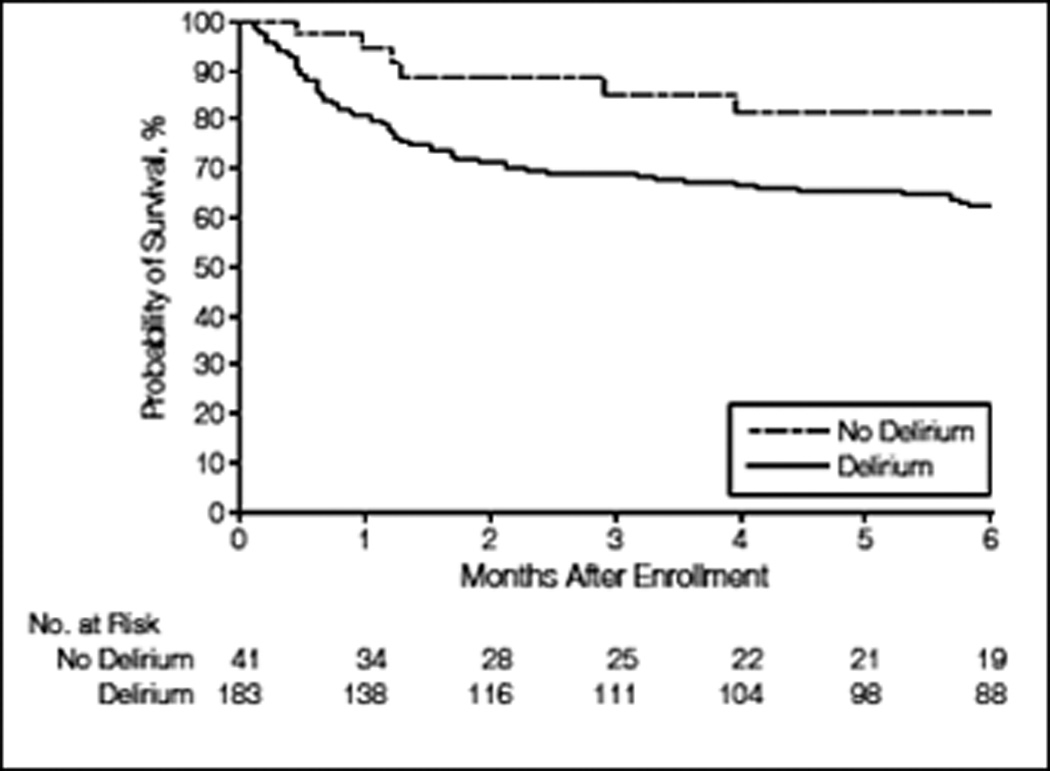
Multivariable Cox-proportional-hazards analysis demonstrated that patients who experience delirium in the ICU were 3-times more likely to die at 6 months (HR, 3.2; 95% confidence interval, 1.4 to 7.7; P=0.008), after adjustment for age, Charlson Comorbidity Index, modified Blessed Dementia Rating Scale score, Acute Physiology and Chronic Health Evaluation II score, Sequential Organ Failure Assessment score, sepsis, acute respiratory distress syndrome, and time-varying covariates for coma and use of sedative and analgesic medications. From Ely et al.(21) Copyright © 2004 American Medical Association. All rights reserved.
In addition to in-hospital brain dysfunction (namely, delirium and coma), over one-half of ICU survivors have long-term brain dysfunction in the form of a functionally debilitating dementia-like illness. This appears to be predicted by delirium duration(23–25) and leads to markedly increased rates of institutional placement.(26,27) The impact on an ICU survivor’s life can be devastating.
Thus, in response to the changing ICU population and alarming cognitive outcomes among ICU survivors, an overarching restructuring of how intensivists assess and manage the brain at the “front-end” and the “back-end” of critical care is required. We propose a 3-step approach to this partially iatrogenic and certainly modifiable phenomenon, an approach including Screening for brain dysfunction, Prevention of brain dysfunction, and Restoration of brain function (SPR). This practical system, which represents a synthesis of recent evidence along with expert opinion, will balance concepts of personalized medicine (i.e., tailoring components to specific patients) with protocolized medicine. The approach will also balance improvements in individual patient’s health with ICU system’s health. Finally, recognizing the infancy of research in ICU brain dysfunction, we stress the importance of incorporating new screening, prevention, and restoration innovations to replace the virtual absence of existing paradigms of care.
Step 1: SCREENING for Brain Dysfunction
Screening for delirium in the SPR model encompasses two core components. The first is risk factor screening prior to delirium onset. The second is ongoing screening for delirium within the ICU using validated instruments.
Screening for Delirium Risk Factors
Multiple studies have investigated factors that may precipitate or prolong delirium. Risk factors are traditionally divided into predisposing and precipitating factors (Table 1).(28) Predisposing factors can be further classified into: a.) Genetics, b.) Demographics, c.) Functional status, and d.) Chronic conditions. Precipitating factors can be classified into: a.) Acute physiology, b.) Biochemical, c.) Acute conditions, d.) Procedures, e.) Medications, and f.) Environment. Note that classification of predisposing versus precipitating factors serves as a framework to understand risk factors and is not a firm dividing line for any single factor.
Table 1.
