Abstract
A structurally transformed lytic bacteriophage having a broad host range of Staphylococcus aureus strains and a penicillin-binding protein (PBP 2a) antibody conjugated latex beads have been utilized to create a biosensor designed for discrimination of methicillin resistant (MRSA) and sensitive (MSSA) S. aureus species 1,2. The lytic phages have been converted into phage spheroids by contact with water-chloroform interface. Phage spheroid monolayers have been moved onto a biosensor surface by Langmuir-Blodgett (LB) technique 3. The created biosensors have been examined by a quartz crystal microbalance with dissipation tracking (QCM-D) to evaluate bacteria-phage interactions. Bacteria-spheroid interactions led to reduced resonance frequency and a rise in dissipation energy for both MRSA and MSSA strains. After the bacterial binding, these sensors have been further exposed to the penicillin-binding protein antibody latex beads. Sensors analyzed with MRSA responded to PBP 2a antibody beads; although sensors inspected with MSSA gave no response. This experimental distinction determines an unambiguous discrimination between methicillin resistant and sensitive S. aureus strains. Equally bound and unbound bacteriophages suppress bacterial growth on surfaces and in water suspensions. Once lytic phages are changed into spheroids, they retain their strong lytic activity and show high bacterial capture capability. The phage and phage spheroids can be utilized for testing and sterilization of antibiotic resistant microorganisms. Other applications may include use in bacteriophage therapy and antimicrobial surfaces.
Keywords: Bioengineering, Issue 75, Microbiology, Infectious Diseases, Infection, Medicine, Immunology, Cellular Biology, Molecular Biology, Genetics, Anatomy, Physiology, Bacteria, Pharmacology, Staphylococcus, Bacteriophages, phage, Binding, Competitive, Biophysics, surface properties (nonmetallic materials), surface wave acoustic devices (electronic design), sensors, Lytic phage spheroids, QCM-D, Langmuir-Blodgett (LB) monolayers, MRSA, Staphylococcus aureus, assay
Introduction
Methicillin resistant strains of Staphylococcus aureus have been suggested as a factor in essential infections and nosocomial outbreaks 4-8. Common ways of the recognition of methicillin resistance, such as the disk diffusion oxacillin agar screen test, or broth microdilution, rely on tailored culture conditions to enhance the expression of resistance. Alterations include the utilization of oxacillin, incubation at 30 or 35 °C rather than 37 °C, and the inclusion of NaCl to the growth medium. Furthermore, for correct detection by these types of techniques, a long incubation period of 24 hr instead of 16 to 18 hr is required. Rapid techniques with appropriate (>96%) level of sensitivity for identification of methicillin resistance include automated microdilution techniques such as the Vitek GPS-SA card, the Rapid ATB Staph system, and the Rapid Microscan Panel system which produce results after 3-11 hr 9-11. The Crystal MRSA ID system is a rapid method based upon recognition of growth of S. aureus in the presence of 2% NaCl and 4 mg of oxacillin per liter with an oxygen-sensitive fluorescence sensor. Claimed sensitivities range between 91 to 100% after 4 hr of incubation 12-14. These phenotypic methods are limited in their accuracies by the impact of prevalent strains that express heterogeneous resistance. Therefore, the best widely accepted methods for the recognition of methicillin resistance is PCR or DNA hybridization of the mecA gene 15. However this technique requires purified DNA and is extremely sensitive to various admixtures (impurities), which include cell debris16.
Furthermore, these techniques need a long time to perform. Strategies to the recognition of the mecA gene product, protein PBP 2a, could be utilized to determine resistance and may be more reliable compared to standard test techniques 17.
It had been earlier shown that bacteriophage 12600 can be utilized as a recognition probe for Staphylococcus aureus strains including those having methicillin resistance 1,2,18. In this work we proposed a novel technique in the specific recognition and detection of MRSA, such as the recognition of bacteria along with conformation of MRSA in real time. For this specific purpose a S. aureus bacteriophage with a wide spectrum of hosts (including MRSA strains) combined with monoclonal antibody against protein (PBP 2a) have been used. PBP 2a is a cell wall protein and it is the cause of antibiotic resistivity of MRSA. However PBP 2a antibody is not specific for S. aureus since some other bacteria have antibiotic binding proteins with sequence similarity to PBP 2a 19,20. Consequently in this work, S. aureus bacteriophage and antibodies against PBP 2a protein have been used. To be able to develop a biosensor to specifically detect and identify MRSA a device with a two-step action has been utilized. The initial step used a S. aureus bacteriophage monolayer as a sensor probe, while the second step employed PBP 2a specific antibodies. Therefore, step one will recognize S. aureus bacteria, as the other one will be sensitive to the antibiotic-binding protein. When signals received from two steps are positive, it indicates the specific detection of MRSA.
