Abstract
Allergic fungal sinusitis (AFS), also referred to as allergic fungal rhinosinusitis (AFRS), is a noninvasive, eosinophilic form of recurrent chronic allergic hypertrophic rhinosinusitis. AFS has distinct clinical, histopathological, and prognostic findings that differentiate it from other forms of sinusitis. The core pathogenesis and optimum treatment strategies remain debated. Concerns surround the use of immunotherapy for AFS because allergen-specific immunoglobulin G (IgG) induced by immunotherapy could theoretically incite a Gell and Coombs type III (complex mediated) reaction. Type I hypersensitivity is established by high serum levels of allergen-specific IgE to various fungal antigens and positive Bipolaris skin test results. Type III hypersensitivity is established by an IgG-mediated process defined by the presence of allergen-specific IgG that forms complexes with fungal antigen inducing an immunologic inflammatory response. These reveal the multiple immunologic pathways through which AFS can impact host responses. Recent literature establishing benefits of fungal immunotherapy and no evidence of type III–mediated reactions, severe local reactions, or delayed reactions, indicate that application of AFS desensitization is a reasonable therapeutic strategy for this difficult to manage entity. Our review should encourage further clinical acceptance of AFS desensitization because the existing literature on this subject shows benefits of fungal immunotherapy and no evidence of type III–mediated reactions, severe local reactions, or delayed reactions.
Keywords: Allergic fungal sinusitis, chronic rhinosinusitis, eosinophilic mucus, literature review
Since its description in 1981 when Millar et al. described five patients with “allergic aspergillosis of the paranasal sinuses,” the characterization, pathophysiology, and ideal treatment of allergic fungal sinusitis remains shrouded in controversy.1,2 Fungal rhinosinusitis includes immune and pathological mechanisms, with continued debate regarding the pathophysiology of its hypersensitivity.3 Currently classified into three invasive (acute necrotizing, chronic invasive, and granulomatous invasive) and two noninvasive (mycetoma and allergic fungal) subgroups, fungal rhinosinusitis is an ever-changing entity. Recent publications question the central pathogenic importance of fungal allergy as a clinically important distinction from other eosinophilic mucin chronic rhinosinusitis entities.4
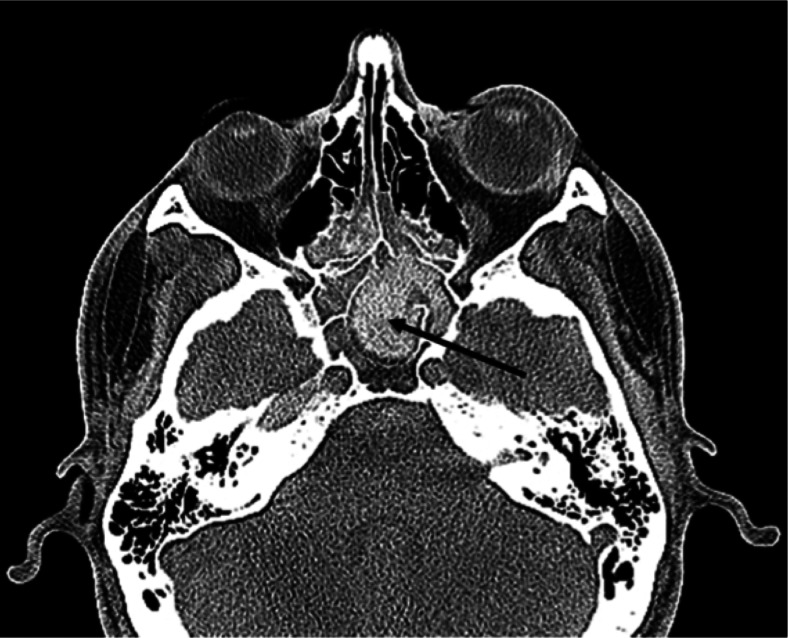
Allergic fungal sinusitis (AFS) or allergic fungal rhinosinusitis is a noninvasive eosinophilic form of highly recurrent chronic allergic hypertrophic rhinosinusitis that is clinically, histopathologically, and prognostically distinct from other forms of sinusitis.5 Five diagnostic criteria originally described by Bent and Khun define AFS: characteristic findings on CT to include opacification of paranasal sinuses, expansion and erosion of the involved sinus, and scattered intrasinus high attenuation areas amid mucosal thickening on unenhanced CT scans (Fig. 1), type I hypersensitivity, nasal polyposis presence of fungi on direct microscopy or culture (Fig. 2), and allergic mucus-containing fungal elements without tissue invasion.6,7 The sequelae of AFS can be significant, including visual symptoms, proptosis, and bony erosion. However, the core pathogenesis and optimum treatment strategy for AFS continue to be debated.2,5,8,9
Figure 1.