Predisposing and Precipitating Risk Factors Associated with ICU Delirium
| Predisposing Factors | |||||
|---|---|---|---|---|---|
| Genetics | Demographics | Functional Status | Chronic Comorbidities | ||
| APOE4(31) | Age(29,33) | None | Alcohol(30) | ||
| Cognitive impairment (16,30) | |||||
| Hypertension(13) | |||||
| Precipitating Factors | |||||
|
Acute Physiology |
Biochemical |
Acute Diagnosis |
Procedures | Medications | Environment |
| APACHE II score(29,32) |
Tryptophan (29) | Anxiety (32) | Number of intravenous infusions (30) |
Opiods(13,29,33) | Isolation (30) |
| Arterial pH(16) | Tyrosine (29) | Coma (32) | Number of tubes and catheters (13,30,34) |
Benzodiazepines(16,33 | Daylight (13,30) |
| Bilirubin(13) | Medical admission(30) |
Dopamine (82) | Family visits (13,30) |
||
| Creatinine(16) | Epidural use(13) | ||||
| Pain level(32) | Antipsychotics(30) | ||||
| Propofol(33) | |||||
The table includes only risk factors that were associated with incident ICU delirium in multivariable analyses. Table is limited to published studies that utilized a validated screening and/or diagnostic instrument for delirium.
Nearly 100 different risk factors have been investigated for potential association with delirium incidence in the ICU. The studies are heterogeneous, differing by study location, design, population, and outcome assessment.(13,16,29–35) Therefore, it is not surprising that at least 27 diverse risk factors have been independently associated with delirium among seven studies that applied validated delirium assessments. Despite this heterogeneity, there are some predisposing risk factors that are of broad importance, with age(29,33) and history of cognitive impairment(16,30) being particularly influential. Among precipitating risk factors, acute physiologic derangements (e.g., APACHE II score),(29, 32, 33) and opioid,(13, 29, 30, 33) and benzodiazepine administration(16, 30, 33) are the most common implicated factors. Only recently have investigators explored the relationship between ICU delirium and genetics, (31) biochemical factors,(29) and the environment.(30) Continued identification of novel risk factors and validation of previously reported ones will play a critical role for at least three future applications in the management of ICU delirium:
Personalized Medicine: Advances in risk factor identification will improve our understanding of the pathophysiologic basis of delirium, for which there are many hypotheses but few firm conclusions.(36) New knowledge brings promise of novel prevention and treatment strategies. For example, elucidation of genetic risk markers, coupled with advances in rapid genetic diagnostic technologies, will facilitate personalized pharmacologic treatments that consider the inherent genetic variation among patients experiencing ICU delirium. Similarly, biomarkers may someday assist in targeting treatments specific to one of the likely many physiologic pathways leading to the clinical syndrome of delirium.
Risk Stratification: Risk factor identification is critical for the development of robust risk-prediction models that will aid future clinicians to identify those at highest risk for delirium upon entry to the ICU and guide prevention strategies accordingly. Delirium risk strata may guide future choices of ICU analgesia and sedation or entry into a specialized delirium care pathway. Many factors to help stratify risk are likely not yet identified. For example, biochemical markers may provide a more sensitive means to signal early pre-clinical brain dysfunction.
Performance Measurement: Whereas mortality and length of stay are currently the most common risk-adjusted outcomes to measure ICU performance(37), delirium offers an additional outcome that is: a) measured with high sensitivity and specificity, b) measured prior to ICU and hospital discharge, c) influenced by changes in ICU environment and care processes, and d) critical to multiple stakeholders. Identification of reliable risk factors for the prediction of incident ICU delirium will enhance risk-adjustment models. Assessment of risk-adjusted cognitive outcomes across ICUs and/or institutions will help stakeholders learn from systems that exhibit exceptional rates of delirium to improve outcomes more broadly across ICUs.
Screen for Acute Cognitive Impairment
Any program designed to improve acute and chronic cognitive outcomes for critically ill patients requires robust measurement of ICU delirium. Although education(11) and implementation(38, 39) barriers exist, widespread availability of simple measurement tools significantly reduces obstacles to routine delirium assessment. Instruments such as the Intensive Care Delirium Screening Checklist (ICDSC) (40), the Nursing Delirium Screening Scale (Nu-DESC),(41) and the Confusion Assessment Method for the ICU (CAM-ICU)(14, 15) offer sensitive and specific instruments specifically developed for the ICU environment, across medical and surgical units, and among mechanically ventilated patients. Tools may differ in their development population, validation studies, and testing characteristics, but all provide an opportunity to improve each of the following:
Recognition of and Communication Regarding Delirium: In the absence of a valid screening instrument, assessments of cognitive status by nurses and physicians are variable and grossly under-recognize delirium.(11, 12) Symptoms of delirium may be incorrectly attributed to dementia or depression, or they may be completely overlooked. Validated ICU delirium instruments provide necessary tools to standardize the examination, and they provide highly sensitive and specific delirium measurements when compared to the gold standard DSM IV diagnostic criteria.(8) In addition to improved recognition, validated instruments provide a standard concept and language for efficient and informative provider-to-provider communication.(42) Without this standard, providers may lack confidence in their ability to assess and communicate delirium.
Clinical Decision Making: Delirium assessment will support providers’ diagnostic and therapeutic maneuvers. New onset of delirium alerts providers to changes in a critical end-organ, much like rises in creatinine or falls in blood pressure. Delay in delirium diagnosis poses a barrier toward efforts to understand the underlying etiology, such as sepsis, medication changes, or metabolic abnormalities. In addition, the decision to initiate or titrate medications (e.g., analgesia, sedation) depends upon accurate assessment of delirium. Without appropriate cognitive status information, treatment will not match the needs of the patient.
Measurement of ICU Performance: A common framework of care quality states that optimization of structure and processes will yield benefits in outcomes.(43) Implicit in this framework is that one reliably measures the outcome of interest. With reliable measures of delirium, ICU systems possess necessary tools to monitor cognitive outcomes. Further advances in risk-adjustment will increase the potential to measure a system’s health and understand how changes made in an ICU’s structure (e.g., hiring 24-hour intensivists) or process (e.g., implementing a standardized sedation protocol) affect cognitive outcomes.
