Abstract
Sinonasal malignant neoplasms comprise only 3% of all head and neck malignancies. Synchronous and metachronous tumors of the head and neck have been described, but rarely have there been reports of a single tumor with two distinct histologies. Here, we describe a case of a sinonasal malignant neoplasm with two distinct histologies. A case report and literature review was performed. We present a case of paranasal sinus neoplasm involving two malignant cell types. An 83-year-old woman presented with a 2-year history of symptoms suggestive of chronic sinusitis, which included nasal congestion and intermittent midface pressure. More recently, her symptoms progressed with the development of left-side epistaxis and she was found to have a mass in the left maxillary and ethmoid regions. A biopsy of the maxillary sinus mass revealed a moderately differentiated squamous cell carcinoma (SCC). She underwent complete resection of the lesion through an extended endoscopic approach. Final pathological analysis showed a malignant neoplasm with two distinct malignant morphologies; a moderately differentiated SCC and small cell neuroendocrine carcinoma. Appropriate diagnosis and treatment of head and neck malignancy depends on accurate tumor classification and staging. We present a case of a sinonasal tumor with two distinct malignant entities and review the available literature on the subject. Additionally, we discuss the etiologic theories and challenges in planning the optimal approach to management in this scenario.
Keywords: Azzopardi, colliding, histology, paranasal, sinus, treatment, tumor
Sinonasal tumors are relatively uncommon, accounting for only 3% of head and neck malignancies and 0.3% of all malignancies.1 The most common malignancies encountered in the nasal sinuses are squamous cell carcinoma (SCC), followed by adenocarcinoma. Neuroendocrine neoplasia, however, is rarely seen in the sinonasal region.2 Small cell neuroendocrine carcinoma (SNEC), in particular, is even more rarely seen. Current classification of neuroendocrine neoplasia is modeled after the current pulmonary classification scheme (carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma, and SNEC) and is based on morphological and biological behaviors of these neoplasms.2,3
Review of the current literature showed only three malignant tumors of combined histological morphology located in the paranasal sinuses. Although the prognosis of SCC of the paranasal sinuses is relatively variable though usually aggressive, those of the sinonasal SNEC seem to be always associated with poor prognosis and high rates of recurrence and, occasionally, distant metastasis.1,2 In contrast to small cell carcinomas of the lung and larynx, distant metastasis of paranasal sinus primary tumors appears to be relatively uncommon.
Here, we present the case of a maxillary sinus malignancy with two distinct histological subtypes and discuss the associated challenges with administration of postoperative adjuvant therapy.
CASE REPORT
Clinical Course
An 83-year-old woman presented to her primary care physician with complaints of left-sided nasal congestion and intermittent maxillary pressure for almost 2 years. She was initially treated with several courses of antibiotics for presumed sinusitis. With progression of symptom severity and the onset of epistaxis, a CT scan was obtained, which showed a left-side mass in the maxillary and ethmoid regions. Biopsy performed by an outside otolaryngologist revealed a moderately differentiated SCC (T3, N0, and Mx), at which time she was referred to our institution for further management.
On physical examination, including nasal endoscopy, she was found to have a left mass located at the anterior head of the middle turbinate and in the middle meatus. On further review of the CT scan, an enhancing soft tissue density centered in the medial maxillary sinus and extending into the anterior ethmoid sinus and nasal cavity was seen (Fig. 1). Bone of the orbit, skull base, and anterior maxilla did not appear to be involved. A magnetic resonance imaging scan was obtained that corroborated the tumor location and confirmed that opacification of neighboring cells was postobstructive in nature. Metastatic workup, including CT scans of the neck and chest, showed no evidence of metastatic disease.
Figure 1.
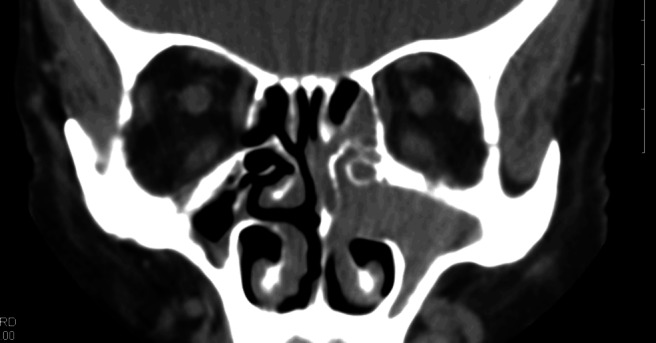
CT coronal cut: soft tissue window.
