Summary
Clinical experience shows that certain diseases involve specific areas of the vascular tree and remarkably spare others. Topographic differences in the vascular environment already suggest a regional specificity of the vascular anatomy. The biological grounds of such regional differences`, although unknown, can account for the specificity of biological responses to stimuli. Such segmental specificities are beyond morphological analysis. They create an invisible discontinuity in an apparently homogenous anatomical, histological and haemodynamic system. We call this property segmental identity and thus vulnerability. Most of this identity is established during development and is preserved throughout life; its expression, however; may vary over time according to various stresses and create various clinical phenotypes.
The memory of the evolutionary steps and their chronology is imprinted on the arterial anatomy and thus potentially readable. One can postulate that since the age of each arterial segment is different, its resistance to time and stimuli is most likely variable. The vulnerability of these segments cannot be permanent both in a qualitative and quantitative way. Some genetic functions only seem to be active during a short period of time: during vasculogenesis for example. Therefore either the trigger is always active and the target vulnerability window of the cells time-limited, or the target is permanently exposed and the trigger agent can either be exogenous and rare, or most of the time inactive or inactivated.
Key words: embryology, cerebral arteries, stroke, anatomy, moyamoya
Introduction
The clinical experience gained over the past 20 years has shown certain diseases to involve specific areas of the vascular tree and remarkably spare others. It is amazing to see that a signal which promotes angiogenesis and collateral circulation, triggers the vasculature in an adjacent vascular territory, beyond the watershed zone and yet remains topographically circumscribed. Cerebral ischaemic status can induce angiogenesis in regions that are not in morphologic contact, yet the