Step 2 - PREVENTION of Brain Dysfunction
To balance patient comfort and minimize iatrogenic brain injury, universal optimization of a synergistic group of evidence-based practices must be implemented across ICUs to prevent acute and/or chronic brain dysfunction. We propose a set of evidence-based processes: Awakening and Breathing Coordination, Delirium monitoring and Exercise / Early mobility (ABCDE) that serve as a critical foundation for a brain dysfunction prevention model. The proposed ABCDE bundle represents years of critical care trials that have led (and are leading) to improvements in the “back-end” of critical care; that is to say, processes of care that focus on minimizing potentially harmful exposures in the ICU and move the patient towards quicker and more complete recovery both within and outside the ICU. Table 2 highlights the evolution of the “back-end” of critical care management and recovery.
Table 2.
Evolution of the “Back-end” of Critical Care: Management and Recovery
| Years | Concept Introduced and Published | “Back-End” Process of Care | Evolutionary Step of the ABCDE Bundle |
|---|---|---|---|
| 1995–99 | Spontaneous Breathing Trials (SBTs) | Liberation from Ventilation | Step B |
| 1999–2004 | Spontaneous Awakening Trials (SATs) | Liberation from Sedation | Step A |
| 2001–07 | Awakening and Breathing Coordination (SATs + SBTs) |
Liberation from Sedation and Ventilation |
Steps ABC |
| 2001–08 | Validation and Implementation of Delirium Assessment / Monitoring Tools |
Delirium monitoring | Step D |
| 2009–10 | Early Mobility and Physical Therapy | Animation | Step E |
| 2010 | Awakening and Breathing Coordination, Delirium Monitoring, and Early Mobility |
Liberation and Animation | ABCDE |
The strength of the ABCDE model’s foundation rests upon evidence developed over time as well as its potential ability to positively impact additional valued outcomes, including mortality, length of stay, and physical function. The model’s foundation should be viewed as a starting point, with future discoveries in genetics, neuroscience, and pharmacology leading to additional strategies to build upon or modify the ABCDE model. In addition, this protocolized approach, although widely applicable in the ICU, is intended to be a guiding design and not a tool that should be rigidly applied without consideration of clinical input and critical analysis. With that said, information gained from sedation and delirium monitoring, as well as during spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs), will greatly facilitate decision -making, providing an information-rich environment to enable improved clinical decisions.
The first three steps in ABCDE, awakening and breathing coordination, refer to the daily performance of spontaneous awakening trials paired with spontaneous breathing trials as coordinated by the ICU team. In 1999, Kress et al demonstrated that, compared with uninterrupted sedation, consistent implementation of scheduled daily spontaneous awakening trials (SATs) reduced average mechanical ventilation duration by more than two days and reduced the number of ICU complications (44) without increased unplanned extubations.(45) In 1996, Ely et al published the first controlled trial to demonstrate that, when compared to physician judgment alone, protocolized SBTs to assess readiness for extubation decreased ventilator days by 1.5 days and led to 50% fewer ventilator-related complications.(46) This management approach has been successful in other settings and when managed by non-physician providers.(47, 48) In 2008, Girard et al, building upon prior evidence, demonstrated that coordinating SATs and SBTs together further improved outcomes compared with usual practices.(49) In this randomized controlled trial, the intervention group received patient-targeted sedation each day accompanied by protocolized, paired SATs and SBTs. The control group also received patient-targeted sedation and daily SBTs. SATs were allowed in the control group but were initiated at the discretion of individual providers as part of usual practice. Compared with the control group, the intervention group achieved reductions in hospital length of stay by four days, reduced median days of coma by one day, 14% absolute risk reduction in death at one year (FIGURE 2), and a reduction in the incidence of long –term brain dysfunction at 3 months.(50)
