Abstract
Atypical presentation of IL-12 receptor β1 deficiency with pneumococcal sepsis and disseminated nontuberculous mycobacterial infection in a 19-month-old girl born to nonconsanguineous US residents
To the Editor
Mendelian susceptibility to mycobacterial diseases (OMIM 209950) is a rare primary immunodeficiency resulting from genetic defects in the IFN-γ/IL-12/23 axis. due to decreased IL12 or IFNγ secretion. In patients with disseminated nontuberculous mycobacterial and salmonella infections, mutations identified include IFNGR1, IFNGR2, IL12β, IL12Rβ1 and STAT1. Severe cases are commonly typically comcommonly associated with complete absence of IFN-γ receptor 1 receptor expression.1 We report a severe, unique presentation apparently associated with an IL-12 receptor β1 (IL-12Rβ1) mutation. This previously healthy girl with no family history of immunodeficiency or consanguinity presented at 19 months of age with fever, vomiting, diarrhea, anemia, thrombocytopenia, and massive hepatospleno-megaly. Blood cultures grew Mycobacterium avium complex and Streptococcus pneumoniae. Peripheral blood smear showed moderate poikilocytosis and anisocytosis but no conclusive signs of functional asplenia (ie, Howell-Jolly bodies). Bone marrow aspirate/biopsy demonstrated significant dyserythropoiesis that normalized on subsequent bone marrow evaluations. The underlying explanation for the cytopenias and red blood cell morphologic changes was a large mycobacterial burden in the bone marrow and sepsis. Bone marrow aspirate and stool culture grew M avium complex.
After ruling out HIV infection, she was initially evaluated for an IFN-γ receptor 1 defect given her age and the severity of her presentation. Flow cytometry for IFN-γ receptor 1 demonstrated detectable receptor on the patient’s monocytes (data not shown). Furthermore, Toll-like receptor 4 engagement via LPS with or without IFN-γ of the patient’s PBMCs in vitro demonstrated robust IL-12p70 secretion (Fig 1, A). In contrast, IFN-γ was markedly decreased after stimulation with phytohemagglutinin or phy-tohemagglutinin plus IL-12 (Fig 1, B). Measurement of the inflammatory cytokine TNF-γ after LPS or phorbol 12-myristate 13-acetate and ionomycin exposure of the patient’s PBMCs in vitro allowed for assay of Toll-like receptor signaling and IL-1 receptor (IL-1R)–associated kinase (IRAK4) functionality. Our patient and the healthy control had comparable production of TNF-α (the ratio of TNF-α production in response to phorbol 12-myristate 13-acetate and ionomycin/TNF-α produced in response to LPS alone was 1.7 for our patient vs 1.83 for the healthy control; data not shown). To assess humoral function, antibody titers were measured. Our patient demonstrated protective antibody responses to 3 of 14 pneumococcal serotypes (>1 μg/mL measured by multiplex immunofluorescent assay; data not shown), and natural blood allohemagglutinin levels were robust (anti-B titer, 1:64; data not shown). Together, these data suggested that IFN-γ receptor and Toll-like receptor signaling were intact, making other molecular defects such as nuclear factor B (NF-kB) essential modulator (NEMO) and IRAK4 unlikely, and implicated a defect in IL-12 receptor signaling.
FIG 1.
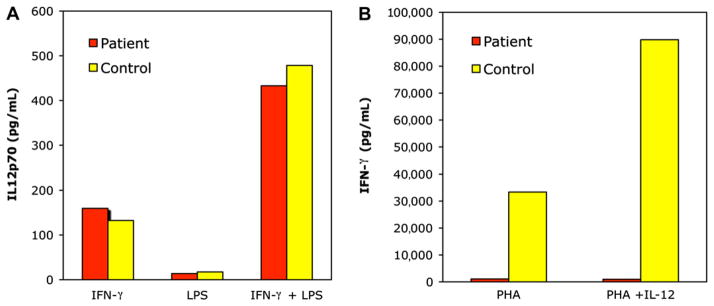
Incubation of PBMCs with IFN-γ, LPS, and IL-12 demonstrates intact IFN-γ receptor signaling and aberrant IL-12 responsiveness in this patient. PBMCs were isolated from the patient and a control subject. A, Cells were stimulated with IFN-γ or LPS alone or the combination. Secreted IL-12p70 was measured by ELISA. B, PBMCs were stimulated with either phytohemagglutinin (PHA) or a combination of PHA and IL-12. Secreted IFN-γ was measured by ELISA.
Flow cytometry for activation-induced phosphorylation of signal transducer and activator of transcription STAT–1 and STAT4 in PBMCs provided a rapid diagnostic test for interrogation of IFN-γ and IL-12 signaling.2,3 The patient’s cells demonstrated normal tyrosine phosphorylation of STAT1 in response to IFN-γ (data not shown) but no appreciable increase in tyrosine phosphorylation of STAT4 in response to IL-12 (Fig 2). These findings suggested that the activation defect was specific for IL-12 signaling and guided our genetic sequencing approach.
FIG 2.
