Abstract
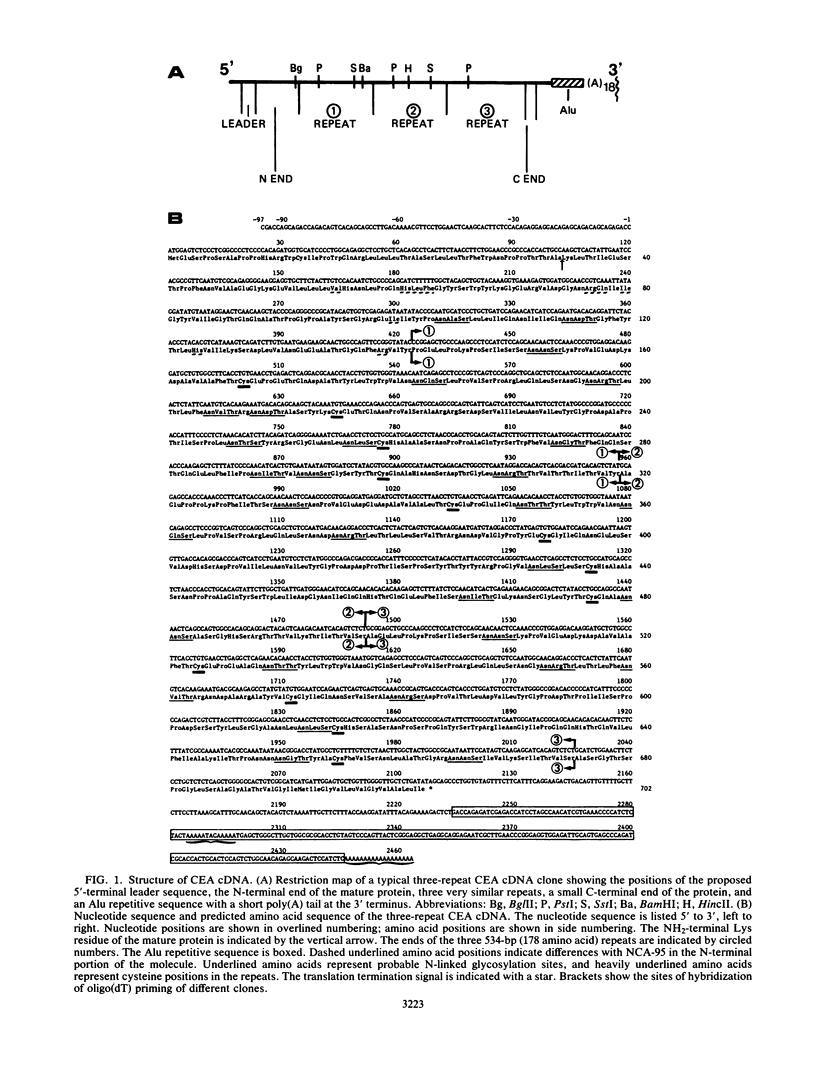
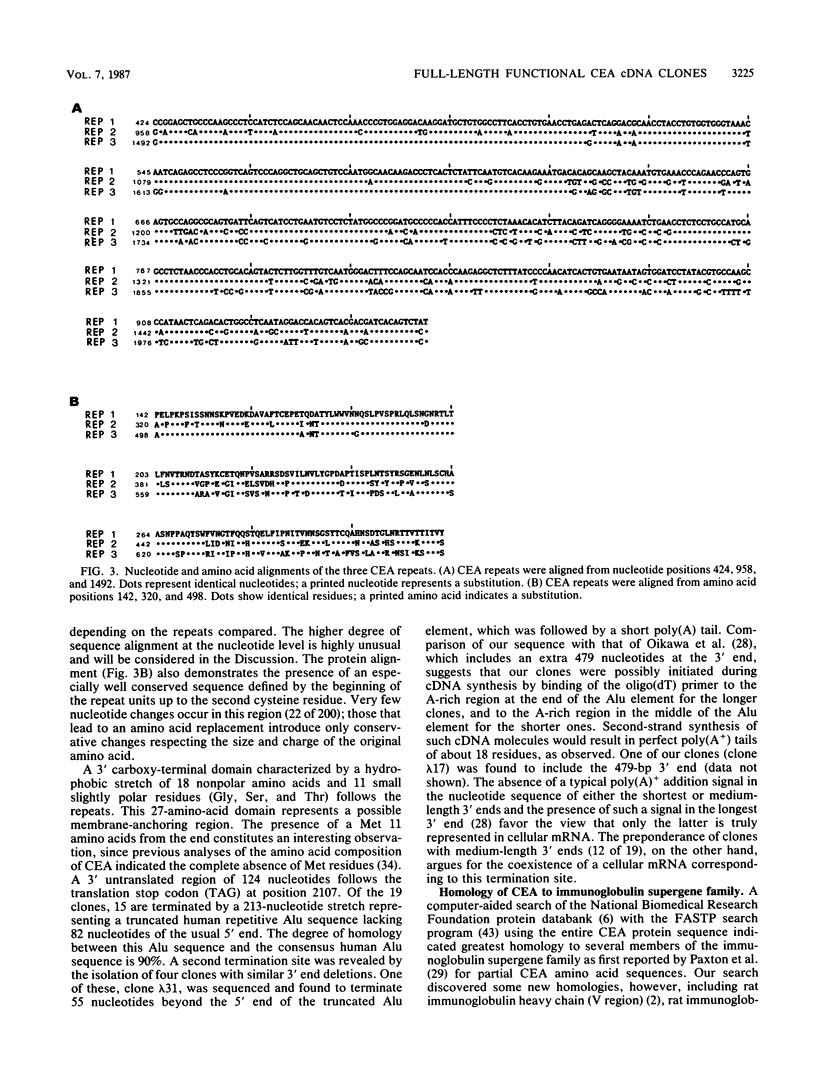
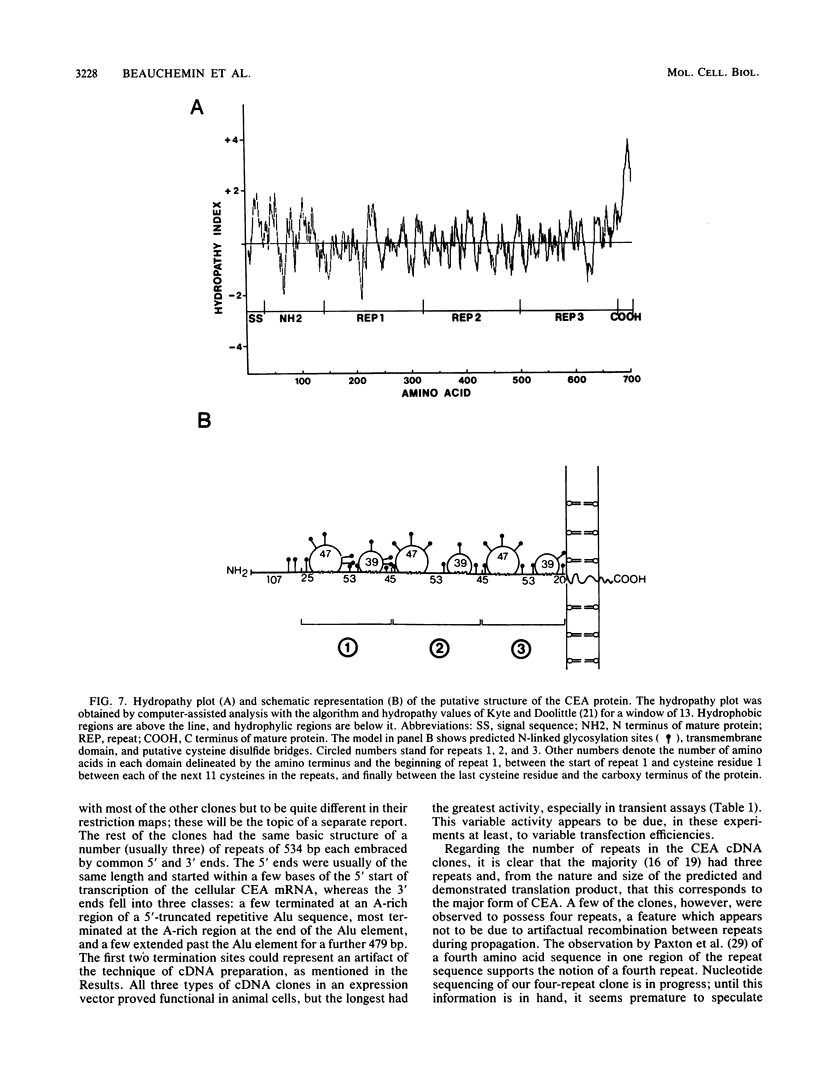
Carcinoembryonic antigen (CEA) expression is perhaps the most prevalent of phenotypic changes observed in human cancer cells. The molecular genetic basis of this phenomenon, however, is completely unknown. Twenty-seven CEA cDNA clones were isolated from a human colon adenocarcinoma cell line. Most of these clones are full length and consist of a number (usually three) of surprisingly similar long (534 base pairs) repeats between a 5' end of 520 base pairs and a 3' end with three different termination points. The predicted translation product of these clones consists of a processed signal sequence of 34 amino acids, an amino-terminal sequence of 107 amino acids, which includes the known terminal amino acid sequence of CEA, three repeated domains of 178 amino acids each, and a membrane-anchoring domain of 27 amino acids, giving a total of 702 amino acids and a molecular weight of 72,813 for the mature protein. The repeated domains have conserved features, including the first 67 amino acids at their N termini and the presence of four cysteine residues. Comparisons with the amino acid sequences of other proteins reveals homology of the repeats with various members of the immunoglobulin supergene family, particularly the human T-cell receptor gamma chain. CEA cDNA clones in the SP-65 vector were shown to produce transcripts in vitro which could be translated in vitro to yield a protein of molecular weight 73,000 which in turn could be precipitated with CEA-specific antibodies. CEA cDNA clones were also inserted into an animal cell expression vector and introduced by transfection into mammalian cell lines. These transfectants produced a CEA-immunoprecipitable glycoprotein which could be visualized by immunofluorescence on the cell surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Cartier M., Chang M. W., Stanners C. P. Use of the Escherichia coli gene for asparagine synthetase as a selective marker in a shuttle vector capable of dominant transfection and amplification in animal cells. Mol Cell Biol. 1987 May;7(5):1623–1628. doi: 10.1128/mcb.7.