Summary
We evaluated the results of Guglielmi detachable coil (GDC) treatment in partially thrombosed aneurysms and determined if there is high rate of recanalisation on follow-up.
Among 149 treated aneurysms in 141 patients, 25 CT- or MR-confirmed partially thrombosed aneurysms were selected for evaluation. The features of thrombosed aneurysms and percentage of occlusion were analysed on initial angiograms. Follow-up angiograms`, which were available in 18 cases, were evaluated for aneurysm lumen recanalisation. The recanalisation rate was compared with that of non-thrombosed aneurysms treated with GDCs.
Locations of aneurysms were as follows: cavernous carotid ten; ophthalmic four; p-com. two; MCA one; A-com. one; basilar tip four; midbasilar two; PICA one. The size of the aneurysm lumen ranged from 5 to 30 mm (mean 16.8 mm) on angiograms, but on cross sectional images the size of aneurysms ranged from 13 to 70 mm (mean 24.6 mm). The extent of aneurysmal thrombosis ranged from 10 to 90 per cent (mean 46.4 per cent). On initial GDC treatment, total to subtotal occlusion was achieved in 18 cases out of 25 (72%). Of the 18 follow-up angiograms, 14 cases (77.8%) showed recanalisation ranging from 10 to 60 per cent of aneurysm size. Luminal recanalisation was due to migration (10 of 14) or compaction (4 of 14) of coil masses. In two cases, symptoms recurred in association with aneurysm recanalisation, but in no instance was haemorrhage noted. Attempts for retreatment were made in ten cases with success in six. In comparison, 14 (15.9%) out of 88 non-thrombosed cases revealed recanalisation on follow-up angiography.
Midterm follow-up angiograms in partially thrombosed aneurysms treated with GDC revealed a fivefold higher rate of recanalisation than in non-thrombosed cases. Close follow-up is necessary in patients with thrombosed aneurysms treated with GDCs.
Key words: interventional, aneurysms, GDC
Introduction
Thrombosis is known to occur in 9% to 13% of intracranial aneurysms with the incidence reported as high as 20% in giant aneurysms1-3. Although surgical clipping is a well-established treatment for intracranial aneurysms, a thrombus within aneurysms presents difficulties for clipping4. For these surgically difficult or inoperable cases endovascular treatment can provide a reasonable alternative. Occlusion of aneurysms with Guglielmi detachable coils (GDCs) is widely accepted as a safe and effective endovascular treatment. However, thrombosed aneurysms are reported to have a tendency to incomplete initial occlusion and frequent recanalisation after embolisation with GDCs5-7. This information is based on sporadic case reports and the true incidence of incomplete initial occlusion or late recanalisation is not well known.
We evaluated the initial treatment result and follow-up recanalisation rate of Guglielmi detachable coil (GDC) treatment in partially thrombosed aneurysms and compared these results with those of non-thrombosed aneurysms.
Patients and Methods
Among 149 intracranial aneurysms in 141 patients treated with GDCs, 25 aneurysms with partial intraluminal thrombosis were selected for evaluation. The remaining 124 non-thrombosed aneurysms in 116 patients were also analysed for comparison. Presence of a thrombus was confirmed by CT and/or MR images. MR criteria for presence of a thrombus included (1) concentric or eccentric signal intensity around flow void signal, or (2) heterogeneous signal intensity in the lumen, and CT evidence of an intraluminal thrombus was an area of non-enhancement 8-11. When equivocal, angiography was used to determine whether a thrombus was present or not: discrepant small size of the aneurysm on angiography compared with that on CT or MR was considered as an evidence of partial thrombosis.
Presenting symptoms of thrombosed aneurysm patients were variable. Subarachnoid haemorrhage was noted in four patients. Other presenting symptoms included cranial nerve palsy in ten, headache/dizziness in nine, aphasia/slurred speech in three, confusion in four, facial/orbital pain in two and ataxia in two. Three aneurysms were found incidentally, and in one patient the aneurysm developed as a complication of transsphenoidal hypophysectomy.
Characteristics of the thrombosed aneurysms
With respect to location, thrombosed aneurysms were found in the cavernous carotid artery (10), ophthalmic artery origin (4), posterior communicating artery origin (2), middle cerebral artery (1), anterior communicating artery (1), basilar tip (4), midbasilar (2), and posterior inferior cerebellar artery (l).The actual size of aneurysm on cross sectional images ranged from 13 to 70 mm (mean 24.6 mm), but patent aneurysm lumen size ranged from 5 to 30 mm (mean 16.8 mm) on angiogram.