response is directed to specific areas. Why do MCA occlusions not always induce lenticulostriate collaterals (figures 1 and 2) as they do in Moya-Moya disease? Collateral patterns are solicited as if they represent predetermined reservoirs. This may represent a qualitative property of the signal rather than a quantitative one. Such signal can hardly be only flow mediated, and most likely uses extra vascular spaces and supporting cell connections. The edges of such angioarchitectural features point to both a special relationship (luminal and abluminal mediated) and inframorphological boundaries within the arterial tree.
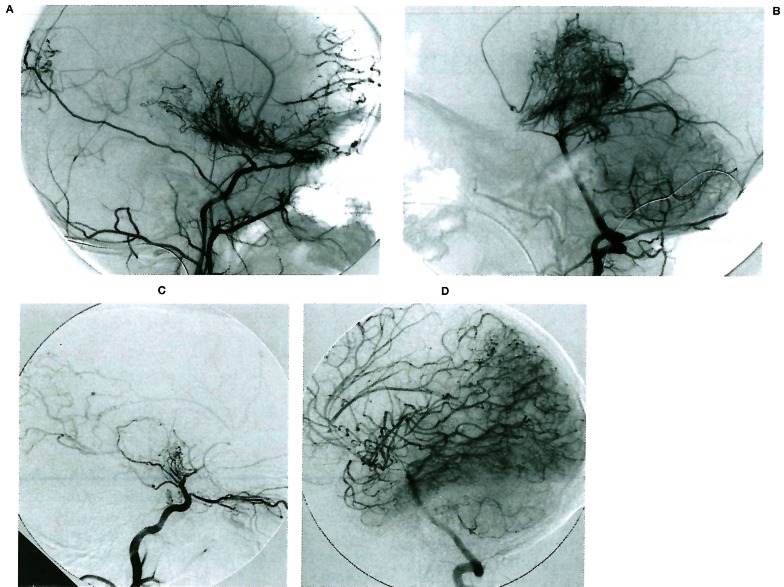
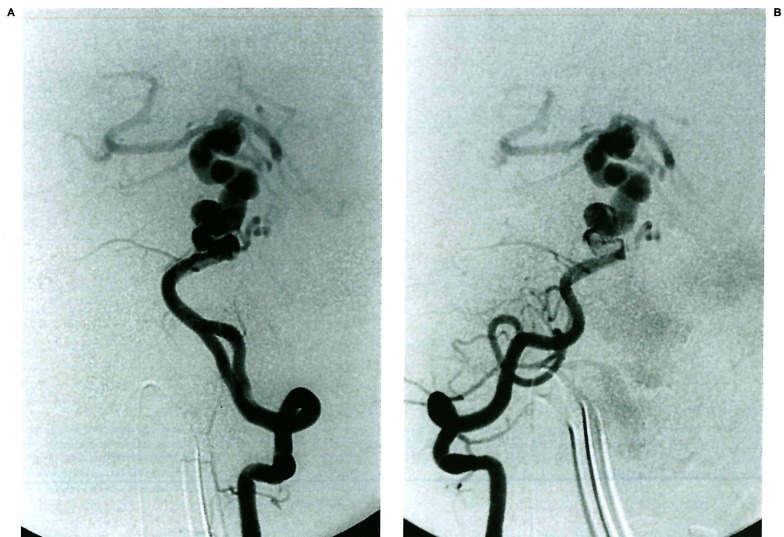
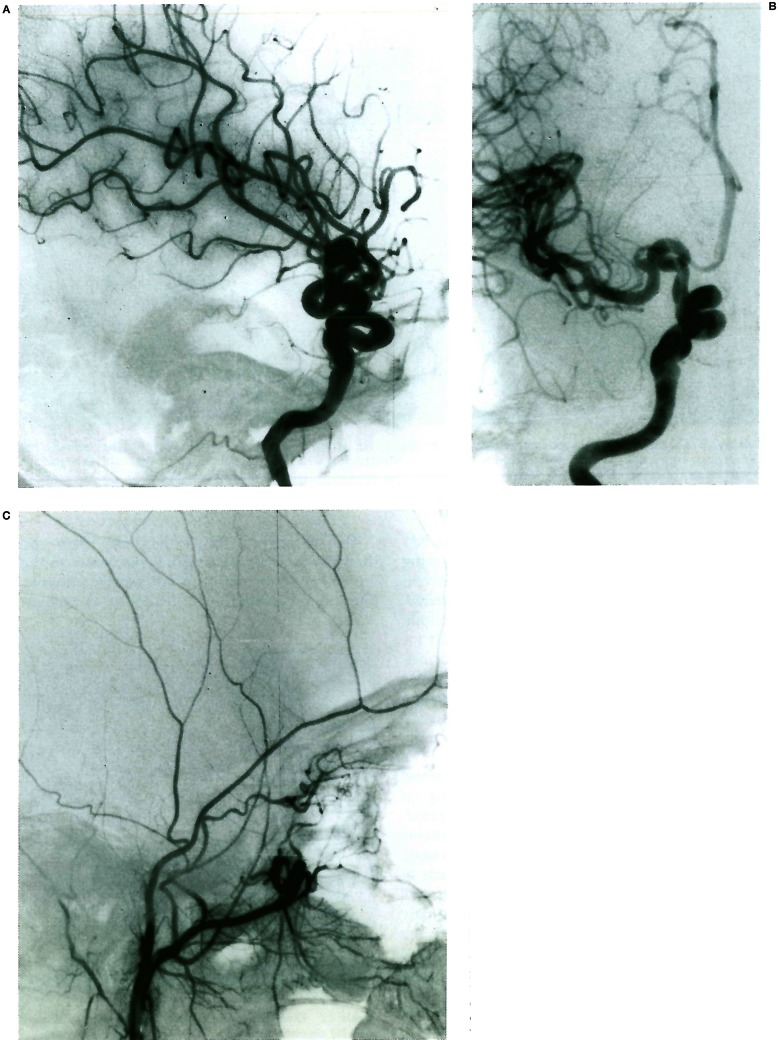
Figure 1.
A, B) Moya-Moya disease in a young child involving the anterior division of the internal carotid artery bilaterally; note the typical aspect of the lenticulostriate network, as well as the transdural angiogenesis remote from the site of the occlusions. C, D) Bilateral occlusion of the anterior division of the internal carotid artery in a young child presenting with repeated deep-seated strokes. Note the absence of lenticulostriate involvement in response to the occlusive stress; there is no transdural supply demonstrated.
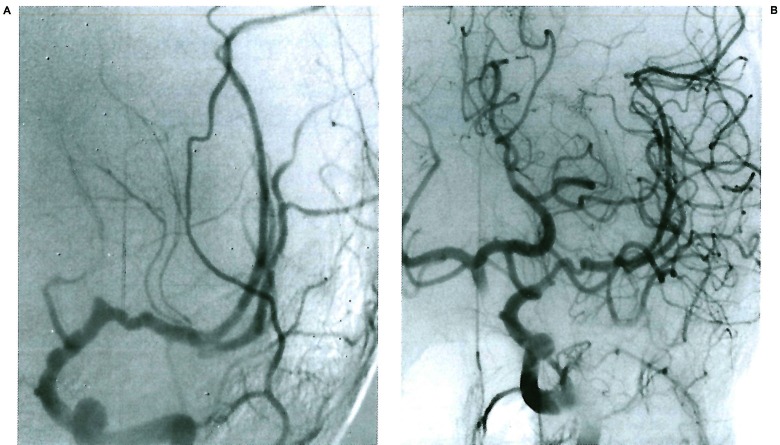
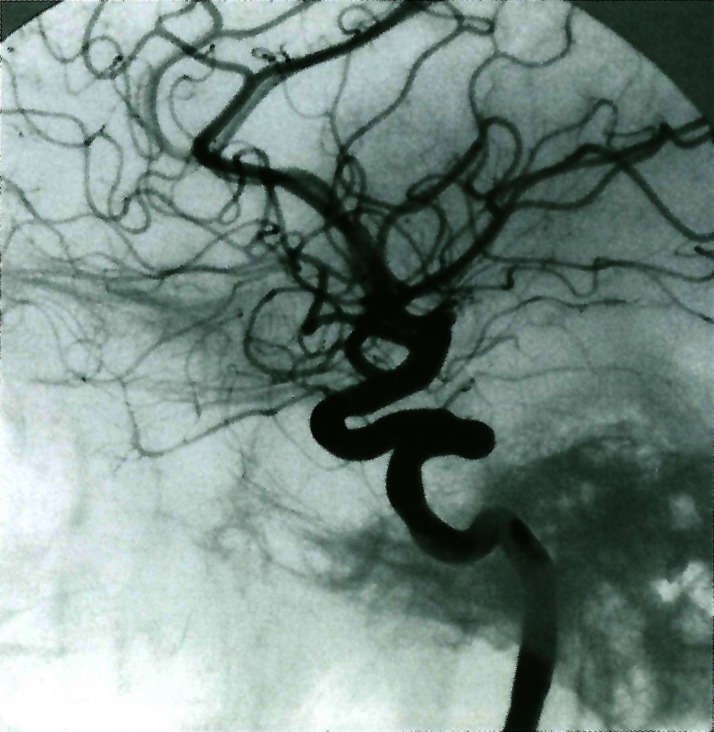
Figure 2.
Young child presenting left sided white matter stroke 9 months after chickenpox infection. The lesion involves the anterior division of the internal carotid artery distal to the posterior communicating artery and is spontaneously repaired over 14 months.
Topographic differences in the vascular environment already suggest a regional specificity of the vascular anatomy. The biological ground of such regional differences can account for the specificity of biological responses to stimuli. Such segmental specificities are beyond morphological analysis. They create an invisible discontinuity in an apparently homogenous anatomical, histological and haemodynamic system. We will call this property segmental identity and thus vulnerability.