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Murre C., Quertermous T., Boss J. M., Leiden J. M., Seidman J. G., Strominger J. L. Cloning and sequence analysis of complementary DNA encoding an aberrantly rearranged human T-cell gamma chain. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2619–2623. doi: 10.1073/pnas.83.8.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Engvall E., Shively J. E., Wrann M. Isolation and characterization of the normal crossreacting antigen: homology of its NH2-terminal amino acid sequence with that of carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1670–1674. doi: 10.1073/pnas.75.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gold P., Krupey J., Ansari H. Position of the carcinoembryonic antigen of the human digestive system in ultrastructure of tumor cell surface. J Natl Cancer Inst. 1970 Aug;45(2):219–225. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haggarty A., Legler C., Krantz M. J., Fuks A. Epitopes of carcinoembryonic antigen defined by monoclonal antibodies prepared from mice immunized with purified carcinoembryonic antigen or HCT-8R cells. Cancer Res. 1986 Jan;46(1):300–309. [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Construction of a modular dihydrofolate reductase cDNA gene: analysis of signals utilized for efficient expression. Mol Cell Biol. 1982 Nov;2(11):1304–1319. doi: 10.1128/mcb.2.11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi T., Chang H. C., Denome R., Silver J. Thy-1 cDNA sequence suggests a novel regulatory mechanism. Nature. 1983 Jan 6;301(5895):80–82. doi: 10.1038/301080a0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Oikawa S., Nakazato H., Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence. Biochem Biophys Res Commun. 1987 Jan 30;142(2):511–518. doi: 10.1016/0006-291x(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Paxton R. J., Mooser G., Pande H., Lee T. D., Shively J. E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Stanners C. P. Characterization of cell lines showing growth control isolated from both the wild type and a leucyl-tRNA synthetase mutant of Chinese hamster ovary cells. J Cell Physiol. 