We classified the aneurysms into three groups by their size. Criteria for small aneurysms were less than 10 mm in the largest diameter, large between 10 to 25 mm, and giant, 25 mm and greater. Angiography was utilized to evaluate initial occlusion rate and follow-up recanalisation rate.
The extent of aneurysmal thrombosis ranged from 10 to 90 per cent (mean 46.4 per cent) of the whole sac based on cross sectional imaging.
Evaluation of initial treatment results
Results of initial GDC treatment were divided into three groups based on the occlusion rate of the lumen: total, 100%; subtotal, 9599%; and incomplete, under 95%. We compared the occlusion rates between thrombosed and non-thrombosed groups. We also evaluated occlusion rates according to aneurysm size in thrombosed aneurysms. Statistical analyses were performed using chi square test or FisherºØs exact test. Complications related to procedure and symptom improvement after treatment was reviewed.
Evaluation of follow-up results
Follow-up angiograms were available in 18 patients and extended from 3 months to 4 years. We estimated aneurysmal lumen recanalisation rate and evaluated the presence of compaction or migration of coil mass on follow-up angiogram. We compared the recanalisation rate between thrombosed and non-thrombosed groups. The recanalisation rate was also compared in different aneurysm size groups. Statistical analyses were performed using chi square test or Fisher's exact test.
Results
Thrombosed aneurysms accounted for 25 cases (16.8%) of the 149 aneurysms treated with GDCs. On initial treatment with GDCs, total to subtotal occlusion (95% plus) was achieved in 18 cases (72%) out of 25 thrombosed aneurysms (table 1). In the non-thrombosed group 104 cases (83.9%) were occluded totally or subtotally. The difference in initial occlusion rate was not statistically significant between the two groups. In the thrombosed group the difference in occlusion rate between small and large/giant aneurysm groups was not significant, either. However, in the non-thrombosed group there was a significant difference between different size groups: that is, small aneurysms showed a higher rate of total to subtotal occlusion than in the large/giant aneurysm group.
Table 1.
Comparison of initial occlusion rate between thrombosed and non-thrombosed aneurysm groups
| Size (mm) | Thrombosed 1) | Non-thrombosed | ||||||
|---|---|---|---|---|---|---|---|---|
|
Initial occlusion (%) |
2) <10 | 10-25 | 25≤ | Total | 3) <10 | 10-25 | 25≤ | Total |
| ≥95 | 3 | 13 | 2 | 18 | 57 | 44 | 3 | 104 |
| ≤95 | 0 | 5 | 2 | 7 | 5 | 12 | 3 | 20 |
| 3 | 18 | 4 | 25 | 62 | 56 | 6 | 124 | |
|
NOTE: 1) Non-significant by Fisher’s exact test, comparison between thrombosed and non-thrombosed groups; 2) Non-significant by Fisher's exact test, comparison between small and large/giant groups; 3) p<0.05 by Chi-square test, comparison between small and large/giant groups. | ||||||||
Three patients underwent carotid artery trapping or surgical clipping of aneurysm during or shortly after the endovascular* procedure either due to associated vascular pathology or to remove mass effect.
After endovascular treatment with GDCs, symptoms improved in 14 cases and in four cases there was no change in symptoms. The remaining seven cases, which were excluded from symptom review, were as follows: three post GDC parent vessel trapping or clipping, three incidentally found aneurysms, and one in which the medical record was not available. Thirteen out of these 18 patients had pre-procedural symptoms which were attributed to mass effect of the aneurysms. Among them, ten showed symptom improvement after the procedure and three patients showed no improvement. Complications related to initial GDC treatment were punture site haematoma or thrombosis in two.
Follow-up angiograms were available in 18 cases out of 25 in the thrombosed group and 88 cases out of 124 in the non-thrombosed group. Recanalisation of the aneurysm lumen was noted in 14 cases (77.8%) of 18 thrombosed aneurysms (table 2). Four cases showed no luminal enlargement or reopening on follow-up (figure 1). In the non-thrombosed group 14 cases (16%) out of 88 showed recanalisation. The difference in recanalisation rate between the two groups was statistically significant. The degree of luminal recanalisation varied from 10 to 60 per cent in the thrombosed group. Luminal recanalisation was due to either migration of the coil mass (10 cases) (figure 2) into the surrounding thrombus or compaction of the coil mass (4 cases) (figure 3), but it usually occurred in combination at least to some degree (figure 4).