Figure 2. Paired Spontaneous Awakening Trials with Spontaneous Breathing Trials Reduce Mortality at 1-year.
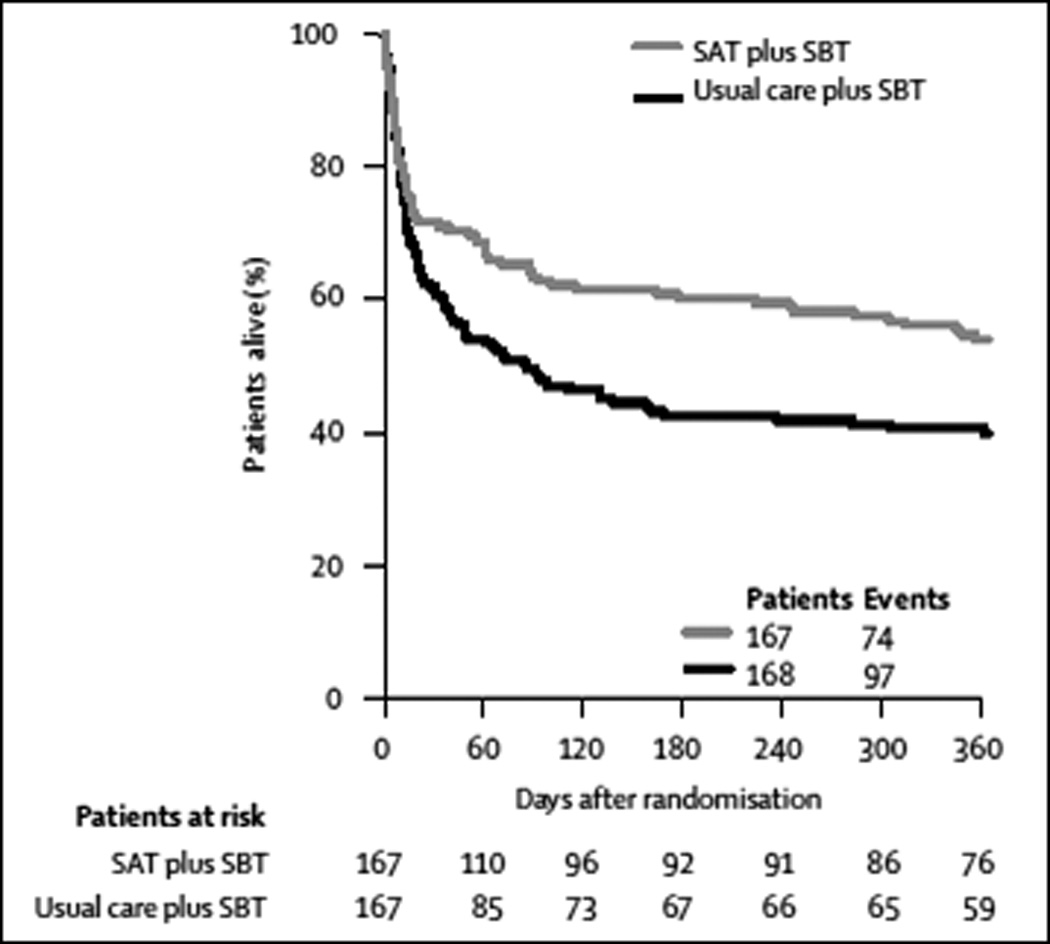
Multivariable Cox-proportional-hazards analysis demonstrated that patients in the intervention group (receiving paired daily spontaneous awakening and breathing trials) were 32% less likely to die during the following year compared to the control group. HR = 0.68; 95% confidence interval, 1.6 to 2.9; P < 0.001. Reprinted from The Lancet, Vol 371, Girard et al"Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial”, Pages 126-134, © 2008, with permission from Elsevier.(49)
These findings highlight the harms of prolonged exposure to sedation. The benefits seen with paired SATs and SBTs, together with consistent titration of sedation to minimum achievable sedation targets, should make continuous deep sedation an uncommon situation that requires specific indications such as ongoing needs for high FiO2 and/or PEEP. Implementation of awakening and breathing coordination also underscores the importance of standardized sedation assessments. Standardized measures allow adjustment of sedative exposure to meet the minimal necessary sedation level using objective measures rather than clinician judgment alone. Sedation monitoring can be achieved with simple, validated, sedation screening instruments (e.g., Sedation Agitation Scale (SAS),(51) Ramsay Score,(52) Richmond Agitation-Sedation Scale,(53) or Minnesota Sedation Assessment Tool(54)). Patients requiring deeper levels of sedation may receive additional monitoring. For example, intermittent bispectral index monitoring(55) may assist clinicians in the prevention of burst suppression, an electroencephalographic finding associated with heavy sedation exposure that is an independent predictor of death.(56)
The fourth step in ABCDE, delirium monitoring, refers to regular screening for delirium in the ICU with a validated screening instrument (e.g., the ICDSC,(40) the Nu-DESC,(41) or CAM-ICU)(15)). First discussed in Step 1 (Screen for Brain Dysfunction), this process should also be viewed as a critical piece of any delirium prevention model. Standardized instruments provide reliable means to communicate important information of brain function across providers,(57) and signal important clinical changes, which may require diagnostic and/or therapeutic adjustments in care. In addition, unit level rates of delirium may be sensitive to newly implemented prevention strategies. For example, implementation of paired awakening and breathing protocols may impact cognitive outcomes that can be followed with routine use of delirium assessment instruments. This feedback mechanism provides invaluable information to the ICU team in making process adjustments and implementing new system -wide changes.
The final step of ABCDE, exercise, refers to early mobilization among ICU patients. Although under-utilized in modern critical care, early mobilization is both feasible (58, 59) and effective. Schweickert et al, (59) recently demonstrated that early mobilization combined with awakening and breathing coordination, compared with awakening and breathing coordination alone, was associated with decreased ICU length of stay, reduced number of ICU delirium days from a median of 4.0 (95% confidence interval; 2.0–7.0) to 2.0 (0.0–6.0), and earlier return to independent functioning across a broad range of basic activities of daily living (FIGURE 3). An additional single-center implementation study demonstrated impressive benefits of early mobilization, with a mean reduction in hospital length of stay by 3.1 (95% confidence interval; 0.3 – 5.9) days and 32% (21 – 53%) fewer days of delirium in the post-implementation group.(60) This compelling evidence suggests that continued efforts to improve physical function yields cognitive, as well as physical, restorative benefits, and that early exercise should be strongly considered as a routine part of ICU care.
Figure 3. Early Mobilization When Added to Paired Spontaneous Awakening and Breathing Trials Improves Independent Function at Hospital Discharge.