Protocol
1. Setting the Stage
Obtain type strain S. aureus ATCC 12600, S. aureus ATCC 27690 and Bacillus subtilis ATCC 6051. Methicillin-resistant strains of S. aureus - MRSA1, MRSA 2, MRSA 5, MRSA 13, MRSA 26, MRSA 34, MRSA 45, B. anthracis Sterne, Salmonella typhimurium LT2, Shigella flexneri, Yersinia enterocolotica, Proteus mirabilis, Klebsiella pneumoniae 13882; The lytic phage 12600.
Obtain PBP 2a antibody conjugated latex beads.
Prepare NZY medium as described 21.
Obtain phosphate buffered saline solution (PBS).
Prepare deionized water as subphase for Langmuir-Blodgett (LB) monolayer deposition.
2. Bacteriophage Propagation and Titration
Incubate 5 ml of overnight culture of S. aureus ATCC 12600 (3.6 x 108 colony forming units (CFU)/ml) with 500 μl of phage (3 x 109 PFU/ml) in 500 ml NZY medium in 2 L flack on shaker-incubator at 37 °C overnight.
Centrifuge culture at 4,424 x g for 10 min at 4 °C.
Re-centrifuged supernatant at 11,325 x g for 10 min at 4 °C.
Filter supernatant through a 0.22 μm Millipore filter.
Centrifuge filtrate at 75,395 x g for 1.5 hr.
Dissolve pellet in 100 μl of distilled water.
Prepare 10-fold dilutions of phage suspension in NZY medium.
Plate 1 ml of overnight (ON) culture of S. aureus ATCC 12600 onto each of 2 plates with NZY agar to make sure all surface is covered. Remove the excess of culture and let the surface dry for 30 min.
Spot a sample (10 μl) of appropriate phage dilution onto the surface of the plate and incubate at 37 oC for 18-24 hr.
Examine the formation of plaques and calculate the phage titer. Bacteriophage titers (T) are estimated on the basis of the number of plaques (N) formed per 10 μl volume (v) of phage suspension and the dilution factor (F). The titer was calculated by formula: T=N/(vxF). For example, for the number of plaques 75 at the dilution 10-7 and the volume 10 μl (10 x 10-3 ml), the titer is equal 75 / (10 x 10-3 x 10-7) = 7.5 x 1010 plaque forming units (PFU) per ml of suspension.
3. Gold-immobilized Phage
Clean gold-coated quartz pieces (60 mm2) by plasma etching in argon for 10 min and then sterilize for 6 hr under UV light in a sterile cabinet.
Add 50 microliters of 1 x 109 PFU/ml phage suspension to the gold surface of each piece in a sterile Petri dish, and then incubate overnight in a humid chamber at room temperature.
Remove and titer the remaining phage suspension.
Wash pieces with bound phage 5 times with PBS to remove unbound phage.
4. Testing of Immobilized Phage Infectivity on a Dry Surface
Spread an O/N culture of S. aureus ATCC 12600 onto an NZY agar plate and allow drying.
Place a gold piece with immobilized phage onto the plate face down.
Observe a zone of lysis after 12 hr incubation at 37 °C that indicates infectivity.
Use gold covered pieces with no phage in control experiments.
5. Testing of Lytic Activity of Free and Bound Phage in Liquid
Add an ON culture of S. aureus ATCC 12600 to 10 ml of NZY in every of four 300 ml sidearm flasks (6 x 106 CFU/ml in each flask).
Add In two flasks 2 x 106 PFU/ml of free phage.
Use the other two flasks as controls.
Monitor the time course of S. aureus cell lysis for 570 min by optical density measurement (OD600) at 30 min intervals for all flasks.
Take a final measurement at 24 hr.
Repeat steps 5.1-5.5, but use gold pieces with bound phages instead of free phages, and gold pieces with no phage in control flasks.
6. Loss of Bound Phages During Incubation
Add 50 μl of 1 x 109 PFU/ml phage onto the surface of sterile gold piece in a Petri dish that is placed into a humid chamber overnight at room temperature.
Remove the suspension with unbound phage from the gold surface and titer.
Wash the gold piece with bound phage 5 times with PBS and place in 1 ml NZY solution in 15 ml tube in shaker-incubator at 37 °C for 8 hr.
Determine the titer of the detached phage as described in 2.
7. Phage Spheroids Preparation
Combining 400 μl of stock phage 12600 suspension (1011 PFU/ml) with an equal volume of spectrophotometric grade chloroform in 1 ml vial at room temperature.
Gently vortex the phage-chloroform suspension 5-6 times (5 sec intervals) over one minute duration.
Allowed the suspension to stabilize for 30 sec and then pipette of the top phase containing spheroids was pipetted off for monolayer preparation.
Take a few microliters of the spheroids suspension for the transmission and scanning electron microscopy.
Use a remaining spheroid suspension for biosensor manufacturing.
8. Biosensor Preparation
Clean QCM-D sensors by plasma etching in argon for 10 min PDC-32G Harrick plasma cleaner.