Table 2.
Comparison of recanalisation rate between thrombosed and non-thrombosed groups
| Size (mm) | Thrombosed 1) | Non-thrombosed | ||||||
|---|---|---|---|---|---|---|---|---|
| Reopen | 2) <10 | 10-25 | 25≤ | Total | 3) <10 | 10-25 | 25≤ | Total |
| Yes | 1 | 10 | 3 | 14 | 2 | 9 | 3 | 14 |
| No | 1 | 3 | 0 | 4 | 45 | 27 | 2 | 74 |
| No FU | 1 | 5 | 1 | 7 | 15 | 20 | 1 | 36 |
| Total | 3 | 18 | 4 | 25 | 62 | 56 | 6 | 124 |
|
NOTE: 1) p<0.01 by Fisher's exact test, comparison between thrombosed and non-thrombosed groups; 2) Non-significant by Fish- er's exact test, comparison between small and large/giant groups; 3) p<0.01 by Chi-square test, comparison between small and large/giant groups; FU: follow-up. | ||||||||
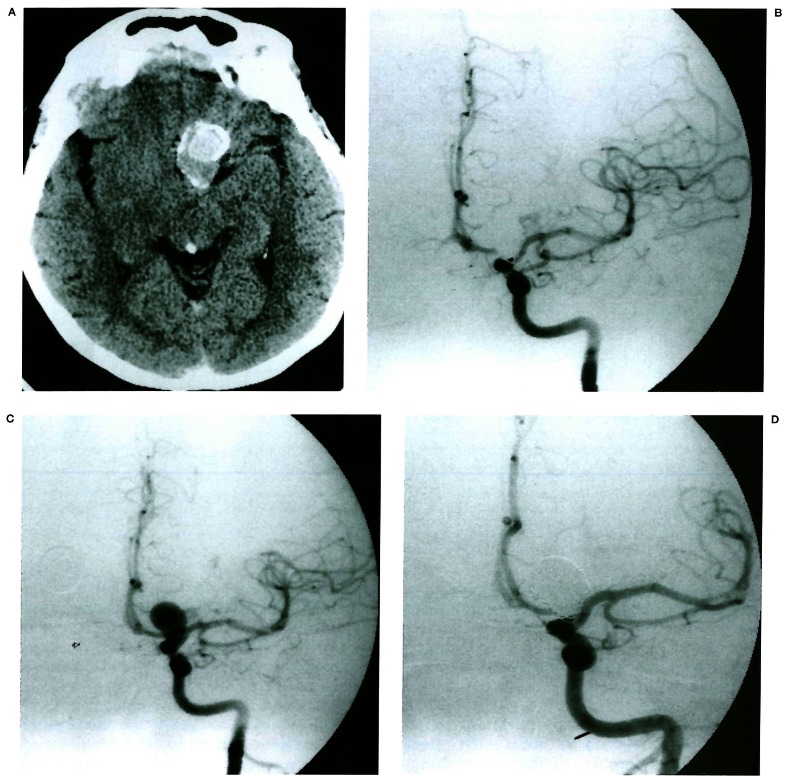
Figure 1.
Non-recanalisation. Pre-contrast CT (A) reveals high density mass with rim calcification in the left suprasellar area. The denser area (arrow) represents the lumen and the surrounding low density area represents a thrombus. Left carotid angiogram (B) shows an aneurysm arising from the ophthalmic artery origin. Post-procedure angiogram (C) shows more than 95% occlusion of lumen with GDCs. On seven month follow-up angiogram (D), there is no change in coil position or shape.