Axial CT sinus with soft tissue windowing in patient with features typical of allergic fungal sinusitis (AFS). Black arrow shows enhancement of fungus-laden mucus in sphenoid sinus.
Figure 2.
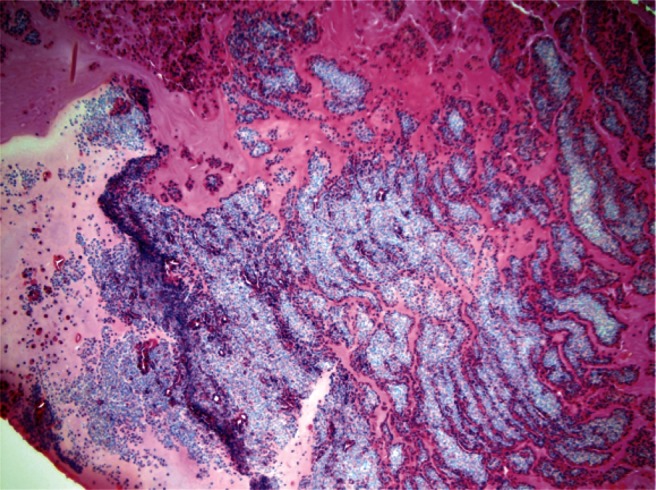
Hematoxylin and eosin stain (100× magnification) showed inspissated mucin containing collections of chronic inflammatory cells in a laminated arrangement. Focal aggregates of eosinophils are present. A special stain for fungi shows fungal elements morphologically consistent with Aspergillus or Bipolaris species.
DISCUSSION
Fungal hypersensitivity is central to the pathogenesis of AFS, but its exact role remains in question. Much of the controversy surrounding the role of fungal hypersensitivity revolves around fungal culture techniques and ambiguous defining characteristics of eosinophilic mucin chronic rhinosinusitis subtypes. The ability to culture fungi from AFS can range from 50 to 100%; however, there is significant debate as to whether fungal organisms obtained from these patients represent nonpathogenic flora versus causative organisms.10 It is evident that there is great risk in identifying commensurate organisms of the nose rather than pathogens with traditional culture techniques.10 In fact, it is hypothesized that the origin of AFS may be caused by subtypes of fungi present in the sinuses for which effective culture techniques are currently lacking. This ambiguity surrounding fungal culture complicates interpretation of those studies that evaluated the role of immunotherapy in AFS.10,11 An additional concern with existing research is that the spectrum of eosinophilic sinusitis remains incomplete, which currently ranges from the rigid Bent and Kuhn's criteria to chronic bacterial rhinosinusitis with nasal polyposis.11–13 In all of these cases, antigenic stimulation is believed to drive Th2 response that amplifies eosinophilic inflammation.
Along with culture technique differences, the lack of available antigens for suspected causative organisms is another complicating factor. Most commercially available mold extracts are variable and generally low in allergen.14 Source materials for these allergenic extracts originate from stock cultures maintained by the producer to ensure their integrity. The criteria and specifications used to select the fungal strains vary depending on the manufacturer. With a high rate of somatic mutation and hyphal fusion new gene combinations are constantly produced. To ensure consistency, genetic stability of the stock cultures must be monitored and controlled. Some strains are inherently less stable in this regard and not apt for manufacturing use.15
Much of what is known about the immunopathogenesis of AFS draws a strong analogy to allergic bronchopulmonary aspergillosis (ABPA). In ABPA, Aspergillus fumigatus is found within bronchial allergic mucin. Peripheral eosinophilia is found with very high titers of serum fungal-specific IgG and IgE. Similarly, in AFS, Bipolaris spicifera is found within sinus-allergic mucin in patients with hypertrophic sinus disease along with high titers of fungal-specific serum IgG. In contrast, fungal-specific serum IgG levels are not as high as in ABPA.5 In one immunohistological analysis, the increased allergic response in immune competent patients has been partly correlated to the known combined toxicities of eosinophilic granule major basic protein and neutrophil elastase.16 One study found that peripheral blood mononuclear cells from AFS patients are stimulated by fungal antigens and then secrete Th2-type cytokines.17 This has been confirmed in more recent studies further supporting a role for humoral immune factors in AFS.17,18
Type I hypersensitivity to fungal elements has been shown in several series by the presence of high serum levels of allergen-specific immunoglobulin E (IgE) to different fungal antigens and via positive Bipolaris skin test results.19,20 The existence of immunoglobulin specific to fungal elements uncovers the possibility that immunotherapy may offer a means to prevent the host inflammatory response.21 In addition to type I IgE-mediated responses, there are also classic clinical parallels in allergic fungal sinusitis and ABPA with type III hypersensitivity. Holman et al. showed this when 100% of patients (n = 16) with AFS showed IgE sensitivity via skin-prick testing to Biploaris, 82% showed allergen-specific IgE, and 93% had allergen-specific serum IgG.19 Concerns exist with the use of immunotherapy for AFS because allergen-specific IgG induced by immunotherapy could theoretically incite a Gell and Coombs type III (complex mediated) reaction. With this in mind, early immunotherapy treatment trials provoked great anxiety. Thus, allergen desensitization in AFS was historically avoided until an extended series of small retrospective clinical studies showed that it was safe; however, these studies provided little convincing evidence of therapeutic efficacy.5,7,22–27 One early study did find worsening of symptoms in five patients who underwent immunotherapy before surgery versus improvement in patients undergoing immunotherapy after surgery.11