Rinse sensor with hexane to remove any organic impurities.
Prepare and clean a trough of the film balance as described in 22.
Fill LB trough with a subphase solution and clean it with a trough barrier and stabilize the trough at 20±0.1 °C
Spread 300 μl aliquot of phage 12600 in aqueous suspension (1011 PFU/ml) onto LB subphase.
Prepare phage monolayer onto LB subphase solution by allowing 300 μl aliquot of phage 12600 aqueous suspension (1011 PFU/ml) to run down an inclined wettable glass rod that is partially submersed into the subphase 22,23.
Stabilize the prepared monolayer for 10 min, and then compress it at a rate of 30 mm/min (45 cm2/min), until a constant pressure 19 N/m is attained.
Perform a vertical film deposition onto perpendicular positioned QCM-D sensor at a rate of 4.5 mm/min by successively dipping sensor in and out of the monolayer seven times. Electron microscopy of deposited phage monolayers before exposure to bacterial samples showed that the layers were continuous, homogeneous, and uniform (data not shown).
Repeat steps 4.5-4.8 with a phage spheroid suspension that is prepared in 3.
Use fabricated phage biosensors for MRSA detection, electron microscopy, and ellipsometry.
9. Biosensor Testing, Discrimination of Methicillin Resistant and Sensitive Staphylococcus Bacteria
In this protocol, biosensors with unmodified and modified phage and phage spheroids are prepared. A quartz crystal microbalance with four sensor flow chambers is used to monitor binding of bacteria to the phage immobilized on QCM sensor surface. PBP 2a antibody conjugated latex beads are utilized to discriminate between methicillin resistant (MRSA) and sensitive staphylococcus bacteria. All measurements are conducted in the flow mode (50 μl/min) at 5 MHz.
Establish a base line resonance frequency of the QCM sensor in water (~30 min).
Draw a suspension of live methicillin sensitive staphylococcus bacterial cells in water (109 CFU/ml) through the test cell.
Continuously monitor changes in the sensors resonance frequency and energy dissipation for the first overtone, using the Q-Soft software.
When the changes in the sensors resonance frequency and dissipation reached saturation levels, add the suspension of PBP antibody conjugated latex beads (6.77 x1010 beads/ml) to the flow during the continuous monitoring of frequency and dissipation.
Repeat steps 9.2-9.5 for methicillin resistant staphylococcus bacterial cells (MRSA).
Define a frequency change through the mass change due to bacteria binding by the Sauerbrey equation 24,25 Δf=-Δm xn/C, where C is the mass sensitivity constant (C=17.7 ng x cm-2 x Hz-1 at 5 MHz), m is mass and n is the overtone number (n = 1).
Define an energy dissipation change (ΔD) from the recording the exponential decay of oscillation (frequency and amplitude dampening, which allowed quantification of the energy dissipated and stored during one period of oscillation, Edissipated and Estored, respectively: ΔD = Edissipated / 2πEstored.ΔD is measured in dissipation units (DU), one DU = 10-6 relative units).
Representative Results
The phage demonstrated lytic activity against all tested strains of S. aureus, including MRSA strains, as indicated by the phage spot test. Plaque sizes generally ranged from 5 to 15 mm. No activity was found against other test-cultures (Table 1).
A normal growth of S. aureus ATCC 12600 in NZY medium on shaker-incubator at 37 °C is shown in Figure 1A (a curve labeled by empty circles). The number of bacteria increased from 3.2 x 106 to 4.0 x 108 CFU/ml. Figure 1A (a curve labeled by filled circles) shows results of the co-cultivation of 2.0 x 106 PFU/ml free phage simultaneously added with the same concentration of S. aureus ATCC 12600. Phage, immobilized on the gold surface, demonstrated the lytic activity comparable with the activity of phage in suspension (Figure 1A, curve labeled by top down triangles). Immobilized phage remained infective when the gold piece with phage was first used in 24-hr growing experiment, then it was washed 5 times and stored in PBS for 6 days at 4 °C, and finally reused with a fresh bacterial suspension. (Figure 1A, curve labeled by top up triangles). It seems that immobilized phages inject their DNA in the bacteria, leaving void capsids, which are incapable to infect new bacteria. We hypothesize that immobilized phages on a gold surface served as primary "catalyzers" to infect a few bacteria, which generate free phages those in turn infect new bacteria and so on. Therefore, the most of immobilized phages were probably not used in a first 24 hr growing experiment and, therefore, can be utilized a second time. The surface density of phages deposited by physical adsorption was about ~0.7 phage particle/μm2. This concentration is high, but it can grow up to 10 times 2. Therefore, the gold plate could incorporate some of the free viable phages released by infected bacteria in the first 24 hr growing experiment.