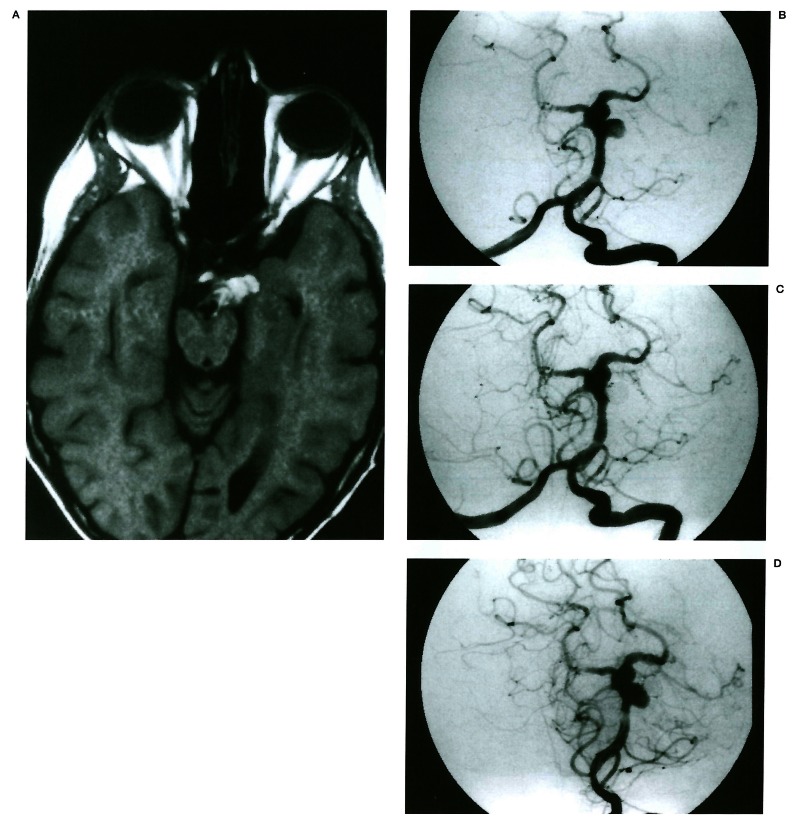
Figure 2.
Migration of coil mesh. Tl-weighted MR (A) shows an elongated shape aneurysm arising from the basilar artery with internal multilayer high signal (arrow). Left vertebral angiogram (B) demonstrates a 7 mm aneurysm arising from the superior cerebellar artery origin. The superior cerebellar artery is not visible. Immediate post-procedure angiogram (C) shows complete occlusion of the aneurysm. On 7 month follow-up angiogram (D), coil mesh migrated laterally without significant change in coil mesh shape and recanalised lumen.
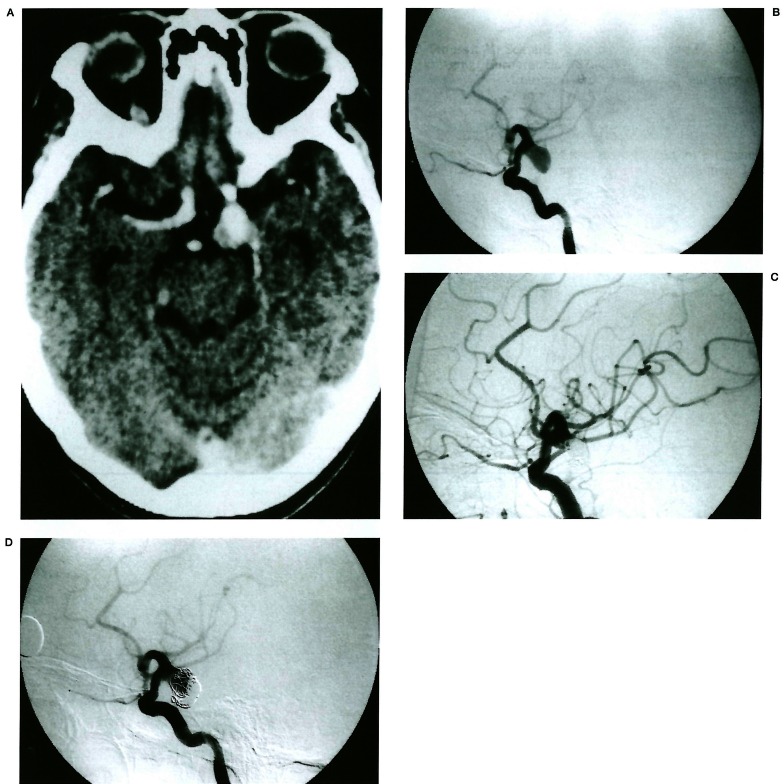
Figure 3.
Compaction of coil mesh. Contrast enhanced CT (A) shows an enhancing aneurysm in the left posterior communicating artery origin area. Note focal non-enhancing portion representing a thrombus. Left internal carotid angiogram (B) shows an aneurysm at the same location. The aneurysm was occluded with GDCs and achieved 95% occlusion (C). Eight month follow-up angiogram (D) shows coil mesh compaction without migration.