Several conditions may account for this property and they can be active during embryogenesis, ontogenesis, or later during life They ground in part the phenotypic maturation of the vessel wall in general, and endothelial cells in particular. They may represent signals from vascularized tissues trough glial (or other) mediated messages.
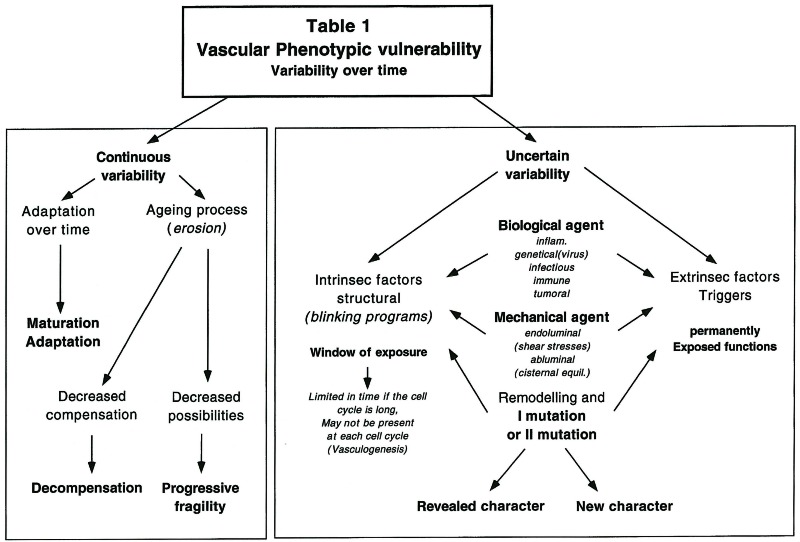
Relationships with transient vascular structures (embryonic arteries, branchial or aortic arches,…), arteries-veins relationships, haemodynamic conditions (diastolic/systolic flow,…), abluminal environment 1,2,6,7 (astrocytes, pia, arachnoid, lymphatics, CSF cisterns and hydrodynamics, interstitial liquids…), vasa vasorum, pericytes, perivascular monocytes and innervation may all contribute simultaneously or consecutively to such segmental identity. Most of this identity is established during development and is preserved throughout life; its expression, however, may vary over time according to various stresses (table 1).
Table 1.
 |
The three stages of vascular development, vasculogenesis 1,2,7, angiogenesis 2,6 and remodelling 3 will each imprint some specificities onto the developing and maturing arteries.
Phytogeny, by expanding over millions of years and multiple species the various orientations taken by the embryo during the first few weeks of life, outlines the different generations or ages that coexist in two consecutive arterial segments. It establishes most of the topographic characteristics as well as their timing during the early stages of development. During evolution, not all vessels contributing to cerebral vascularisation develop simultaneously as they go through various selection and maturation processes. The events that normally occur at spinal cord level are the oldest ones in terms of phylogeny and they can be considered the most stable programs. The large arteries supplying the brain are phylogenetically more recent and they constitute specific segmental entities that can be clearly recognised according to their perivascular relationships or branching boundaries (figure 3).
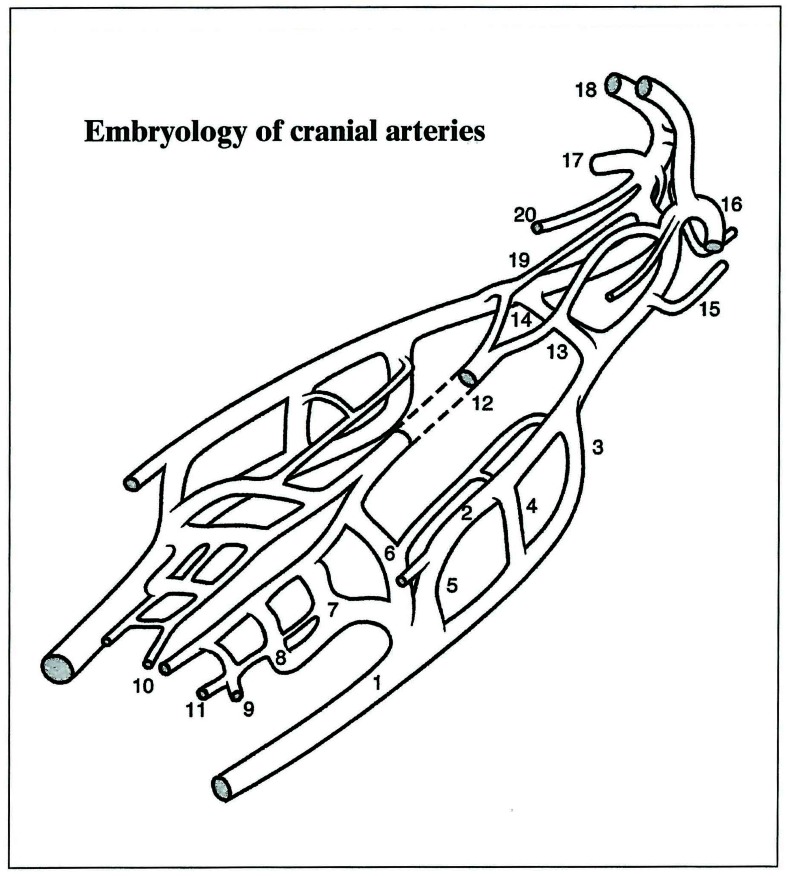
Figure 3.