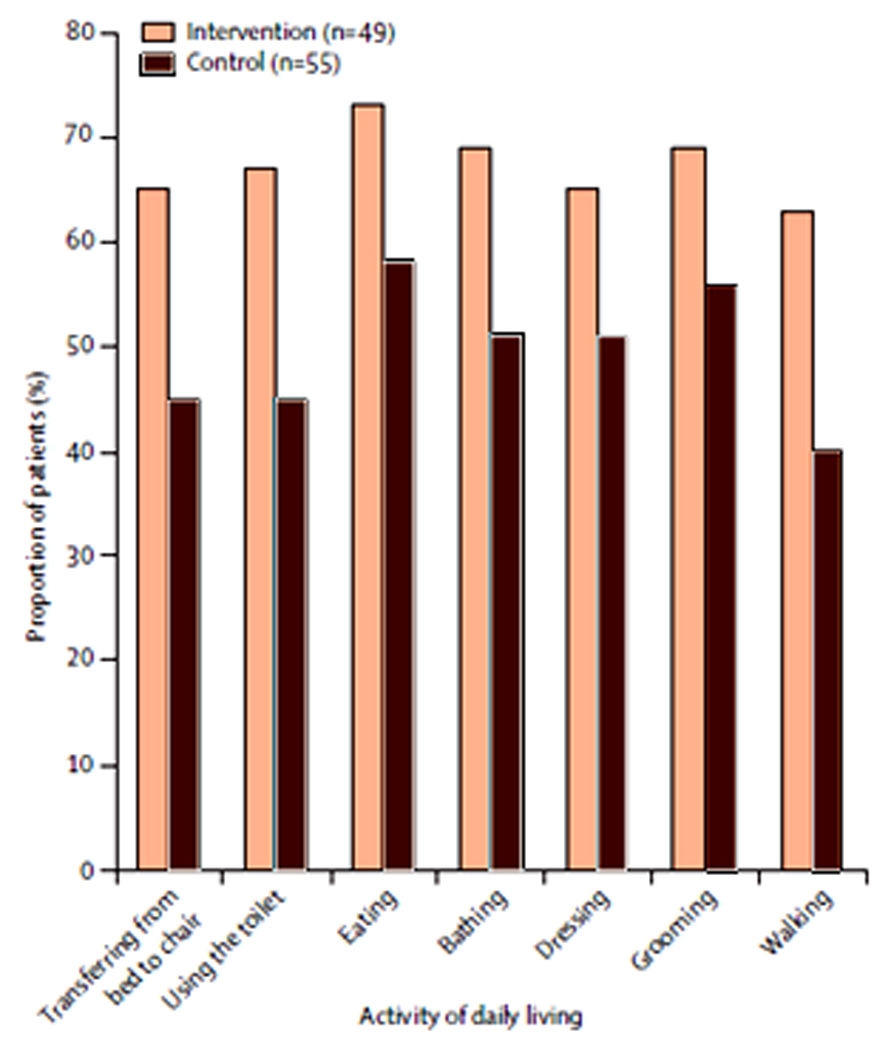
Comparison of functional outcomes between intervention group receiving coordinated spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs), delirium monitoring, and protocolized early mobilization compared to control group receiving paired SATs and SBTs and delirium monitoring alone. Functional outcomes are consistently improved in the intervention group. Reprinted from The Lancet, Vol 373, Schweickert et al"Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial”, Pages 1874-1882, ©2009, with permission from Elsevier.(59)
The ABCDE brain dysfunction prevention model must be viewed as a framework upon which new prevention strategies can be added as we learn more. New strategies will focus on detrimental neuro-regulatory stressor effects of specific sedative medications, environmental factors, and disease-specific interactions, including those with sepsis. These factors have been implicated as specific risk factors for delirium, and each may lend themselves to modifications in treatment or the ICU environment itself. For example, sedative management may prove to be particularly important in influencing ICU delirium incidence and/or duration. Therefore the “C” of ABCDE may be considered an indicator of sedation “Choice.” Recent controlled trials, in fact, investigating analgosedation,(61) as well as light sedation(62) protocols demonstrated significant reductions in mechanical ventilator days and ICU length of stay without associated psychological harm. Although analgosedation in one study resulted in increased “agitated delirium,” the effect on the more prevalent form of delirium (hypoactive delirium) is unknown, and long -term cognitive and psychological outcomes have not been reported to date.(61)
Other sedative strategies have shown important effects on brain dysfunction. Studies investigating dexmedetomidine, a novel selective α2-adrenergic receptor agonist, show promising reductions in daily prevalence of delirium when compared to traditional benzodiazepine sedation.(63–65) In a randomized control trial by Riker et al., (64) sedation with dexmedetomidine rather than midazolam reduced daily prevalence of delirium by 24.9% (FIGURE 4). Dexmedetomidine may be a promising agent to reduce delirium, but is only one of many commonly used sedative agents that need to be rigorously studied with respect to their effects on acute and chronic brain dysfunction. With strong evidence that individual agents may have variable effects on cognition, research must expose how other alternative pharmacologic strategies (e.g., propofol, antipsychotics, or analgosedation) promote or prevent brain dysfunction. Sedation protocols must be regularly refined and updated to incorporate evolving evidence and integrate best practices of sedation and delirium monitoring as well as tightly linked SAT and SBT practices (FIGURE 5).
Figure 4. Treatment with Dexmedetomidine Reduces the Daily Prevalence of Delirium when Compared to Treatment with Midazolam.
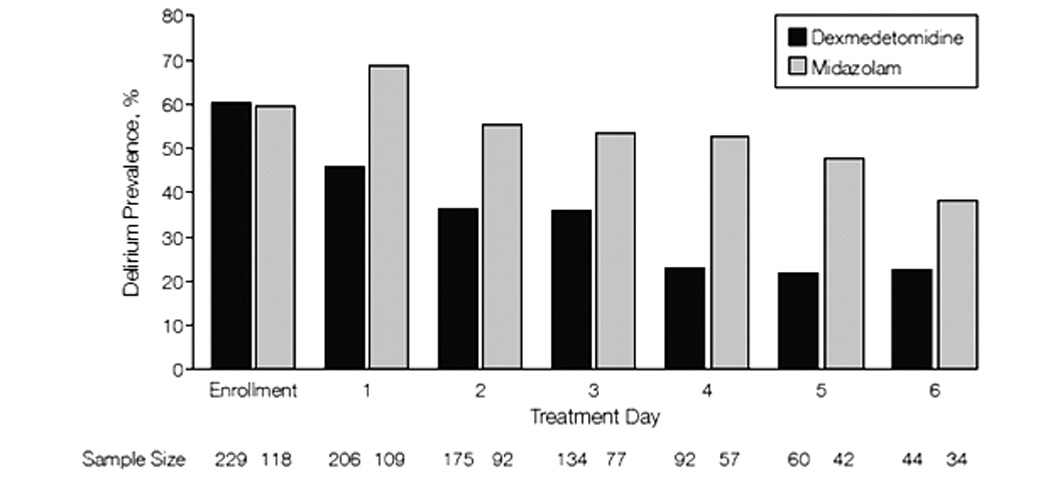
Comparison of daily delirium prevalence of intervention group receiving dexmedetomidine-based sedation compared to the control group receiving midazolam. The dexmedetomidine sedation arm experienced a 24.9% relative decrease (95% confidence interval; 16%-34%; P < 0.001) in daily delirium compared to midazolam. From Riker et al.(64) Copyright © 2009 American Medical Association. All rights reserved.