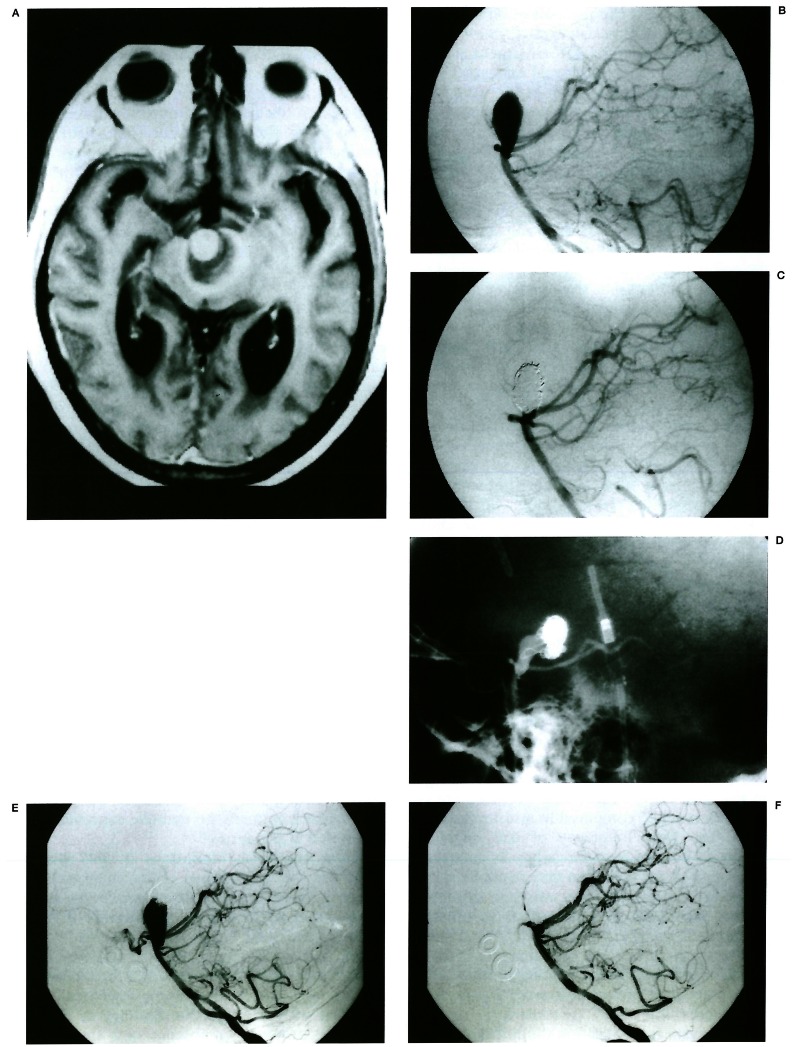
Figure 4.
Predominant migration of coil mesh. T1-weighted MR (A) with contrast enhancement shows a partially thrombosed aneurysm in the interpeduncular fossa. The thrombus shows low signal with marginal high signal and residual lumen shows enhancement. Lateral angiogram of the vertebral artery (B) reveals an aneurysm arising from the basilar tip. Immediate post-procedure angiogram (C) shows 95% occlusion of the aneurysm. Fifteen months follow-up angiogram (D) shows migration of coil mesh toward the fundus and lumen recanalisation. In addition the coil mesh shows compaction. This patient was treated again. On 4-year follow-up angiogram (E), coils migrated to the dome and the lumen recanalised. Final angiogram (F) after the repacking procedure shows more than 95% occlusion of the lumen.
Symptoms recurred in two cases in association with aneurysm recanalisation, but in no instance was haemorrhage noted. Attempt for retreatment was made in ten cases with success in six. However, in one case of basilar tip aneurysm, recanalisation occurred necessitating re treatment with GDCs on three subsequent occasions. In this patient actual growth of the aneurysm was noted (figure 4). Similar post-treatment aneurysm growth was noted in four other cases.
Discussion
Some degree of spontaneous thrombosis occurs in 9% to 13% of intracranial aneurysms. Thrombosed aneurysms tend to be large12 and conversely, larger aneurysms tend to throm-bose more frequently. The size distribution of the aneurysms in general is as follows: small 6078%; large 20-30%; giant 2-13%4. Compared to this, actual size (i.e., on CT or MR) of all thrombosed aneurysms in our series was large or giant and giant aneurysms accounted for 36%. Our result corresponds well with other reports that 40% of thrombosed aneurysms are giant4. The distribution of thrombosed aneurysms was very different from that of general aneurysms. Generally, about 70% of intracranial aneurysms are found in or above the circle of Willis, in the anterior circulation, that is, anterior communicating artery, posterior communicating artery and middle cerebral artery bifurcation4. On the contrary, most of the thrombosed aneurysms in our series were found in the internal carotid artery below the posterior communicating artery and in the vertebrobasilar system. Cavernous carotid artery was thesingle most common location accounting for ten cases (40%), followed by the posterior circulation (seven cases, 28%) including basilar tip.