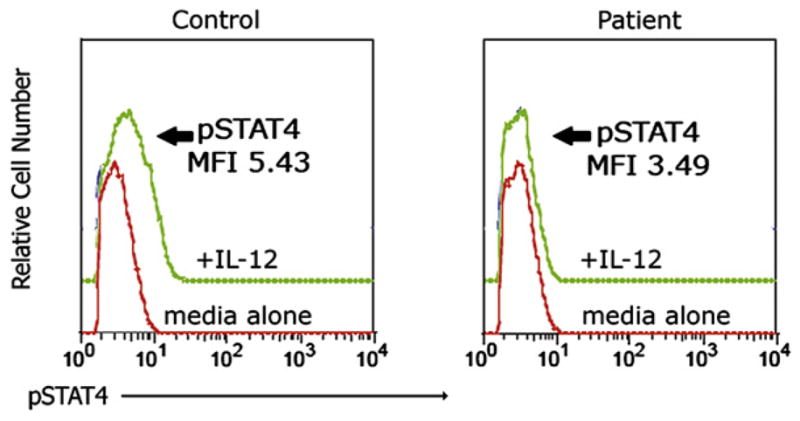
Diminished phosphorylation of STAT4 after IL-12 stimulation in this patient. PBMCs from the patient and a control subject were stimulated for 5 days with phytohemagglutinin +IL-2. After stimulation, 10 ng/mL IL-12 was added for 20 minutes, and cells were fixed, permeabilized, and stained with anti-pSTAT4 antibody (BD Biosciences, San Jose, Calif). Unstimulated cells served as a control. The histogram for pSTAT4 staining in the IL-12–stimulated lymphocytes is shown in comparison with the unstimulated control. The mean fluorescence intensity (MFI) for pSTAT4 is shown for each sample.
Genomic DNA sequencing of the IL12RB1 gene revealed a previously described autosomal recessive nonsense mutation at exon 14 (1623_1624delinsTT) leading to a premature stop codon at position glutamine 541 (Q541X).4 A recently characterized founder effect has been described for this mutation in the Argentinean population and has its origins in several European countries.5 Both parents of our patient have European ancestry but no known distinct familial immigration commonality. The patient currently remains on multiple antimicrobial agents, including ciprofloxa-cin, clarithromycin, rifampin, and ethambutol. IFN-γ was added after she demonstrated persistence of M avium complex in her blood.
In summary, this patient had an early and severe presentation of disseminated mycobacterial disease and pneumococcal sepsis associated with IL-12Rβ1 deficiency. Defects in IL-12Rβ1 are typically associated with a milder phenotype than those in IFN-γ receptor 1 and IFN-γ receptor 2. The use of phospho-flow cytometry was helpful in guiding genetic diagnosis and highlights the utility of functional flow cytometry assays as a rapid diagnostic tool. In addition, this is the first case of IL-12Rβ1 deficiency documented in a nonimmigrant, nonconsanguineous US patient. This case also represents the first documented case of pneumococcal sepsis in a patient with IL-12Rβ1 deficiency. The role of the IFN-γ/IL-12/23 axis in human pneumococcal disease is unknown, and studies using murine models have demonstrated conflicting results regarding a protective role for IL-12.6,7 It is unclear whether the pneumococcal sepsis in our patient was secondary to functional asplenia, secondary to an additional immune deficit not yet defined, or a direct consequence of IL-12Rβ1 deficiency.
Footnotes
Disclosure of potential conflict of interest: C. M. Seroogy and D. A. Gruenberg have received research support from the National Institutes of Health, the American Academy of Allergy, Asthma & Immunology, and Midwest Athletes Against Childhood Cancer. T. R. Torgerson is on a scientific advisory board for Baxter Healthcare and has received research support from CSL Behring and Grifols. The rest of the authors have declared that they have no conflict of interest.
References
- 1.Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113:620–6. doi: 10.1016/j.jaci.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Uzel G, Frucht DM, Fleisher TA, Holland SM. Detection of intracellular phosphor-ylated STAT-4 by flow cytometry. Clin Immunol. 2001;100:270–6. doi: 10.1006/clim.2001.5078. [DOI] [PubMed] [Google Scholar]
- 3.Fleisher TA, Dorman SE, Anderson JA, Vail M, Brown MR, Holland SM. Detection of intracellular phosphorylated STAT-1 by flow cytometry. Clin Immunol. 1999;90:425–30. doi: 10.1006/clim.1998.4654. [DOI] [PubMed] [Google Scholar]
- 4.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–35. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancoski J, Rocco C, Bernasconi A, Oleastro M, Bezrodnik L, Vratnica C, et al. A 475 years-old founder effect involving IL12Rb1: a highly prevalent mutation conferring mendelian susceptibility to mycobacterial diseases in European descendants. Infect Genet Evol. 2009;9:574–80. doi: 10.1016/j.meegid.2009.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauw FN, Branger J, Florquin S, Speelman P, Van Deventer SJ, Akira S, et al. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol. 2002;168:373–8. doi: 10.4049/jimmunol.168.1.372. [DOI] [PubMed] [Google Scholar]
- 7.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007;75:1196–202. doi: 10.1016/j.jaci.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]