The most recognized study of immunotherapy in the treatment of AFS was a 4-year retrospective study by Mabry.7,23–25,28 After the 1st year, nine patients previously treated with functional endoscopic sinus surgery for AFS completed 4–12 months of immunotherapy with the closest commercially available antigen to Bipolaris (Helminthosporium). While receiving immunotherapy, these patients had a decrease in polyp recurrence, nasal crusting, and decreased allergic mucin. Importantly, no patients developed immune complex or other adverse reactions. After the 2nd year, nonfungal antigens were added to immunotherapy simultaneously if indicated. Two of 10 patients receiving immunotherapy required systemic steroids and surgery for recurrence of disease. There continued to have no adverse reaction to immunotherapy, and the use of systemic corticosteroids was significantly less in the immunotherapy population. At the end of 3 years of observation, three patients that discontinued immunotherapy before 7 months were used as controls. Only 2 of 11 immunotherapy patients ever needed additional steroids. In the control group, each patient required multiple courses of oral steroids or had worsening clinical exams. Immunologic changes in allergen-specific IgE increased and never decreased whereas IgG to Alternaria and Helminthosporium was inconsistent. When immunotherapy was discontinued none of the original patients had recurrence of polyps, allergic mucin, or fungal casts 17 months after discontinuation.
Since the late 1990s there has been a notable gap in studies involving immunotherapy in AFS, potentially a result of Mabry's later study group's failure to show significant benefit from immunotherapy. When 17 patients were followed for ≥4 years in a study at the turn of the century, immunotherapy did not show a significant impact on longer-term controls.29 Also, Mabry's studies relied on the end point titration immunotherapy technique that uses far more dilute concentrations of immunotherapy.23,24,28,29 Current guidelines advocate more potent allergen concentrations.30 Concerns with recreating or initiating new studies using these higher allergen concentrations may have led researches to fear inciting an adverse reaction. Several other small retrospective studies have also been performed. One study by Folker et al. compared 11 patients receiving immunotherapy for at least 12 months to a control group (identified through pathology archives) of 11 patients with similar disease states who had been treated similarly with surgery, but had not received immunotherapy.31 The group receiving immunotherapy showed statistically significant improvements in endoscopic disease scoring and chronic sinusitis survey symptom scores, while using less corticosteroids. None of these patients reported any adverse reactions to immunotherapy. In a more recent review by Greenhaw et al. in 2011, 14 patients with AFS were treated with high-dose subcutaneous fungal immunotherapy and compared with 14 CRS patients without fungal allergy treated with fungal immunotherapy. There was no difference in the number of local reactions, large reactions, or late local reactions.26
CONCLUSION
Currently, the mainstay of treatment for AFS is surgical debridement followed by either oral corticosteroids, intranasal corticosteroids, or a combination of both. Topical and systemic antifungal therapy are proven ineffective in AFS,11,32,33 but limited case reports and retrospective studies using fungal immunotherapy as a treatment option have shown improvement in nasal crust formation, polyp formation, decreased systemic corticosteroid use, and improved quality of life. Aside from the single study where subjects receiving immunotherapy had worsening of symptoms, no study reported any type III complex–mediated reactions or more severe local or delayed reactions. In the current literature, there are no double-blind, placebo-controlled studies comparing fungal desensitization in AFS patients with a control group, illustrating that there is a need for continued investigation and research for this confounding and fascinating disease.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Millar J, Lamb D. Allergic bronchopulmonary aspergillosis of the maxillary sinuses. Thorax 36:710, 1981 [Google Scholar]