Endovascular treatment of partially thrombosed aneurysms entails various difficulties compared with the non-thrombosed group. Recanalisation is frequently reported with GDC treatment. Additionally, initial occlusion is more difficult and packing of the aneurysm carries a high risk of embolism 13. Some authors claim it is difficult to eliminate the mass effect in case of large or giant aneurysms. The overall reported incidence of aneurysm luminal recanalisation after GDC treatment varies widely, ranging from 5% in small neck aneurysms and up to 65% in wide neck aneurysms5,14-18. It is well known that the size of the aneurysm neck is the most important factor for the degree of aneurysm occlusion and follow-up recanalisation 16,19-21. The size of aneurysm, initial occlusion rate and presence of luminal thrombus can also affect the recanalisation rate after GDC treatment. Although recanalisation of the aneurysm lumen after GDC treatment is commonly reported in thrombosed aneurysms, the incidence is not well known. In our study, the rate of recanalisation was 78%, five times higher than in the non-thrombosed group.
The mechanism of luminal recanalisation after occlusion of the aneurysm with GDCs is still unclear. It is generally accepted that coil compaction causes regrowth of the aneurysm22-25. Compaction is believed to occur by arterial pressure and the constant pulsatile flow against the coil mass17,19,26. In our 14 cases of luminal recanalisation after GDC treatment, we observed two patterns of changes in coil mass, which caused recanalisation: one was shrinkage (compaction) of the coil mass (four cases) and the other was migration of the coil mass (ten cases) into the thrombus. In reviewing the literature little distinction is made between coil compaction and migration and the term compaction seems to be generally by many authors used to express both shrinkage and displacement of coils. In our experience they represent separate entities in partially thrombosed aneurysms. We define migration as the entire coil mass moving inside the aneurysm, whereas, compaction represents a shrinking of the coil mass. Typically one predominates, but at least to some degree these phenomena occur in combination.
Compaction of the coil mass may be in part caused by underpacking of the coils22. Szikora 27, in his experimental model, showed that only 23-26% endovascular volume is occupied by coil even though packing seemed dense. Compaction occurs more easily when a large or giant aneurysm is partially occluded 11,14,15. Because thrombosed aneurysms are usually larger in size, it is easily conceivable that they are often underpacked compared to non-thrombosed ones. The high frequency of coil migration seems to be due to a thrombus in aneurysm. Migration of the coil mass may result from embedding of the coil mass into soft thrombus, partial resolution of a thrombus or enlargement of the aneurysm itself7,22,26,28-30. We consider coil migration one of the characteristics of thrombosed aneurysms. In addition, a wide neck may have influenced more frequent recanalisation after GDC treatment.
Aneurysm recanalisation did not directly result in clinical recurrence. Although recanalisation of the lumen was noted in 14 patients, only two patients had symptomatic recurrence related to recanalisation. We attempted retreatment in ten patients with success in six. In the four failed cases, the neck was too wide (three cases) or access was not possible (one case). Including four cases without recanalisation, we achieved more than 95% of luminal occlusion in ten cases (40%).
Another possible problem related to endovascular treatment of thrombosed aneurysm is difficulty in eliminating mass effect because thrombo*sed aneurysms tend to be large or giant in size 5,31. A large percentage of clinical symptoms are related to the mass effect of large or giant aneurysms. In one case with a 7-cm giant ophthalmic artery origin aneurysm, surgery was performed after successful embolisation with GDCs because of mass effect. Initial GDC treatment relieved symptoms related to mass effect in ten of 13 patients. The reason for improvement of symptoms is probably that by relieving pulsation, mass effect also was relieved. Others reported an actual decrease in aneurysm volume in some cases on follow-up32. We believe successful treatment with GDC can reduce the mass-related symptoms unless the aneurysm is exceptionally large.
Our initial occlusion rate was slightly lower than in the non-thrombosed group. However there was no statistically significant difference in occlusion rate between the thrombosed and non-thrombosed groups. The main reason for lower initial occlusion rate can be explained as broad neck and large size of the thrombosed aneurysm22.