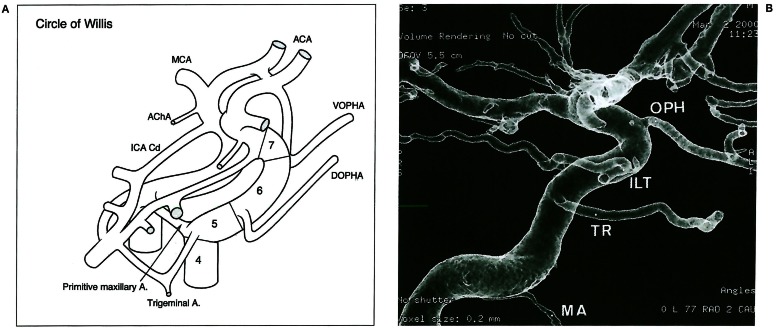
Overall schematic view of the cranial arteries. (1) Ventral aorta VA; (2) Dorsal aorta DA; (3) First aortic arch 1AA; (4) Second aortic arch 2AA; (5) Third aortic arch 3AA; (6) Hypoglossal artery HA; (7) Pro-atlantal artery, type I PA 1; (8) Pro-atlantal artery, type II PA 2; (9) Third cervical segmental artery; (10) Longitudinal neural arteries LNA; (11) Para-ventral (lateral) neural artery; (12) Basilar artery (fused ventral arteries) BA; (13) Trigeminal artery Trig.A; (14) Primitive maxillary artery PMA; (15) Dorsal ophthalmic artery DOPHA; (16) Ventral ophthalmic artery VOPHA; (17) Middle cerebral artery MCA; (18) Anterior cerebral artery ACA; (19) Internal carotid posterior (caudal) division ICA Cd; (20) Anterior choroidal artery. AChA
Following a cephalic metameric (regionalised) organisation 3, the circle of Willis and its branches will also develop according to the evolutionary sequence of events. The Ml segment of the middle cerebral artery precedes by a few hundred million years the appearence of the M2 segment. The former expresses the lenticulostriate and lateral olfactory vascular supply role played by the “Ml” segment in fish. The latter appears much later, in flying birds, when the “hyperstriatum” development takes place. Only thereafter does the M2 segment sprout from this highly functional existing bud to contribute and accompany parietal, temporal and later frontal lobe growth. Thus, there is as much difference between the Ml and M2 segments as between a fin and a wing or an arm. However, considered in a broad fashion they could all be looked at as limbs 5. Additional changes during phylogeny include the anterior choroidal artery territory shift towards the posterior cerebral artery, the various ophthalmic artery anastomoses, the basilar artery reversal of flow, and the branchial artery regressions. Each of these phenomena is both an anatomic event and a landmark and time marker.
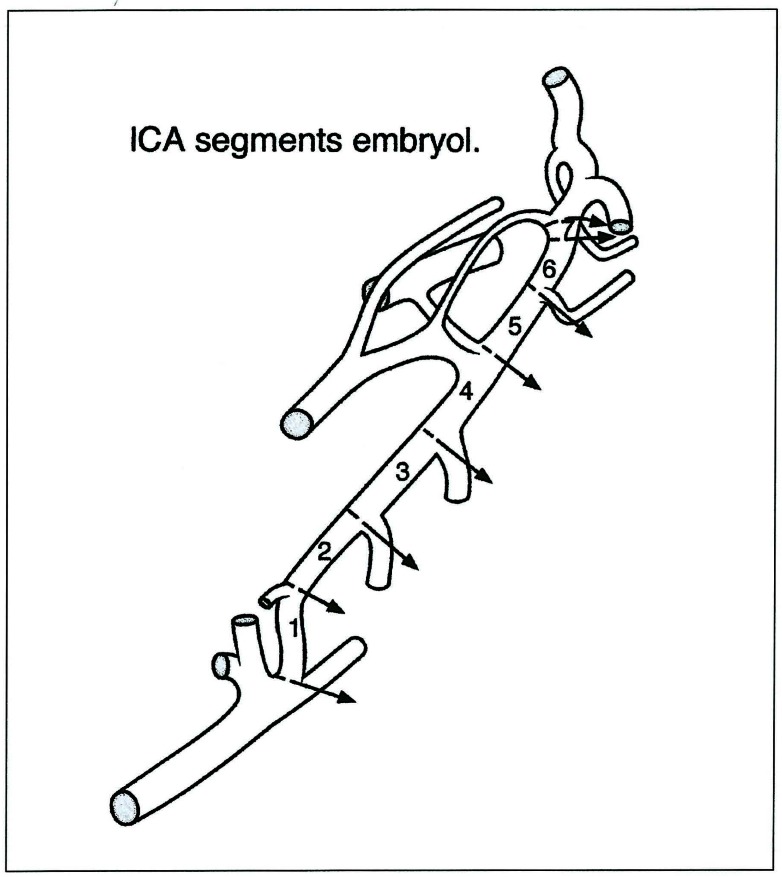
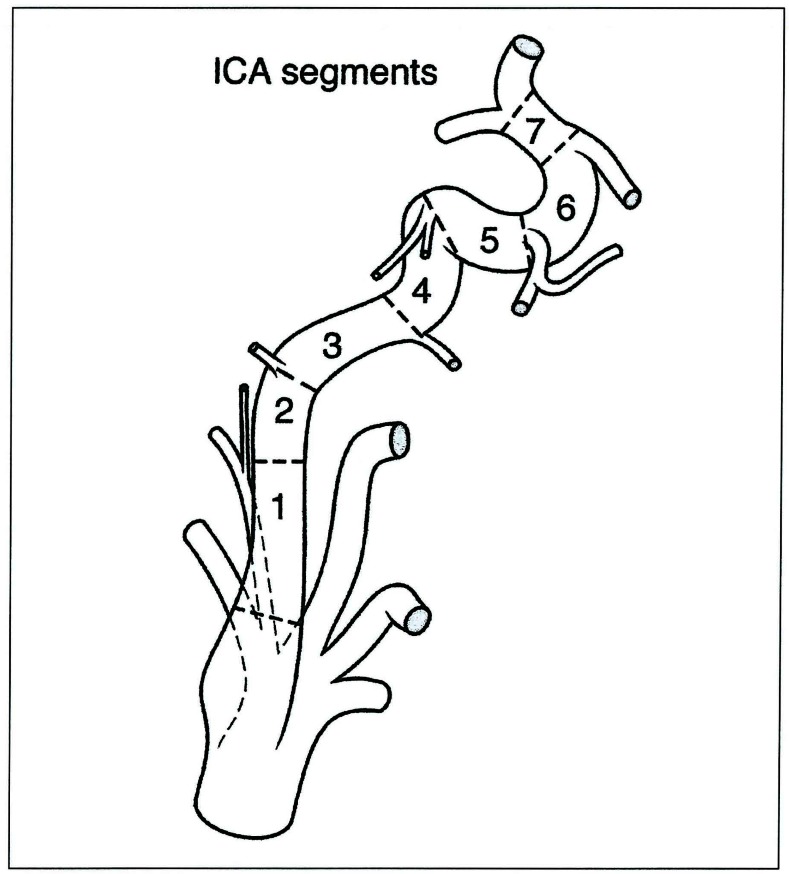