The patient was taken to the operating room for endoscopic medial maxillectomy and tumor resection. Complete removal of the mass was achieved and all frozen section margins were negative on completion of the surgery. The final pathological analysis exhibited a malignant neoplasm with combined moderately differentiated SCC and SNEC.
The decision was made by the patient's oncologists to administer cisplatin because of the aggressive nature of the tumor and tolerability expectations based on the patient's age and medical comorbidities. On early follow-up, ∼6 months after therapy, the patient was found to be clinically free of disease; however, on positron emission tomography imaging 7 months after therapy, multiple foci of physiological uptake were identified in the right rib, sternum, and thoracic vertebrae. A fine-needle aspirate was performed on the sternum, which showed tumor cells similar in appearance to the original biopsy specimen, which upstaged her diagnosis to stage IVC, T3N0M1. We discussed the risks and benefits of additional aggressive chemotherapy for an attempt at cure. Given the low likelihood of cure and high risk of associated side effects with aggressive chemotherapy, the patient elected for tumor surveillance and palliative care.
Pathological Findings
The examined specimen obtained from the endoscopic nasal resection of the tumor mass was in fragments that measured in aggregate 8 cm exhibited no invasion of bone or neural tissue and showed two distinct malignant histologies on the examined hematoxylin and eosin–prepared slides, a moderately differentiated SCC and SNEC. Some fragments showed a conventional moderately differentiated SCC exhibiting the classic morphology of infiltrating cobblestone pattern sheets of atypical squamous cells with spinous processes, glassy eosinophilic cytoplasms, occasional dyskeratotic cells, and surrounding stromal desmoplasia (Fig. 2). Other fragments showed infiltrating small blue tumor cells exhibiting small-sized cells with salt-and-pepper–appearing nuclei, scant cytoplasm, and marked apoptosis. Focal nuclear molding and fragile nuclei as shown by the presence of nuclear chromatin crushing and streaking artifact was found (so-called Azzopardi effect)4 (Fig. 3).
Figure 2.
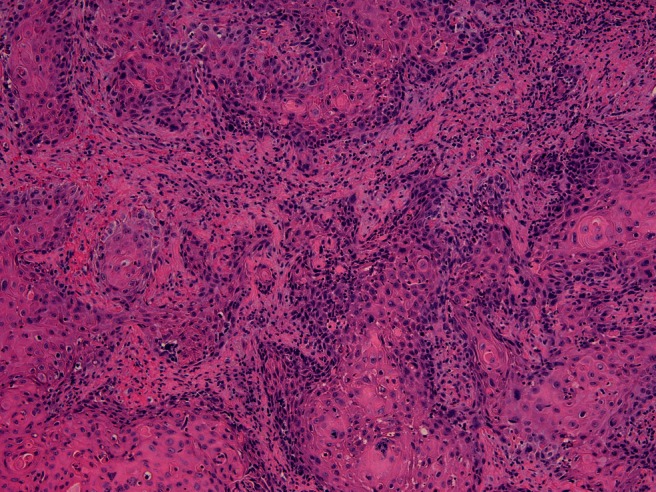
Part of the tumor showed features of conventional squamous cell carcinoma (hematoxylin and eosin stain, 20× magnification).
Figure 3.