Risk of thromboembolism is high with surgical treatment of thrombosed aneurysms33. Although there is also a potential risk of embolism from aneurysmal thrombus during endovascular treatment, no embolic complication was noted in our series31.
There are some limitations in this study. Since the MR appearance of the aneurysms is variable, there is a chance of mistaking non-thrombosed aneurysms as thrombosed if they have atypical MR signal characteristics 8,10. To minimize this possibility we used more or less strict criteria for the presence of a thrombus on CT or MR and if they were equivocal, we matched with angiographic findings to determine the presence or absence of a thrombus. On the other hand, because we used strict criteria, there is a chance of missing patients with thrombosis if CT or MR was not available or they had a small size thrombus with equivocal CT/MR and angiographic appearance.
Another possible source of error is the small sample size of the thrombosed aneurysm group. To reduce possible error, we simplified the size group into small and large/giant in comparing initial occlusion rate and recanalisation rate within the group and with the non-thrombosed group. In our series of thrombosed aneurysms, although there was a tendency for lower initial occlusion rate and higher recanalisation rate, statistically, there was no significant difference between different size groups. This seems to be mainly due to the small number of patients. We were also unable to analyse different behaviors of aneurysms based on their locations, again because of small sample size.
Conclusions
In thrombosed aneurysms initial occlusion rates with GDCs are slightly lower than those in the non-thrombosed group, but the incidence of recanalisation in the thrombosed group is up to fivefold greater than in the non-thrombosed group.
The pattern of recanalisation was mainly migration of coil mass into the thrombus. Close follow-up is required in patients treated with GDC and if necessary, retreatment should be performed.
References
- 1.Atkinson JLD, Lane JI, et al. Spontaneous thrombosis of posterior cerebral artery aneurysm with angiographic reappearance. J Neurosurg. 1993;79:434–437. doi: 10.3171/jns.1993.79.3.0434. [DOI] [PubMed] [Google Scholar]
- 2.Housepain EM, Pool JL. A systemic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital. 1914-1956. J Neuropathol Exp Neurol. 1958;17:409–423. doi: 10.1097/00005072-195807000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Spallone A, Cantore G. The role of extracranial carotid abnormalities in the genesis of cerebral aneurysms. J Neurosurg. 1981;55:693–700. doi: 10.3171/jns.1981.55.5.0693. [DOI] [PubMed] [Google Scholar]
- 4.Weir B, Macdonald RL. Intracranial aneurysms and subarachnoid haemorrhage: an overview. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. Vol. 2. New York, NY: McGraw-Hill; 1996. pp. 2191–2213. [Google Scholar]
- 5.Malisch TW, Guglielmi G, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg. 1997;87:176–183. doi: 10.3171/jns.1997.87.2.0176. [DOI] [PubMed] [Google Scholar]
- 6.Taki W, Nishi S, et al. Selection and combination of various endovascular techniques in the treatment of giant aneurysms. J Neurosurg. 1992;77:37–42. doi: 10.3171/jns.1992.77.1.0037. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux AJ, Ellison DW, et al. Histologic findings in giant aneurysms treated with Guglielmi detachable coils. J Neurosurg. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- 8.Atlas SW, Grossman RI, et al. Partially thrombosed giant intracranial aneurysms: correlation of MR and pathologic findings. Radiology. 1987;162:111–114. doi: 10.1148/radiology.162.1.3786749. [DOI] [PubMed] [Google Scholar]
- 9.Atlas SW. Magnetic resonance imaging of intracranial aneurysms. Neuroimaging Clin North Am. 1997;7:709–720. [PubMed] [Google Scholar]
- 10.Rolen PB, Sze G. Small, patent cerebral aneurysms: atypical appearance at 1.5-T MR imaging. Radiology. 1998;208:129–136. doi: 10.1148/radiology.208.1.9646803. [DOI] [PubMed] [Google Scholar]
- 11.Strother CM, Eldevik P, et al. Thrombus formation and structure and the evolution of mass effect in intracranial aneurysms treated by balloon embolisation: emphasis on MR findings. Am J Neuroradiol. 1989;10:787–796. [PMC free article] [PubMed] [Google Scholar]