As can be demonstrated with the internal carotid artery development, the meaning of these events and landmarks is easily illustrated. The artery is constituted by seven segments (figure 4-7). Each of them is located in between embryonic arteries or their remnants; each of these segments can be absent, where it will then represent a focal agenesis. Such anomaly does not usually compromise the supply to the brain significantly, but is sometimes associated with severe disorders such as the PHACES Syndrome. It then becomes a potential time marker for a developmental disease associating proliferative events with malformations.
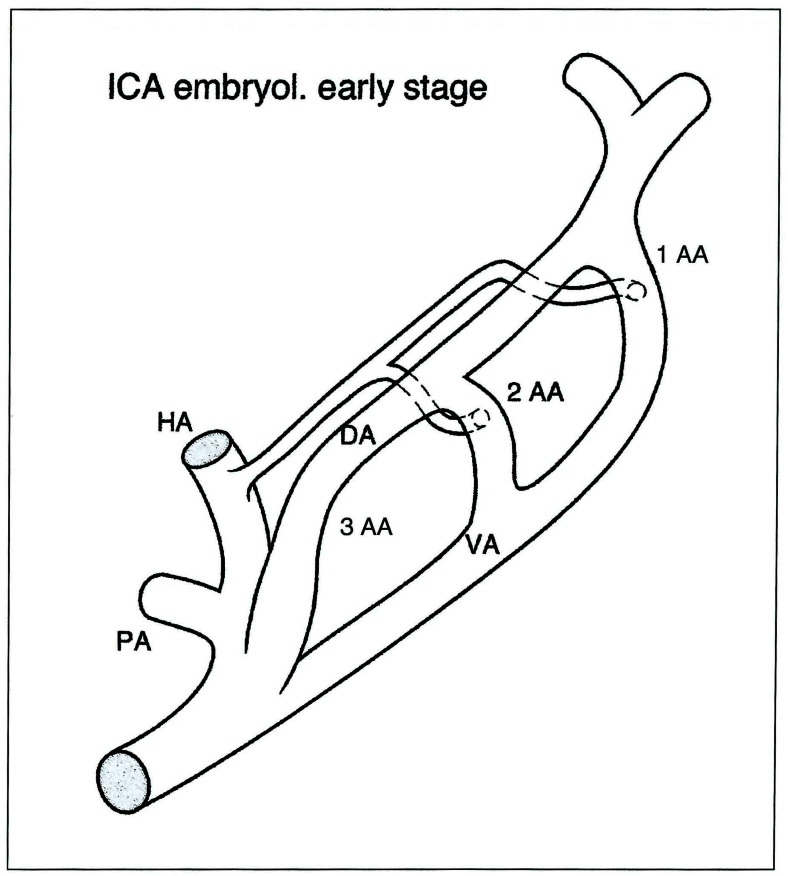
Figure 4.
Internal carotid artery embryology: early stage. PA: proatlantal artery - HA: hypoglossal artery - VA: ventral aorta - DA: dorsal aorta - 1 AA: first aortic arch - 2AA: second aortic arch - 3AA: third aortic arch.
Figure 5.
A) Intra cranial segments (4 to 7) successively extending between the mandibular (not seen) and the trigeminal-primitive maxillary arteries, the dorsal ophthalmic artery, the ventral ophthalmic artery and the bifurcation. B) internal carotid angiography with the 5th cranial segments arterial boundaries visible: Mandibular artery MA, trigeminal remnant TR, Infero lateral trunk ILT, ophthalmic artery OPH.
Figure 6.
Internal carotid artery segments derived from figure 3. Note that each segment lies between the origin of an embryonic vessel; these embryonic arteries determine precisely the segment boundaries.
Figure 7.
Projection of the embryologic segment of the internal carotid artery on its final disposition: 1: cervical - 2: ascending intrapetrous - 3: horizontal intrapetrous - 4: ascending foramen lacerum - 5: horizontal intra-cavernous- 6: clinoid - 7: termination. Note that the first cervical segment starts above the carotid glomus.
The memory of the evolutionary steps and their chronology is therefore imprinted on the arterial anatomy and thus potentially readable. One can postulate that since the age of each arterial segment is different, its resistance to time and stimuli will most likely be variable. Simultaneously exposed to the same aggression they will probably respond differently. Embryology having reproduced the evolutionary sequence of events, exposes both “old” and “recent” systems at different times (window of exposure or vulnerability) 1
Later, the remodelling processes probably preserve these phenotypic properties. If the window of exposure is reopened during each cell cycle, an “acquired disease” may very well “segmentally” affect the vasculature.