- 2. Goldstein MF, Atkins PC, Cogen FC, et al. Allergic Aspergillus sinusitis. J Allergy Clin Immunol 76:515–524, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 119:1809–1818, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pant H, Kette FE, Smith WB, et al. Eosinophilic mucus chronic rhinosinusitis: Clinical subgroups or a homogeneous pathogenic entity? Laryngoscope 116:1241–1247, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Schubert MS. Allergic fungal sinusitis: Pathogenesis and management strategies. Drugs 64:363–374, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Manning SC, Merkel M, Kriesel K, et al. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope 107:170–176, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Bent JP, III, Kuhn F. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg 111:580–588, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Bozeman S, deShazo R, Stringer S, Wright L. Complications of allergic fungal sinusitis. Am J Med 124:359–368, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Ryan MW. Allergic fungal rhinosinusitis. Otolaryngol Clin North Am 44:697–710, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin 74:877–884, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Marple BF. Allergic fungal rhinosinusitis: Current theories and management strategies. Laryngoscope 111:1006–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Glass D, Amedee RG. Allergic fungal rhinosinusitis: A review. Ochsner J 11:271–275, 2011 [PMC free article] [PubMed] [Google Scholar]
- 13. Ferguson BJ. Eosinophilic mucin rhinosinusitis: A distinct clinicopathological entity. Laryngoscope 110:799–813, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: A practice parameter third update. J Allergy Clin Immunol 127(suppl):S1–S55, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Esch RE. Manufacturing and standardizing fungal allergen products. J Allergy Clin Immunol 113:210–215, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Khan DA, Cody DT, II, George TJ, et al. Allergic fungal sinusitis: An immunohistologic analysis. J Allergy Clin Immunol 106:1096–1101, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy 23:281–287, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Porter PC, Ongeri V, Luong A, et al. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol 32:43–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gourley DS, Whisman BA, Jorgensen NL, et al. Allergic Bipolaris sinusitis: Clinical and immunopathologic characteristics. J Allergy Clin Immunol 85:583–591, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Manning SC, Mabry RL, Schaefer SD, Close LG. Evidence of IgE-mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope 103:717–721, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Ryan MW. Allergic fungal rhinosinusitis. Otolaryngol Clin North Am 44:697–710, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Bassichis BA, Marple BF, Mabry RL, et al. Use of immunotherapy in previously treated patients with allergic fungal sinusitis. Otolaryngol Head Neck Surg 125:487–490, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Mabry RL, Mabry CS. Immunotherapy for allergic fungal sinusitis: The second year. Otolaryngol Head Neck Surg 117:367–371, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Mabry RL, Marple BF, Folker RJ, Mabry CS. Immunotherapy for allergic fungal sinusitis: Three years' experience. Otolaryngol Head Neck Surgc 119:648–651, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Mabry RL, Marple BF, Mabry CS. Outcomes after discontinuing immunotherapy for allergic fungal sinusitis. Otolaryngol Head Neck Surg 122:104–106, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Greenhaw B, deShazo RD, Arnold J, Wright L. Fungal immunotherapy in patients with allergic fungal sinusitis. Ann Allergy Asthma Immunol 107:432–436, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Schubert MS. Medical treatment of allergic fungal sinusitis. Ann Allergy Asthma Immunol 85:90–97, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Mabry RL, Mabry CS. Allergic fungal sinusitis the role of immunotherapy. Otolaryngol Clin North Am 33:433–440, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Marple B, Newcomer M, Schwade N, Mabry R. Natural history of allergic fungal rhinosinusitis: A 4- to 10-year follow-up. Otolaryngol Head Neck Surg 127:361–366, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Hall AG, deShazo RD. Immunotherapy for allergic fungal sinusitis. Curr Opin Allergy Clin Immunol 12:629–634, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Folker RJ, Marple BF, Mabry RL, Mabry CS. Treatment of allergic fungal sinusitis: A comparison trial of postoperative immunotherapy with specific fungal antigens. Laryngoscope 108:1623–1627, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Bent JP, III, Kuhn F. Antifungal activity against allergic fungal sinusitis organisms. Laryngoscope. 106:1331–1334, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Morpeth J, Rupp N, Dolen WK, et al. Fungal sinusitis: An update. Ann Allergy Asthma Immunol 76:128–140, 1996 [DOI] [PubMed] [Google Scholar]