Figure 5. Analgesia and Sedation Protocol – Incorporating Routine Sedation and Delirium Monitoring.
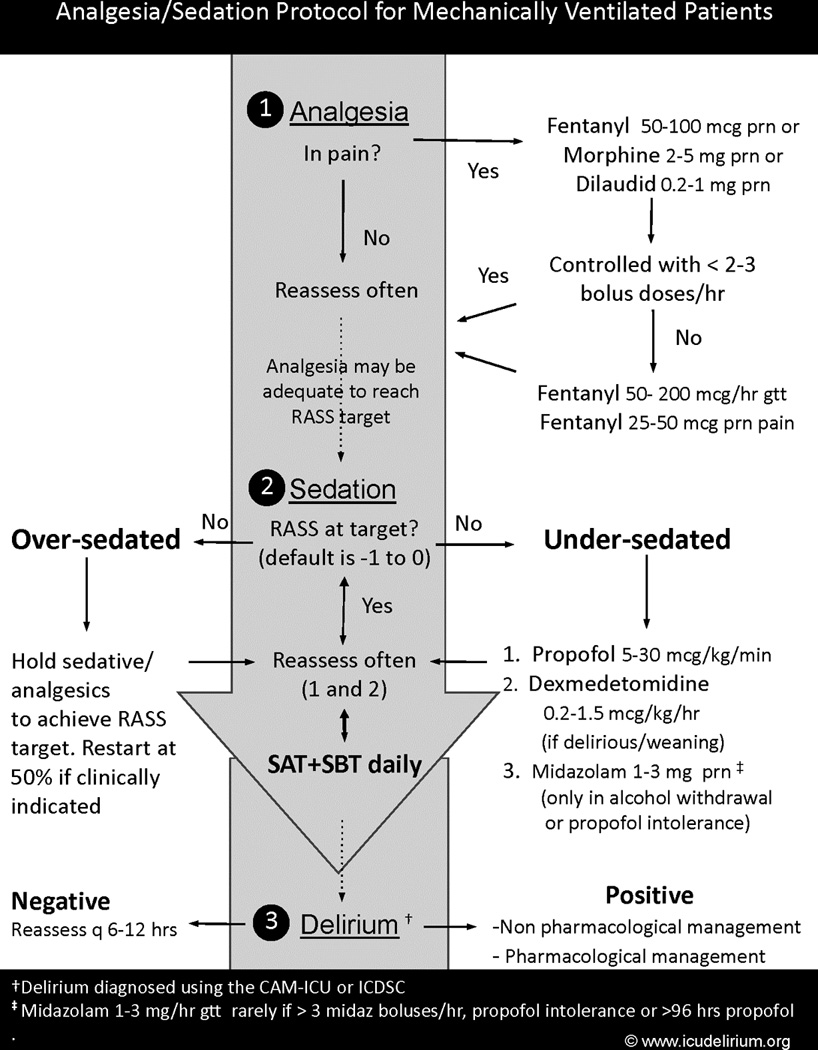
This analgesia and sedation protocol includes principles of sedation and delirium monitoring, targeted sedation using the minimal sedation required with avoidance of benzodiazepines, as well as routine spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs). Copyright © 2010 icudelirium.org. All rights reserved.
In addition to pharmacologic agents, there are nonpharmacologic strategies that demonstrate potential to prevent delirium. Early mobility, already included in the ABCDE model, as well as strategies to optimize sleep, reduce noise, maintain diurnal rhythms, and control pain are among a few promising nonpharmacologic strategies. Some of these have already been shown to reduce delirium in the non-ICU environment.(66, 67) The ICU community must test these and other novel strategies in appropriately designed trials to understand if similar benefits are afforded to the critically ill population.
Step 3 - RESTORATION of Brain Function
The impact of care in the ICU does not end at the threshold of ICU discharge. Increasingly, it is understood that patients cared for in the ICU experience a host of psychiatric (e.g., depression(68), post -traumatic stress disorder(69)) and functional impairments (e.g., long -term cognitive impairment(5), dementia(6), and weakness,(70)) that affect ICU survivors long after hospital discharge. In fact, ICU delirium may be a key link between critical illness and long-term cognitive impairment.(23, 25) Identifying patients at risk for long-term cognitive impairment is critical to address the needs of this population. Unfortunately, providers rarely screen patients for cognitive impairment at discharge(5, 71), and only a few patients who may benefit from cognitive rehabilitation currently receive it.(72) Unrecognized deficits in neuropsychological abilities such as attention, memory, and executive function may pose significant risk to the patient’s ability to carry out instrumental activities of daily living and impact upon their safety, quality of life, and future income.
In light of this gap in care, intensivists must embrace the fact that attention to cognitive function after ICU care is a professional obligation. A new paradigm of post-ICU care is needed, one that incorporates a) identification of populations at high risk for long-term cognitive impairment, b) treatment of “at risk” individuals with cognitive and physical rehabilitation directed towards executive and memory deficits, and c) incorporation of pharmacologic means of brain restoration following appropriate clinical trials.