Figure 1B shows the lysis zone around the gold piece, indicating that immobilized phages were capable of lysing bacterial cells. In the control experiments, the empty gold plate did not inhibit bacterial growth. Hence the effective decrease of bacterial growth found at the co-culture of bacteria and immobilized phage is a result of primary interaction of water suspended bacteria and bound phage as shown in Figure 1C. The phage detachment from the gold surface during co-incubation of bound phage and bacteria was small. In order to estimate how many phages were detached from the gold surface during incubation, samples with bound phage were immersed in NZY solution, shaken in the shaker-incubator at 37 °C for 8 hr, and a free phage concentration in supernatant was assessed. We determined that 4.1 x 107 PFU were bound to a 60 mm2 gold piece (~0.7 phage particle/μm2), when only 3 x 104 PFU were detached (0.007% detachment).
The transmission and scanning electron micrographs of intact lytic phage 12600 on the gold substrate surface is shown in Figures 2A and 2B. When the phage suspension was subjected to chloroform treatment, the phage physical appearance was changed. The tail contracted in length and thickened. The polygonal head became rounded (Figures 2D and 2E). We termed the structurally modified lytic phage "spheroid" similar to the name of the chloroform treated filamentous phage 26. In spite of the significant structural changes resulted from chloroform treatment, the spheroid lytic activity measured by plaque numbers has not changed (Figures 2C and 2F). The mean lytic activity of phage and spheroids were (7.4±1.5(SD)) x 1010 (N=4) and (7.5±1.0(SD)) x 1010 (N=7), PFU/ml. At the 0.05 level, the activities of phage and spheroids were not significantly different (Figure 2C and 2F).
The QCM sensors with immobilized lytic phages showed no significant changes in the resonance frequency or energy dissipation when they were exposed to MRSA (Figure 3A). These data indicate that MRSA/phage interaction resulted in a no mass change according to the QCM, and yet the electron micrographs of post assayed biosensors revealed significant bacterial binding at the sensor surface (Figure 3B).
When MRSA suspensions were injected into the flow cell with phage spheroid biosensors, a substantial decrease in the frequency (Δf ≈ -105 Hz) and an increase in the dissipation (ΔD ≈ 26 DU) were observed (Figure 3C). Following phage spheroid-bacterial (MRSA) binding interactions, assayed sensors were exposed to PBP2a antibody conjugated latex beads suspensions. These MRSA assayed biosensors responded to the PBP2a antibody conjugated latex beads suspensions, since a further decrease in the frequency (Δf ≈ -45 Hz) and an increase in the dissipation (ΔD ≈ 15 DU) were observed (Figure 3C). Binding of MRSA to phage probes and PBP2a antibody conjugated latex beads was confirmed using scanning electron microscopy investigations (Figure 3D).
When the phage spheroid biosensor was exposed to MSSA suspensions, a substantial decrease in the frequency (Δf ≈ -200 Hz) and an increase in the dissipation (ΔD ≈ 55 DU) were observed (Figure 3E). Following phage spheroid-MSSA binding interactions, assayed sensors were challenged with PBP2a antibody conjugated latex beads suspensions. Initially, the MSSA assayed biosensor showed short transients of increase in frequency and decrease in dissipation. After a few minutes of MSSA and PBP2a antibody conjugated latex beads interactions, frequency returned to post PBP2a antibody introduction levels, but the dissipative energy increased (Figure 3E). Binding of MSSA to phage probes was confirmed with scanning electron microscopy, but no binding between MSSA and PBP2a antibody conjugated latex beads was observed (Figure 3F). Ellipsometric thickness profile, 3D thickness map of lytic phages and staphylococcus bacteria are shown in Supplementary Figures S1 and S2.
Following contact of sensors with LB immobilized phages or spheroids to the suspension of 109 cells/ml of MRSA or MSSA, the bacteria were observed to bind phages or spheroids at density (ρ) of 9.1 x 107 (MRSA/intact phages), 7.9 x 107 (MRSA/spheroids), and 7.2 x 107 (MSSA/spheroids) cells/cm2. In tests using 5 different lytic phages and 2 host bacteria 27, researchers subjected phages immobilized by covalent binding method to 109 cells/ml bacteria, and determined the phage capture efficiency in a range of (2.5-8.9) x 105 cells/cm2. The phage capture efficiency presented in this work is ~100 times higher.