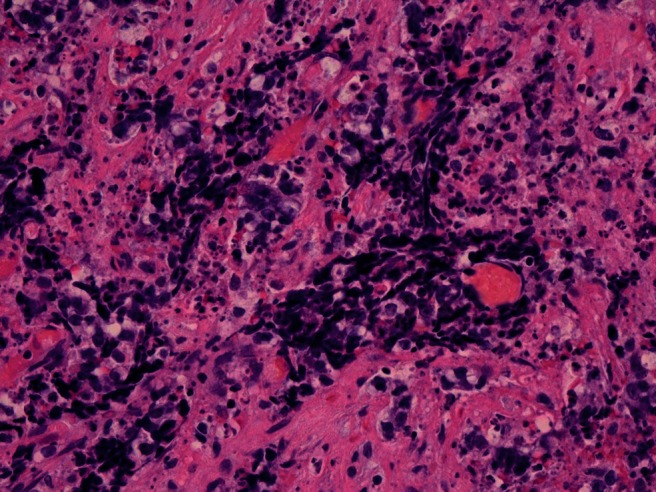
A high power of small cell carcinoma showing the small cell size with salt-and-pepper appearance, the fragile smeared chromatin, and the marked apoptosis (hematoxylin and eosin stain, 40× magnification).
The two entities exhibited not only distinct morphologies, but also distinct immunohistochemical profiles. The small cell carcinoma portion was positive for CD56 (Fig. 4), Cam5.2, and NSE and negative for CK5/6. The SCC portion was negative for CD56, Cam5.2, and neuron-specific enolase and positive for CK 5/6. Additional stains for CD45, chromogranin, synaptophysin, and BCL2 were negative in both tumor entities.
Figure 4.
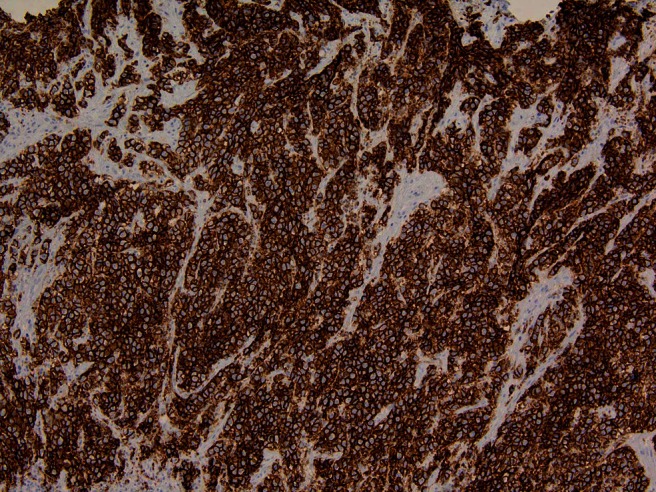
CD 56 immunohistochemical stain (neuroendocrine marker) is strongly positive in small cell carcinoma (20× magnification).
Our diagnosis of small cell carcinoma was based on two parameters; first, the morphology of the cells is essential in the morphological diagnosis of small cell carcinoma (i.e., small cells with basaloid blue–appearing morphology and showing the other associated features seen with these tumors including salt-and-pepper chromatin, nuclear molding, increased cellular apoptosis, and smearing artifacts or Azzopardi effect). It is the constellation of the morphological features that makes the diagnosis and not only one of them. The findings were further supported by immunohistochemical evidence, positivity to CD56 and NSE. The tumor was negative for chromogranin and synaptophysin; however, this is not uncommon in small cell carcinoma or many neuroendocrine tumors in which its reactivity for neuroendocrine markers can be variable to the extent that ∼10% of small cell carcinoma can be totally negative for neuroendocrine markers.5 Indeed, in the lung and other organs, many small cell carcinomas are positive for CD56 and negative for chromogranin and synaptophysin.5–7 In a study of CD56 as a diagnostic marker for small cell carcinoma,6 the positive rate for CD56 (86.3%) was higher than those for synaptophysin (78%), chromogranin A (73%), and NSE (81%).