Some genetically based diseases require a secondary event to become expressed 2; the selection of the sites where the lesions appear do not illustrate the germinal or somatic mutation which theoretically impairs all the cells, but the segmental characteristics of the vasculature over time. The distribution of multifocal lesions is sometimes suggestive of a non random impairment. The territories spared obviously carry important functional information which protects the cells with the dormant defect from growing the disease. Similar observations can be made for some unusual, non genetical arterial lesions. Serpentine arterial aneurysm, arterial dysplasia (figure 8), basilar artery dysplasia (figure 9), Moya-Moya disease (figure 1), dolichoarterial segments (figures 10-13), rete mirabile (figure 14, figure 15), viral or immune arterial lesions (figure 2) may all represent diseases demonstrating this segmental vulnerability and timing. They illustrate the focal aspect of the disorder, the edges of the vascular response (vulnerability) and eventually suggest the mechanism of vascular impairment.
Figure 8.
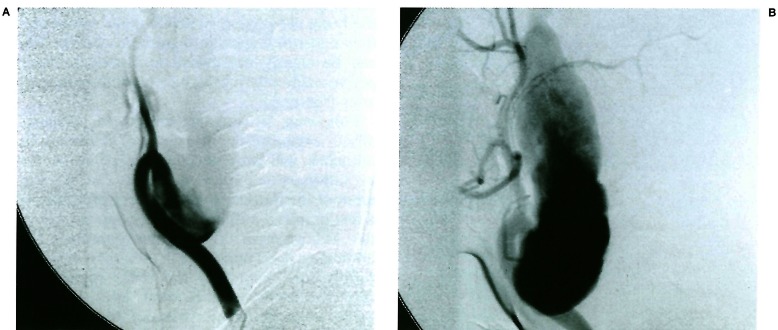
Common carotid injection in a nine year old child presenting with a cervical pulsatile mass. Note the dysplastic change involving the cervical internal carotid artery and its extension from above the carotid glomus to reach the skull base. The rest of the internal carotid system is normal. This lesion involves the first segment of the internal carotid artery (3rd aortic arch). Courtesy of P. Chewit.
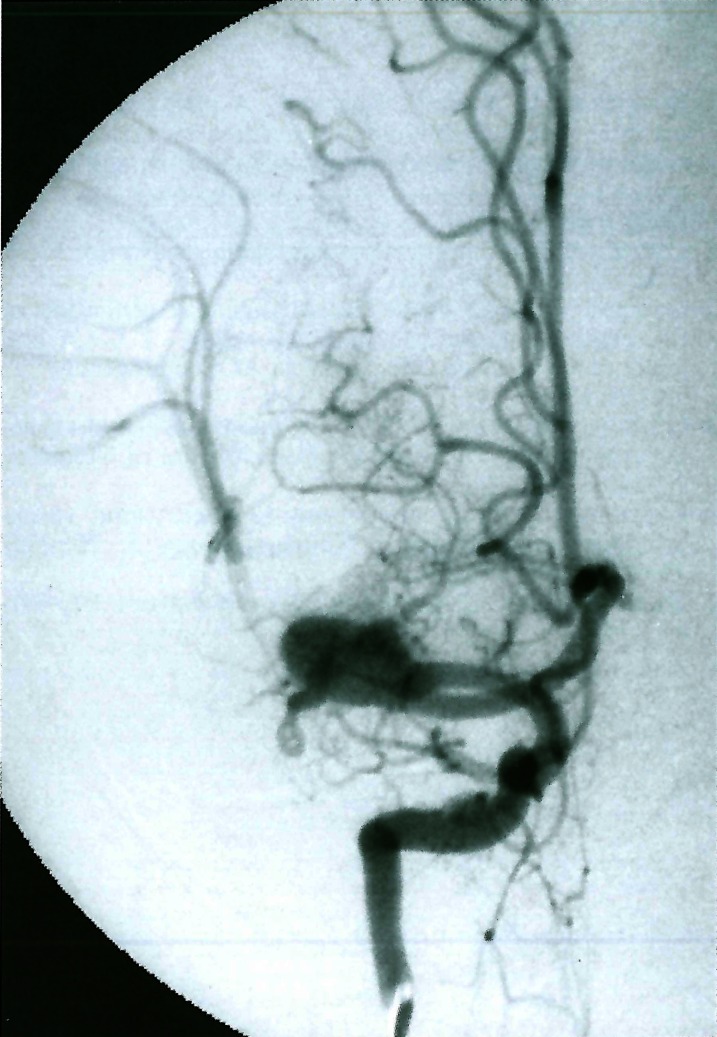
Figure 9.
The vertebral artery injection demonstrates a dolichodistal basilar artery above the origin of the trigeminal artery remnant. Note the intradural duplication of the left vertebral artery. (Courtesy of T. Hyogo).
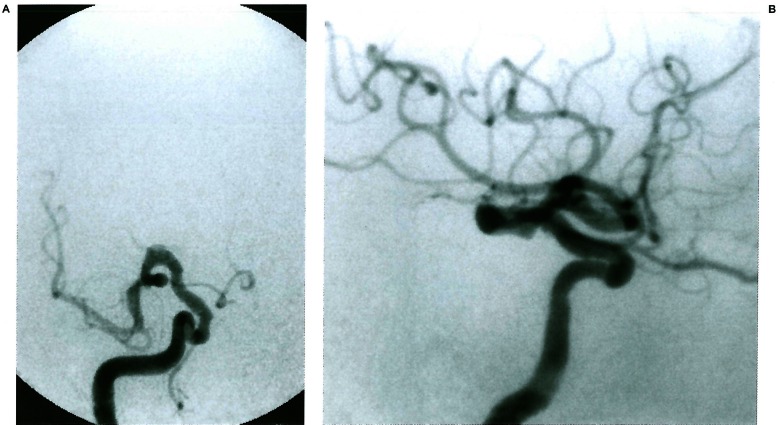
Figure 10.