| Host | Strain | Strain details | Methicillin sensitivity | Phage sensitivity |
| S. aureus | 12600 | ATCC | S | + |
| S. aureus | 27690 | ATCC | S | + |
| S. aureus | 10292 | IA | S | + |
| S.aureus | 10378 | IA | S | + |
| S. aureus | 10497 | IA | S | + |
| S. aureus | 10686 | IA | S | + |
| S. aureus | MRSA 1 | AU | R | + |
| S.aureus | MRSA 2 | AU | R | + |
| S. aureus | MRSA 5 | AU | R | + |
| S. aureus | MRSA 13 | AU | R | + |
| S.aureus | MRSA 26 | AU | R | + |
| S. aureus | MRSA 34 | AU | R | + |
| S. aureus | MRSA 45 | AU | R | + |
| B. anthracis | Sterne | AU | NA | - |
| Salmonella typhimurium | LT2 | AU | NA | - |
| Shigella flexneri | unknown | AU | NA | - |
| Yersinia enterocolitica | unknown | AU | NA | - |
| Proteus mirabilis | unknown | AU | NA | - |
| Klebsiella pneumoniae | 13882 | AU | NA | - |
| Bacillus subtilis | 6051 | ATCC | NA | - |
Table 1. Phage 12600 sensitivity of bacterial strains. IA - isolated from animals; AU - bacterial culture collection of Auburn University; NA - not applicable; a - lytic activity of phage was defined by plaques formation, +, sensitive, -, not sensitive. Overnight cultures of tested strains were plated onto the plates with NZY agar. After the surface dried, a sample (10 μl) of 1011 PFU phage suspension was spotted on the surface of the plate. Plates were incubated at 37 °C for 18-24 hr. Lytic activity of the phages was detected by formation of plaques.
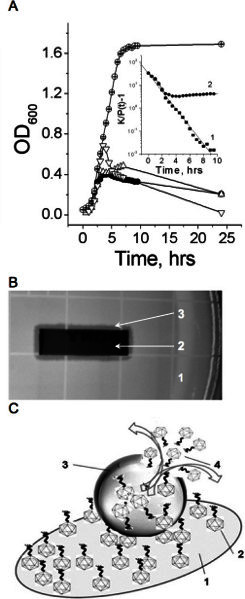 Figure 1. Infective properties of bound and free phage.A. Bacterial growth in the absence (empty circles) and presence (filled circles) of free phage, in the presence of phage bound to gold surface (top up triangles), and in the presence of phage bound to gold surface after 6 hours at 4 °C (top down triangles). Insert: The line (1) shows a linear fit of the no phage growth experimental data to the equation (4) (R=-0.99, p<0.0001). The line (2) shows a fit of the experimental data of bacterial growth at the free phage presence to the same equation. Bacterial growth constant (k) in the absence and presence of free phage, are equal 0.88 and 0.64, (-0.048, decline phase). Representative data of three independent experiments are shown in panel A. A mean relative error OD600 measurements did not exceed 5%. B. Phage immobilized to the gold surface is infective as indicated by the lysis zone around the gold piece. 1- the fragment the agar plate with bacteria; 2 - the gold piece with immobilized phage; 3 - the inhibition zone around the gold piece. C. Schematic representation of bacterium lysis by phage attached to the gold surface. 1 - gold-coated quartz piece, 2 - phage bound to the gold surface, 3 - bacterium anchored by bound phages, 4 - free phages have released by bursting infected bacterium. Used with permission from: Guntupalli, R., et al. 2012. Click here to view larger figure.
Figure 1. Infective properties of bound and free phage.A. Bacterial growth in the absence (empty circles) and presence (filled circles) of free phage, in the presence of phage bound to gold surface (top up triangles), and in the presence of phage bound to gold surface after 6 hours at 4 °C (top down triangles). Insert: The line (1) shows a linear fit of the no phage growth experimental data to the equation (4) (R=-0.99, p<0.0001). The line (2) shows a fit of the experimental data of bacterial growth at the free phage presence to the same equation. Bacterial growth constant (k) in the absence and presence of free phage, are equal 0.88 and 0.64, (-0.048, decline phase). Representative data of three independent experiments are shown in panel A. A mean relative error OD600 measurements did not exceed 5%. B. Phage immobilized to the gold surface is infective as indicated by the lysis zone around the gold piece. 1- the fragment the agar plate with bacteria; 2 - the gold piece with immobilized phage; 3 - the inhibition zone around the gold piece. C. Schematic representation of bacterium lysis by phage attached to the gold surface. 1 - gold-coated quartz piece, 2 - phage bound to the gold surface, 3 - bacterium anchored by bound phages, 4 - free phages have released by bursting infected bacterium. Used with permission from: Guntupalli, R., et al. 2012. Click here to view larger figure.