The ICU community must work to identify those at greatest risk for cognitive impairment and increase subsequent referrals for neuropsychological testing and cognitive rehabilitation. Although more research is needed to understand risk factors for long-term cognitive impairment, it is clear that severity of illness, acute diagnoses, advanced age, sepsis, acute respiratory distress syndrome, ICU delirium, and pre-morbid cognitive dysfunction are initial target populations to consider.(5, 25, 72, 73) In addition, brief cognitive screening instruments such as the Montreal Cognitive Assessment (MoCA),(74) Folstein Mini-Mental State Exam,(75) and the Mini-cog(76)) are additional tools to assist in identification of at-risk individuals for long-term cognitive impairment. A new model will exist of post-ICU neuropsychological testing for the following populations: (a) those identified at high risk by risk-factor identification and/or brief cognitive screening, (b) those with sustained delirium (e.g., 3+ days) due to a near 100% risk of neuropsychological dysfunction,(25) and (c) those returning to a job or objective task that would be jeopardized or unsafe in the presence of executive dysfunction and memory loss. Appropriate testing and treatment thresholds must be investigated and validated and additional risk factors considered, which may both improve the sensitivity and specificity of the screening process over time. For example, advances in anatomic and functional MRI may be incorporated at certain centers to characterize the nature of the patient’s injury and recovery processes.(77) Better understanding of the anatomic pathology may help guide neurocognitive rehabilitation, predict response to therapy, and provide improved understanding of longer-term prognosis for ICU survivors with acute cognitive impairment.
It is important to continue to track the patient’s cognitive status during the weeks to months following ICU and hospital discharge. When patients are believed to have cognitive impairment, they should be referred to a clinical neuropsychologist or clinical psychologist for consultation and further neuropsychological evaluations. When cognitive impairment is appropriately identified, patients must be referred for cognitive and physical rehabilitation directed towards improving executive and memory deficits. Cognitive rehabilitation is an established therapy, which has been shown to improve cognitive function(78, 79) and may be appropriate for individuals with cognitive impairment due to a wide variety of causes (e.g., traumatic brain injury, cerebrovascular accident, hypoxia). It is considered to be safe and effective in improving neuropsychological abilities such as attention, concentration, memory, and executive function.(80)
As awareness of chronic brain dysfunction increases among ICU survivors and physicians, demand for new therapies will increase in parallel. Unfortunately, no randomized trials have been performed to date, demonstrating a large unmet need for drug development and investigation in this area. As an initial step, pharmacological means of brain restoration currently used for dementia (cholinesterase inhibitors) and tested in post-operative settings(81) should be incorporated following appropriate ICU trials. Other medications to consider include those that interrupt/modify dopaminergic (e.g., typical and atypical antipsychotics) or serotonergic pathways, two neurotransmitters that have been implicated in the pathophysiology of delirium. In addition, treatments may be directed towards inflammation, coagulation, or the sympathetic nervous system, other targets with mechanistic relations to delirium. Further understanding of genetic determinants and specific biomarkers will expand this list and hopefully assist in reversing the ongoing epidemic.
Conclusion
The adverse effects of critical illness on acute and chronic brain function are increasingly apparent. In response, we have presented a comprehensive approach aimed at screening and preventing acute and chronic brain dysfunction. The outlined approach requires improvements in both individual patient care as well as ICU systems. Although much needs to be discovered, existing evidence-based strategies are ready for implementation to improve cognitive outcomes at the “back-end” of critical care. Intensivists are uniquely positioned to lead improvements and embrace restoration of acute and chronic cognitive function as an essential component of their practice.
Supplementary Material
Acknowledgments
Financial Support: Dr. Vasilevskis was supported by the Veterans Affairs Clinical Research Center of Excellence and the Geriatric Research Education and Clinical Center, Veterans Affairs, Tennessee Valley Healthcare, Nashville, Tennessee. Dr. Girard is supported by the National Institutes of Health (AG034257) and the VA Geriatric Research, Education and Clinical Center.
Footnotes
Location: The work was performed at Vanderbilt University Medical Center and the Department of Veterans Affairs Medical Center, Tennessee Valley Healthcare System in Nashville, TN.
Conflicts of interest:
Dr. Vasilevskis has no conflicts of interest to disclose. Dr. Ely has received grant support and honoraria from Eli Lilly and Company, Pfizer, Hospira, Aspect Medical Systems, and GlaxoSmithKline. Dr. Pandharipande has received honorarium from Hospira Inc and GlaxoSmithKline. Dr. Girard has received honoraria from Hospira inc.