Dolicho siphon of the internal carotid involving the segment leaning between the trigeminal remnant and the dorsal ophthalmic artery remnant (5th segment).
Figure 11.
The internal carotid artery presents a dolicho segment in its distal portion. The segment involved lies between the ophthalmic artery and the posterior communicating artery. It corresponds to the last segment of the carotid (7th segment). Courtesy of C. Campos.
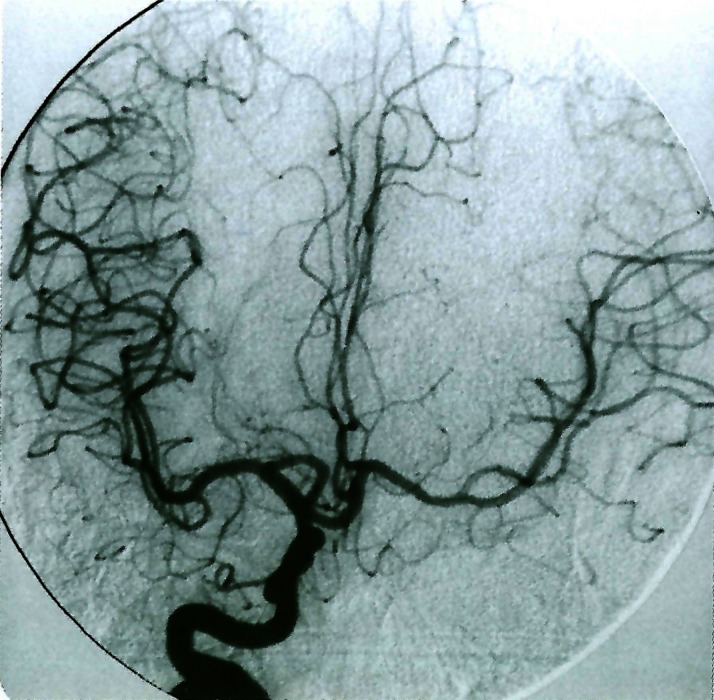
Figure 12.
Dolicho segment of the A1 portion of the right anterior cerebral artery.
Figure 13.
Dolicho segment of the clinoid internal carotid artery extending to its most distal portion (6th and 7th segments). The anomalous segment has opposed to the proper migration of the dorsal ophthalmic artery. The supply to the entire orbital contents arises from the accessory meningeal artery.
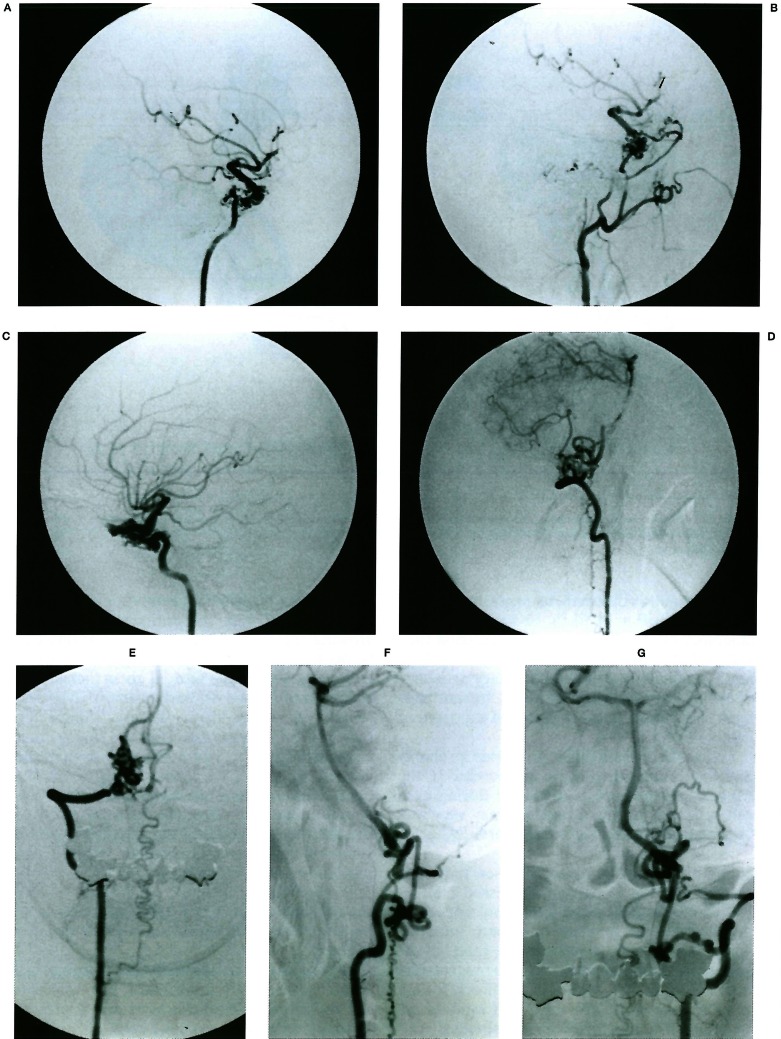
Figure 14.