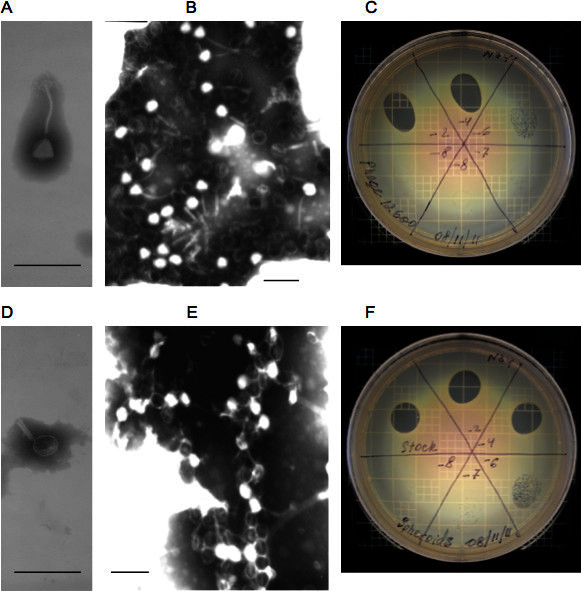 Figure 2. Properties of intact and modified phage.A and B - Transmission and scanning electron micrographs of intact phage, respectively; C - phage lytic activity on an agar plate with MRSA. D and E - Transmission and scanning electron micrographs of intact phage spheroids, respectively; F - phage spheroids lytic activity on an agar plate with MRSA. The mean activity of phage and spheroids are (7.4±1.5(SD)) x 1010 (N=4) and (7.5±1.0(SD)) x 1010 (N=7) PFU/ml. T-test: t = 0.15682, p = 0.87851. At the 0.05 level, the activities of phage and spheroids are NOT significantly different. Bars: A, B, D, and E: 200 nm. Used with permission from: Guntupalli, R., et al. 2012.
Figure 2. Properties of intact and modified phage.A and B - Transmission and scanning electron micrographs of intact phage, respectively; C - phage lytic activity on an agar plate with MRSA. D and E - Transmission and scanning electron micrographs of intact phage spheroids, respectively; F - phage spheroids lytic activity on an agar plate with MRSA. The mean activity of phage and spheroids are (7.4±1.5(SD)) x 1010 (N=4) and (7.5±1.0(SD)) x 1010 (N=7) PFU/ml. T-test: t = 0.15682, p = 0.87851. At the 0.05 level, the activities of phage and spheroids are NOT significantly different. Bars: A, B, D, and E: 200 nm. Used with permission from: Guntupalli, R., et al. 2012.
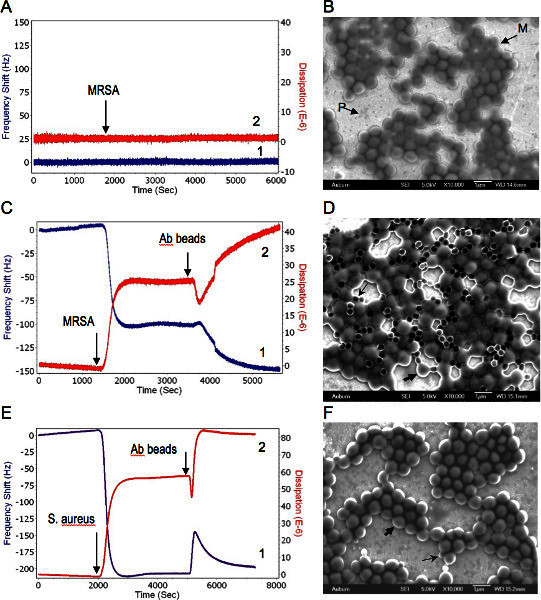
Figure 3. Combined QCM-D and EM analysis of phage-bacteria interactions. Bacteria were delivered to the sensor at concentration of 109 CFU/ml suspensions in water at a flow rate of 50 μl/min. 1, 2 represent changes in the resonance frequency and the energy dissipation, respectively. A. Phage coated QCM-D sensor response to MRSA. Arrow shows the MRSA delivery time to the sensor surface. B. Scanning electron micrograph of post assayed MRSA bound to lytic phage immobilized on the QCM sensor. M-MRSA, P-phages on the sensor surface. C. Successive responses of the phage spheroids coated QCM-D sensor to MRSA first and then to PBP antibody beads. Arrows indicate MRSA and PBP antibody delivery time to the sensor surface, respectively. D. Scanning electron micrograph of post assayed biosensor with phage spheroids, MRSA, and PBP antibody beads. Thick and thin arrows shows typical MRSA cell and antibody bead, respectively. E. Successive responses of the phage spheroids coated QCM-D sensor to S. aureus first and then to PBP antibody beads. Arrows indicate S. aureus and PBP antibody delivery time to the sensor surface, respectively. F. Scanning electron micrograph of post assayed biosensor with phage spheroids, S. aureus, and PBP antibody beads. Thick and thin arrows shows typical S. aureus cell and antibody bead, respectively. Used with permission from: Guntupalli, R., et al. 2012. Click here to view larger figure.
Supplementary Figures
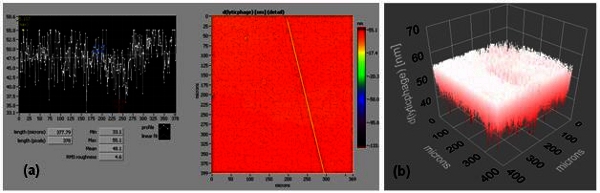 Figure S1. Supplementary Figure 1.(a) and (b) are an ellipsometric thickness profile and 3D thickness map of a lytic phage, respectively (effective refractive index = 1.05). Thickness profiles show mean, RMS roughness, minimum, and maximum thickness of the monolayer. A line across the thickness map was drawn to generate the thickness profile.