1979 Mar;98(3):571–585. doi: 10.1002/jcp.1040980315. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Beatty J. D. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2(4):355–399. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Kessler M. J., Todd C. W. Amino-terminal sequences of the major tryptic peptides obtained from carcinoembryonic antigen by digestion with trypsin in the presence of Triton X-100. Cancer Res. 1978 Aug;38(8):2199–2208. [PubMed] [Google Scholar]
- Shore G. C., Power F., Bendayan M., Carignan P. Biogenesis of a 35-kilodalton protein associated with outer mitochondrial membrane in rat liver. J Biol Chem. 1981 Aug 25;256(16):8761–8766. [PubMed] [Google Scholar]
- Shuster J., Thomson D. M., Fuks A., Gold P. Immunologic approaches to diagnosis of malignancy. Prog Exp Tumor Res. 1980;25:89–139. doi: 10.1159/000403178. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Starace V., Querinjean P. The primary structure of a rat kappa Bence Jones protein: phylogenetic relationships of V- and C-region genes. J Immunol. 1975 Jul;115(1):59–62. [PubMed] [Google Scholar]
- Terry W. D., Henkart P. A., Coligan J. E., Todd C. W. Carcinoembryonic antigen: characterization and clinical applications. Transplant Rev. 1974;20(0):100–129. doi: 10.1111/j.1600-065x.1974.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Pande H., Paxton R. J., Shively L., Padma A., Simmer R. L., Todd C. W., Riggs A. D., Shively J. E. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc Natl Acad Sci U S A. 1987 May;84(9):2965–2969. doi: 10.1073/pnas.84.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Wyman A. R., Botstein D. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene. 1986;49(2):253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Ortlieb B., Friedrich R., von Kleist S. Isolation and characterization of cDNA clones encoding the human carcinoembryonic antigen reveal a highly conserved repeating structure. Proc Natl Acad Sci U S A. 1987 May;84(9):2960–2964. doi: 10.1073/pnas.84.9.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]