DISCUSSION
Sinonasal tumors, in general, are a rare finding in that they account for only 3% of head and neck malignancies and 0.3% of all malignancies.1 The most commonly encountered sinonasal malignancies are SCC, followed by adenocarcinoma. By contrast, most neuroendocrine carcinomas in the head and neck are found in the larynx, followed by the salivary glands.8 In the sinonasal region, they tend to be quite rare including primary SNEC. Small cell carcinoma of the paranasal sinuses was first reported in 1965. The median age at presentation is 55 years, with no gender preference, and the outcome is usually poor. There appears to be no proven etiologic association with either tobacco use or the patient's occupation as seen in SCC and adenocarcinomas of the nasal sinuses, respectively.8,9 The small number of cases of small cell carcinoma in the sinonasal region presents a challenge in defining their typical clinical features, but they appear to be quite aggressive with a high potential for destructive local recurrence and a potential for metastatic disease.10,11
Although the development of SCC and adenocarcinoma in the sinonasal region seems to stem from squamous metaplasia of the respiratory mucosa with subsequent malignant change, the development of neuroendocrine tumors is less clear. In this context, two theories were proposed: metaplasia to neuroendocrine cells and malignant degeneration of a common pluripotent precursor cell. In general, extrapulmonary site neuroendocrine carcinomas are thought to be derived from neuroendocrine amine precursor uptake and decarboxylation and they show characteristic nuclear features (the so-called salt-and-pepper appearance of the nuclear chromatin).12 Immunohistochemistry is essential in supporting a definitive diagnosis of neuroendocrine malignancy when considering other entities of similar morphology occurring in the sinonasal tract. This includes rhabdomyosarcoma, natural killer-cell lymphoma, sinonasal undifferentiated carcinoma, olfactory neuroblastoma, Ewing sarcoma and primitive neuroectodermal tumor, and undifferentiated nasopharyngeal carcinoma. SNEC characteristically stains positive for CD56, Cam5.2, and focally for pancytokeratin and negative or weakly negative for chromogranin and synaptophysin and negative for CK 5/6 and P63. The aforementioned entities stain quite differently.13
Synchronous and metachronous tumors of the head and neck have been rarely described. Two theories can be put forward in our current case. The first is a tumor arising from common pluripotent stem cells with subsequent divergent differentiation, in this case into SCC and small cell carcinoma or, alternatively, a collision tumor, where two separate tumors arise synchronously in the same region through two independent molecular processes. This finding, although extremely rare and sometimes hard to prove, can be seen, e.g., at the esophagogastric junction when a gastric adenocarcinoma collides with an esophageal SCC. In this context, Yazawa et al. investigated the clonality of colliding primary lung cancers of adenosquamous carcinoma and large cell neuroendocrine carcinoma.14 Their results showed different clonality of the adenosquamous components from the neuroendocrine components. They classified this finding as a colliding tumor secondary to the difference in clonality.15
Paranasal sinus squamous cell cancer is typically managed with multimodality therapy. This treatment consists of surgical resection followed by chemotherapy and radiation therapy in all but the smallest of tumors. There are many chemotherapy agents that have been used to treat paranasal SCC, which can be used alone or in combination including carboplatin, cisplatin, 5-fluorouracil, docetaxel, and paclitaxel. Some of other chemotherapy agents that have shown positive results are bleomycin, cyclophosphamide, vinblastine, and methotrexate. Radiation therapy can be used preoperatively to decrease the tumor burden or postoperatively in combination with chemotherapy. Radiation therapy is typically given in excess of 60 gray to the primary site and any sites of nodal disease.1,16,17
In cases of nonsmall lung cancer and colon cancer, epidermal growth factor receptor (EGFR) antagonists and monoclonal antibodies have been found to show promising results.18,19 In head and neck SCC, several EGFR inhibitors have been studied alone or in combination with cisplatin/carboplatin, showing modest response rates.16,20 In the treatment of head and neck cancers, cetuximab, erlotinib, and gefitinib have proven to have less toxic side effects than the majority of chemotherapy agents. Cetuximab with concomitant high-dose radiotherapy has recently been shown to reduce mortality and improve control of locoregional disease in head and neck squamous cell cancers.17 Shiang-Fu et al. investigated EGFR targeting agents in a similar case of a colliding tumor. This study showed the rarity of a colliding tumor with a poor prognosis. The patient in their study had poor response to treatment and they concluded that the tumor's diverse components accounted for its aggressive behavior and lack of response to chemotherapy. They found no EGFR amplification in their tumor but had conclusions of a possible treatment role.15
To date, there is no consensus on the treatment of SNEC of the head and neck. As a result, treatment widely varies from institution to institution. General protocols include surgery followed by radiation therapy, concurrent chemotherapy and radiation therapy, and chemotherapy followed by surgery or radiation therapy. Various types of chemotherapy have been attempted including cisplatin and etoposide.10 Head and neck SCC and SNEC carry a poor prognosis secondary to a high rate of metastasis.2,10,12,13
This case highlights the rarity of the finding of a sinonasal tumor with two malignant histologies and presents the challenge in selection of optimal therapy. Our patient underwent extirpation surgical resection followed by cisplatin.