- 12.Sekhar LN, Heros RC. Origin, growth, and rupture of saccular aneurysms: a review. Neurosurgery. 1981;8:248–260. doi: 10.1227/00006123-198102000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Brugieres P, Blustajn J, et al. Magnetic resonance angiography of giant intracranial aneurysms. Neuroradiology. 1998;40:96–102. doi: 10.1007/s002340050547. [DOI] [PubMed] [Google Scholar]
- 14.Moret J, Pierot L, et al. Endovascular treatment of anterior communicating artery aneurysms using Guglielmi detachable coils. Neuroradiology. 1996;38:800–805. doi: 10.1007/s002340050352. [DOI] [PubMed] [Google Scholar]
- 15.Martin D, Rodesch G, et al. Preliminary results of embolisation of nonsurgical intracranial aneurysms with GD coils: the 1st year of their use. Neuroradiology. 1996;38:S142–150. doi: 10.1007/BF02278143. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmi G, Viñuela F. Intracranial aneurysms: Guglielmi electrothrombotic coils. Neurosurg Clin North Am. 1994;5:427–435. [PubMed] [Google Scholar]
- 17.Choi IS. Endovascular treatment of aneurysms. In: Ojemann RG, Ogilvy CS, et al., editors. Surgical management of neurovascular disease. 3rd ed. Baltimore, MD: Williams & Wilkins; 1995. pp. 138–151. [Google Scholar]
- 18.McDougall CG, Halbach VV, et al. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg. 1996;84:393–399. doi: 10.3171/jns.1996.84.3.0393. [DOI] [PubMed] [Google Scholar]
- 19.Zubillaga AF, Guglielmi G, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. Am J Neuroradiol. 1994;15:815–820. [PMC free article] [PubMed] [Google Scholar]
- 20.Cognard C, Weill A, et al. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology. 1998;206:499–510. doi: 10.1148/radiology.206.2.9457205. [DOI] [PubMed] [Google Scholar]
- 21.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolisation of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 22.Hope JKA, Byrne JV, Molyneux A J. Factors influencing successful angiographic occlusion of aneurysms treated by coil. Am J Neuroradiol. 1999;20:391–399. [PMC free article] [PubMed] [Google Scholar]
- 23.Graves VB, Strother CM, Rappe AH. Treatment of experimental canine carotid aneurysms with platinum coils. Am J Neuroradiol. 1993;14:787–793. [PMC free article] [PubMed] [Google Scholar]
- 24.Mawad ME, Mawad JK, et al. Long-term histopathological changes in canine aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Derdeyn CP, Grave VB, et al. MA angiography of saccular aneurysms after treatment wit Guglielmi detachable coils: preliminary experience. Am J Neuroradiol. 1997;18:279–286. [PMC free article] [PubMed] [Google Scholar]
- 26.Mericle RA, Wakhloo AK, et al. Delayed aneurysmal regrowth and recanalization after Guglielmi detachable coil treatment. J Neurosurg. 1998;89:142–145. doi: 10.3171/jns.1998.89.1.0142. [DOI] [PubMed] [Google Scholar]
- 27.Szikora I, Wakhloo K, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. Am J Neuroradiol. 1997;18:667–672. [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki J, Ohara H. Clinicopathological study of cerebral aneurysms: origin, rupture, repair, and growth. J Neurosurg. 1978;48:505–514. doi: 10.3171/jns.1978.48.4.0505. [DOI] [PubMed] [Google Scholar]
- 29.Strother CM, Graves VB, Rappe A. Aneurysm haemodynamics: an experimental study. Am J Neuroradiol. 1992;13:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- 30.Katayama K, Tsubokawa T, et al. Growth of totally thrombosed giant aneurysm within the posterior cranial fossa. Neuroradiology. 1991;33:168–170. doi: 10.1007/BF00588260. [DOI] [PubMed] [Google Scholar]
- 31.Bavinzski G, Killer M, et al. Endovascular therapy of idiopathic cavernous aneurysms over 11 years. Am J Neuroradiol. 1998;19:559–565. [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuura M, Terada T, et al. Magnetic resonance signal intensity and volume changes after endovascular treatment of intracranial aneurysms causing mass effect. Neuroradiology. 1998;40:184–188. doi: 10.1007/s002340050565. [DOI] [PubMed] [Google Scholar]
- 33.Zubcov YN, Smith RR. Surgical management of complex and giant anterior circulation aneurysms. In: Schmidek HH, Sweet WH, editors. Operative neurosurgical techniques. 3rd ed. Vol. 2. Philadelphia, PA: WB Saunders Co.; 1995. pp. 1041–1053. [Google Scholar]