Four vessel angiogram demonstrating a rete mirabile type of network compensating for segmental agenesis of epidural segments of the internal carotid and vertebral arteries bilaterally. (Courtesy of T. Hyogo. Published in Neuroradiology 38: 433-436,1996). A, B) On the right side the absence of the portion proximal to the inferolateral trunk promotes reconstitution of the distal siphon through the maxillary artery cavernous branches. C) On the left side the clinoid portion of the carotid is absent, distal to the inferolateral trunk; reconstitution of the siphon bridges the segmental agenesis through the orbit with the deep recurrent ophthalmic artery and retrogradely into the supracavernous ophthalmic artery. D, E, F, G) The vertebral arteries, collateralisation uses the lateral spinal artery channels on the left and a local epidural network on the right.
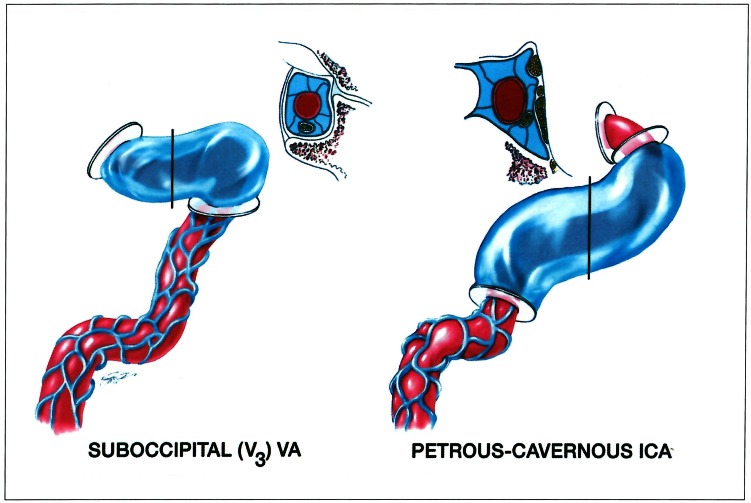
Figure 15.
The disposition illustrated in figure 14 points to the environmental characteristics of both trans dural portions of the internal carotid and vertebral arteries as illustrated by K. Arnautovic (reproduced from J Neurosurg 86: 252-262,1997; with permission).
Depending on the type of disease, we can theoretically suspect two gross mechanisms to explain their focal impact on a given vascular segment
1) The defect occurs early in a mother cell and is subsequently transmitted. The weakness has therefore a clonal character and is present in topographically regrouped cells,
2) A group of normal cells share a specific character that creates a permanently recognisable identity (or a transient one with a window of vulnerability).
In the first situation a secondary trigger reveals the underlying mutation. Such trigger can be non specific or poorly specific: it reveals a structural weakness.
In the second type it identifies the character and uses this property to impair that arterial segment. Such trigger must be specific to the focal character or hit the region at a specific time during the window of vulnerability. Such mechanism is involved in an immune type of recognition and autoimmune diseases.
The vulnerability of the target cannot be permanent both in a qualitative and quantitative way. Some genetic functions only seem to be active during a short period of time: during vasculogenesis for example. Therefore either the trigger is always active and the target vulnerability window of the cells time-limited, or the target is permanently exposed and the trigger agent can either be exogenous and rare, or most of the time inactive or inactivated (table 1). The postnatal genetic stability of endothelial cells, particularly in the brain and in comparison to the instability of tumoral cells, make endothelial cell clonal mutations probably rare. On the other hand such mutation will have a high chance of occurring during vasculogenesis as well as the early (antenatal and perinatal) active angiogenetic phases of vascular modelling. However, it is likely that, as for home-obox-containing genes, some vascular modelling programs are copied in several samples and placed within the genome in such a way that unless multiple alterations occur, a single mutation will seldom emerge as a malformation. If it did, it would certainly betray an early failure of the compensating program or extensive primary damage; fetal and neonatal vascular malformations (vein of galen aneurysmal malformation, dural sinus malformation, cerebral AVMs, ...) support such a hypothesis. On the other hand, some programs, after they have reached their objective, are not normally read again, allowing for a single opportunity to obtain a given pattern of impairment as the result of their damage.
In treatment strategies, the phenotypic segmental characteristics of the vascular wall may represent an obstacle to the expected homogenous VEGF response since luminal signalling will have to be supported by extra luminal ones. Specific topographical or timing adjustments will have to be foreseen to obtain focal correction or repair of a segmental arterial disease. Finally, angiogenesis in its broadest sense can generate various responses in a given segment: length increase (dolicho artery), centrifugal diameter increase with lumen enlargement (aneurysm), or centripetal increase with lumen reduction (occlusion). The three of them may coexist in contiguous segments showing the difficulty of appreciating where the specificity of the trigger, that of the target edges, lies or the time taken to produce the detectable architectural change (figure 16).
Figure 16.
Fifteen month old infant girl, who presented three epileptic events, with a left sided brachial paresis following the last episode. The three types of vascular growth are expressed in this case: dolicho Al artery segment, internal carotid artery anterior division fusiform aneurysm, Ml (centrifugal) occlusion without evidence of distal embolic obstruction. (Courtesy of R. Piske and C. Campos).
References
- 1.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGE Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Mechanisms of angiogenesis. Nature Medicine. 2000;6(3):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 3.Couly G, Coltey P, et al. The angiogenetic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mechanisms of Development. 1995;53:97–112. doi: 10.1016/0925-4773(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons GH, Dzay VJ. The emerging concept of vascular remodeling. The New England Journal of Medicine. 1994;20:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 5.Lasjaunias P. From aneurysms to aneurysmal vasculopathies. Interventional Neuroradiology. 1999;5:105–108. doi: 10.1177/159101999900500201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 7.Robertson P, Du-Bois M, et al. Angiogenesis in developing rat brain: an in vivo and in vitro study. Brain Res. 1985;355(2):219–223. doi: 10.1016/0165-3806(85)90044-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang HU, Chen Z, Anderson D. Molecular distinction and angiogenetic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]