Figure S1. Supplementary Figure 1.(a) and (b) are an ellipsometric thickness profile and 3D thickness map of a lytic phage, respectively (effective refractive index = 1.05). Thickness profiles show mean, RMS roughness, minimum, and maximum thickness of the monolayer. A line across the thickness map was drawn to generate the thickness profile.
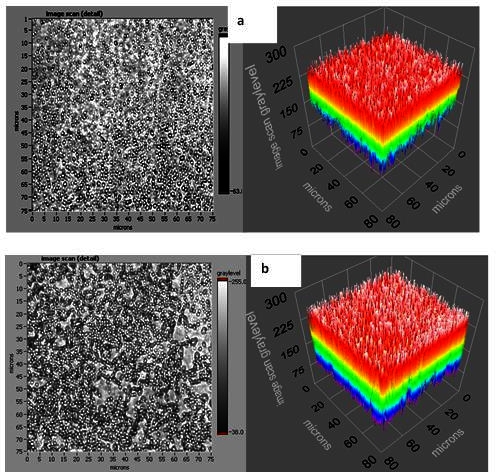 Figure S2. Supplementary Figure 2.
Figure S2. Supplementary Figure 2.
Discussion
It is well known that phages can be used as biosensor probes for bacterial pathogens 28. It is demonstrated in this work that phage together with PBP 2a antibodies can be utilized to resolve the old problem: rapid discrimination antibiotic resistant and sensitive strains.
It was found however those normal unmodified staphylococcal phages are not suitable for bacteria detection with QCM devices, even though they bind bacteria. The phage tail is so long that acoustic waves cannot "reach" the bacteria bound to the end of phage tails. This condition resulted in a "missing mass" effect 29 and inability to register resonance frequency and dissipation change in spite of the significant binding showed by EM images (Figures 3A and 3B). This problem was easily solved by replacing the intact phage with spheroids, the phage modified by chloroform-water treatment. This treatment resulted in fully functional phage with short, thick and non-flexible tail (Figures 2D, 2E, and 2F). When phages were replaced with spheroids in biosensors, the MRSA were easily detected by QCM-D device.
When phage particles were bound to gold surfaces at a proper orientation, their receptors were accessible to the host bacteria. If direct physical contact between a solid surface with phages and a dried bacterial layer occur, the immobilized phages are capable of injecting viral genome into host bacteria (Figure 1C). These results agree well with those obtained with lytic phages immobilized to glass disk surfaces by a covalent binding technique 27. The capability of bound lytic phages to capture host bacteria were also demonstrated by using a biotinylated phage immobilization technique 30. In contrast, a very simple physical adsorption method for attaching properly oriented phages to solid surfaces has been utilized 31. The high phage spheroid capture efficiency can be used for making effective antimicrobial surfaces. A total time-to-answer for the proposed assay is about 16 min per sample. This time can be dramatically shortened by using QCM devices with a large number of chambers. The anticipated shelf life for the phage sensors is about of 3-4 months at room temperature. With a biopolymer protection it could be prolong up to a few years 32. The detection limit of S. aureus was measured for this phage by a surface plasmon resonance spectroscopy, and found to be 104 CFU/ml 18.
One of the most important conditions for using methods described in this article is to comply with clean and sterile conditions 33 requirements for all experiments with phages and bacteria.
Commonly used methods for detection of MRSA, such as the disk diffusion oxacillin agar screen test, or broth microdilution take normally up to 24 hr to carry out the test. Rapid techniques that include automated techniques such as the Vitek GPS-SA card, the Rapid ATB Staph system, the Rapid Microscan Panel system, and the Crystal MRSA ID system also produce results after 3-11 hr 9-14. PCR or DNA hybridization of the mecA gene 15 is a relatively fast and accurate method but requires purified DNA and is extremely sensitive impurities. In contrast, the method described in this work is rapid, does not need DNA extraction, and it is not sensitive to admixtures.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The work reported herein was supported by grants from Auburn University AUDFS and USAF CRADA 07-277-60MDG-01. The views expressed in this article are those of the authors, and do not reflect the official policy or position of the United States Air Force, Department of Defense, or the U.S. Government.