CONCLUSION
A head and neck site simultaneously involved with two distinct malignant entities is an exceedingly rare event. In our case, both SCC and SNEC were simultaneously diagnosed involving the left paranasal region. We discuss the diagnosis, potential prognostic implications, and management of this rare circumstance. Effective management of head and neck malignancies depends on accurate tumor identification and staging followed by appropriate combined treatment modalities. In the setting of two malignant histologies, an experienced multidisciplinary team is required to formulate the optimal treatment plan.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Day TA, Beas RA, Schlosser RJ, et al. Management of paranasal sinus malignancy. Curr Treat Opt Oncol 6:3–18, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Mineta H, Miura K, Takebayashi S, et al. Immunohistochemical analysis of small cell carcinoma of the head and neck: A report of four patients and a review of sixteen patients in the literature with ectopic hormone production. Ann Otol Rhinol Laryngol 110:76–82, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Chen DA, Mandell-Brown M, Moore SF, Johnson JT. “Composite” tumor-mixed squamous cell and small-cell anaplastic carcinoma of the larynx. Otolaryngol Head Neck Surg 95:99–103, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Azzopardi JG. Oat-cell carcinoma of the bronchus. J Pathol Bacteriol 78:513–519, 1959 [DOI] [PubMed] [Google Scholar]
- 5. Guinee DJ, Perkins SL, Travis WD, et al. The spectrum of immunohistochemical staining of small cell lung carcinoma in specimens from transbronchial biopsy and open lung biopsies. Am J Surg Pathol 102:406–414 [DOI] [PubMed] [Google Scholar]
- 6. Yun JP, Xiang J, Hou JH, Fu J. Expression of CD56, as a diagnostic marker in small cell carcinoma. Ai Zheng 9:1140–1143, 2005 [PubMed] [Google Scholar]
- 7. Kontogianni K, Nicholson AG, Butcher D, Sheppard MN. CD56: A useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artifact. J Clin Pathol 58:978–980, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol 15:264–278, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Gnepp DR. Small cell neuroendocrine carcinoma of the larynx. A critical review of the literature. ORL J Otorhinolaryngol Relat Spec 53:210–219, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Babin E, Rouleau V, Vedrine PO, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol 120:289–297, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Georgiou AF, Walker DM, Collins AP, et al. Primary small cell undifferentiated (neuroendocrine) carcinoma of the maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:572–578, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Rejowski JE, Campanella RS, Block LJ. Small cell carcinoma of the nose and paranasal sinuses. Otolaryngol Head Neck Surg 90:516–517, 1982 [DOI] [PubMed] [Google Scholar]
- 13. Avitia S, Osborne RF. Blindness: A sequela of sinonasal small cell neuroendocrine carcinoma. Ear Nose Throat J 83:530–532, 2004 [PubMed] [Google Scholar]
- 14. Yazawa T, Ishii H, Ito T, et al. Colliding primary lung cancers of adenosquamous carcinoma and large cell neuroendocrine carcinoma. Pathol Int 53:58–65, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Huang Shiang-Fu, Chuang Wen-Yu, Cheng Sou-De, et al. A colliding maxillary sinus cancer of adenosquamous carcinoma and small cell neuroendocrine carcinoma—A case report with EGFR copy number analysis. World J Surg Oncol 8:92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22:77–85, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101:13306–13311, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cappuzzo F, Finocchiaro G, Rossi E, et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol 19:717–723, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 21:1980–1987, 2003 [DOI] [PubMed] [Google Scholar]