Reference List
- 1.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: An analysis of bed numbers, use, and costs. Crit Care Med. 2004;32:1254–1259. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- 2.El Solh AA, Ramadan FH. Overview of respiratory failure in older adults. J Intensive Care Med. 2006;21:345–351. doi: 10.1177/0885066606292873. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Kelley MA, Schmitz RJ, et al. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA. 2000;284:2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 4.Lerolle N, Trinquart L, Bornstain C, et al. Increased intensity of treatment and decreased mortality in elderly patients in an intensive care unit over a decade. Crit Care Med. 2010;38:59–54. doi: 10.1097/CCM.0b013e3181b088ec. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31:1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 6.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington (DC): American Psychiatric Press; 2000. [Google Scholar]
- 9.Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin.Clin Neuropsychiatry. 2000;5:75–85. doi: 10.153/SCNP00500075. [DOI] [PubMed] [Google Scholar]
- 10.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: A study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 11.Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35:1276–1280. doi: 10.1007/s00134-009-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eijk MM, van Marum RJ, Klijn IA, et al. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37:1881–1885. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]
- 13.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: A study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 14.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 16.Pisani MA, Murphy TE, Van Ness PH, et al. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 17.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: A prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 22.Pisani MA, Kong SYJ, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson JC, Gordon SM, Hart RP, et al. The association between delirium and cognitive decline: A review of the empirical literature. Neuropsychol.Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: An independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;9999 doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard TD, Jackson JC, Pandharipande PP, et al. Duration of delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097–1101. [PubMed] [Google Scholar]
- 27.Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–857. [PubMed] [Google Scholar]
- 29.Pandharipande PP, Morandi A, Adams JR, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009;35:1886–1892. doi: 10.1007/s00134-009-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Rompaey B, Elseviers M, Schuurmans M, et al. Risk factors for delirium in intensive care patients: A prospective cohort study. Crit Care. 2009;13:R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ely EW, Girard TD, Shintani AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- 32.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 33.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 34.McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: Occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51:591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 35.Aldemir M, Ozen S, Kara I, et al. Predisposing factors for delirium in the surgical intensive care unit. Crit Care. 2001;5:265–270. doi: 10.1186/cc1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin. 2008;24:45–65. doi: 10.1016/j.ccc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman JE, Alzola C, Von Rueden KT. The use of benchmarking to identify top performing critical care units: A preliminary assessment of their policies and practices. J Crit Care. 2003;18:76–86. doi: 10.1053/jcrc.2003.50005. [DOI] [PubMed] [Google Scholar]
- 38.Devlin JW, Fong JJ, Howard EP, et al. Assessment of delirium in the intensive care unit: Nursing practices and perceptions. Am J Crit Care. 2008;17:555–565. [PubMed] [Google Scholar]
- 39.Riekerk B, Pen EJ, Hofhuis JGM, et al. Limitations and practicalities of CAM-ICU implementation, a delirium scoring system, in a Dutch intensive care unit. Intensive Crit Care Nurs. 2009;25:242–249. doi: 10.1016/j.iccn.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: Evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 41.Luetz A, Heymann A, Radtke FM, et al. Different assessment tools for intensive care unit delirium: Which score to use? Crit Care Med. 2010;38:409–418. doi: 10.1097/CCM.0b013e3181cabb42. [DOI] [PubMed] [Google Scholar]
- 42.Pun BT, Gordon SM, Peterson JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: A report from two medical centers. Crit Care Med. 2005;33:1199–1205. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 43.Donabedian A. The definition of quality and approaches to its assessment. Ann Arbor, MI: Health Administration Press; 1980. [Google Scholar]
- 44.Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272–1276. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 45.Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 46.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 47.Ely EW, Bennett PA, Bowton DL, et al. Large scale implementation of a respiratory therapistdriven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999;159:439–446. doi: 10.1164/ajrccm.159.2.9805120. [DOI] [PubMed] [Google Scholar]
- 48.Stoller JK, Mascha EJ, Kester L, et al. Randomized controlled trial of physician-directed versus respiratory therapy consult service-directed respiratory care to adult non-ICU inpatients. Am J Respir Crit Care Med. 1998;158:1068. doi: 10.1164/ajrccm.158.4.9709076. [DOI] [PubMed] [Google Scholar]
- 49.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 50.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.200903-0442OC. E-published ahead of print on March 1, :2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 52.Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 54.Weinert C, McFarland L. The state of intubated ICU patients. Chest. 2004;126:1883–1890. doi: 10.1378/chest.126.6.1883. [DOI] [PubMed] [Google Scholar]
- 55.Deogaonkar A, Gupta R, DeGeorgia M, et al. Bispectral Index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32:2403–2406. doi: 10.1097/01.ccm.0000147442.14921.a5. [DOI] [PubMed] [Google Scholar]
- 56.Watson PL, Shintani AK, Tyson R, et al. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–3177. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quenot JPM, Ladoire SM, Devoucoux FM, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007;35:2031–2036. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 58.Morris PE, Herridge MS. Early intensive care unit mobility: Future directions. Crit Care Clin. 2007;23:97–110. doi: 10.1016/j.ccc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: A quality improvement project. Arch Phys Med Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010 doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 62.Treggiari MM, Romand J-A, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37:2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 63.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 64.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 65.Pandharipande P, Girard TD, Sanders RD, et al. Comparison of sedation with dexmedetomidine versus lorazepam in septic ICU patients. Crit Care. 2008;12:275. [Google Scholar]
- 66.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 67.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 68.Davydow DS, Gifford JM, Desai SV, et al. Depression in general intensive care unit survivors: A systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: A prospective cohort study. Crit Care. 2007;11:R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Jonghe B, Lacherade JC, Sharshar T, et al. Intensive care unit-acquired weakness: Risk factors and prevention. Crit Care Med. 2009;37:S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 71.Boustani M, Baker MS, Campbell N, et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5:69–75. doi: 10.1002/jhm.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 73.Hopkins RO, Weaver LK, Pope D, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 74.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 75.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 76.Borson S, Scanlan J, Brush M, et al. The Mini-Cog: A cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 77.Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57:337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 78.Ho MR, Bennett TL. Efficacy of neuropsychological rehabilitation for mild-moderate traumatic brain injury. Arch Clin Neuropsychol. 1997;12:1–11. [PubMed] [Google Scholar]
- 79.Cappa SF, Benke T, Clarke S, et al. EFNS guidelines on cognitive rehabilitation: Report of an EFNS task force. Eur J Neurol. 2003;10:11–23. doi: 10.1046/j.1468-1331.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 80.Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Arch Clin Neuropsychol. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343–349. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 82.Sommer BRM, Wise LCD, Kraemer HCP. Is dopamine administration possibly a risk factor for delirium? Crit Care Med. 2002;30:1508–1511. doi: 10.1097/00003246-200207000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