References
- Guntupalli R, Sorokulova I, Krumnow A, Pustovyy O, Olsen E, Vodyanoy V. Real-time optical detection of methicillin-resistant Staphylococcus aureus using lytic phage probes. Biosens. Bioelectron. 2008;24:151–154. doi: 10.1016/j.bios.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Guntupalli R, Sorokulova I, et al. Detection and identification of methicillin resistant and sensitive strains of Staphylococcus aureus using tandem measurements. J. Microbiol. Methods. 2012;90:182–191. doi: 10.1016/j.mimet.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Guntupalli R, Sorokulova I, Long R, Olsen E, Neely W, Vodyanoy V. Phage Langmuir monolayers and Langmuir-Blodgett films. Colloids and Surfaces, B: Biointerfaces. 2011;82:182–189. doi: 10.1016/j.colsurfb.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Barie PS. Antibiotic-resistant gram-positive cocci: implications for surgical practice. World. J. Surg. 1998;22:118–126. doi: 10.1007/s002689900359. [DOI] [PubMed] [Google Scholar]
- Byun DE, Kim SH, Shin JH, Suh SP, Ryang DW. Molecular epidemiologic analysis of Staphylococcus aureus isolated from clinical specimens. J. Korean Med. Sci. 1997;12:190–198. doi: 10.3346/jkms.1997.12.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Lei H, Huang E, Yi G, Fan W. Drug resistance of Staphylococcus aureus from lower respiratory tract. Zhonghua Yiyuanganranxue Zazhi. 2011;21:1667–1668. [Google Scholar]
- Giamarellou H, Papapetropoulou M, Daikos GK. Methicillin resistant' Staphylococcus aureus infections during 1978-79: clinical and bacteriologic observations. J. Antimicrob. Chemother. 1981;7:649–655. doi: 10.1093/jac/7.6.649. [DOI] [PubMed] [Google Scholar]
- Knopf HJ. Nosocomial infections caused by multiresistant pathogens. Clinical management exemplified by multiresistant Staphylococcus aureus. Urologe A. 1997;36:248–254. doi: 10.1007/s001200050099. [DOI] [PubMed] [Google Scholar]
- Knapp CC, Ludwig MD, Washington JA. Evaluation of differential inoculum disk diffusion method and Vitek GPS-SA card for detection of oxacillin-resistant staphylococci. J. Clin. Microbiol. 1994;32:433–436. doi: 10.1128/jcm.32.2.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struelens MJ, Nonhoff C, Van DA, Philippe Mertens R, Serruys E. Evaluation of rapid ATB Staph for 5-hour antimicrobial susceptibility testing of Staphylococcus aureus. J. Clin. Microbiol. 1995;33:2395–2399. doi: 10.1128/jcm.33.9.2395-2399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods GL, LaTemple D, Cruz C. Evaluation of MicroScan rapid gram-positive panels for detection of oxacillin-resistant staphylococci. J. Clin. Microbiol. 1994;32:1058–1059. doi: 10.1128/jcm.32.4.1058-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CC, Ludwig MD, Washington JA. Evaluation of BBL crystal MRSA ID system. J. Clin. Microbiol. 1994;32:2588–2589. doi: 10.1128/jcm.32.10.2588-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri SM, Ueno Y, Imambaccus H, Almodovar E. Rapid detection of methicillin-resistant Staphylococcus aureus by Crystal MRSA ID System. J. Clin. Microbiol. 1994;32:1830–1832. doi: 10.1128/jcm.32.7.1830-1832.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambardi G, Fleurette J, et al. European multicentre evaluation of a commercial system for identification of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 1996;15:747–749. doi: 10.1007/BF01691964. [DOI] [PubMed] [Google Scholar]
- Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DFJ, Edwards DI, et al. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA) J. Antimicrob. Chemother. 2005;56:1000–1018. doi: 10.1093/jac/dki372. [DOI] [PubMed] [Google Scholar]
- Gerberding JL, Miick C, Liu HH, Chambers HF. Comparison of conventional susceptibility tests with direct detection of penicillin-binding protein 2a in borderline oxacillin-resistant strains of Staphylococcus aureus. Antimicrobial Agents & Chemotherapy. 1991;35:2574–2579. doi: 10.1128/aac.35.12.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Sorokulova IB, Vodyanoy VJ, Simonian AL. Lytic phage as a specific and selective probe for detection of Staphylococcus aureus-A surface plasmon resonance spectroscopic study. Biosens. Bioelectron. 2007;22:948–955. doi: 10.1016/j.bios.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Popham DL, Young KD. Role of penicillin-binding proteins in bacterial cell morphogenesis. Current Opinion in Microbiology. 2003;6:594–599. doi: 10.1016/j.mib.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Wei Y, Havasy T, McPherson DC, Popham DL. Rod shape determination by the Bacillus subtilis class B penicillin-binding proteins encoded by pbpA and pbpH. J. Bacteriol. 2003;185:4717–4726. doi: 10.1128/JB.185.16.4717-4726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco SHH, Lee S, Dunbar WS, MacGillivray RTA, Curtis SB. Maximizing filamentous phage yield during computer-controlled fermentation. Bioprocess and Biosystems Engineering. 2009;32:773–779. doi: 10.1007/s00449-009-0303-3. [DOI] [PubMed] [Google Scholar]
- Olsen EV, Pathirana ST, Samoylov AM, Barbaree JM, Chin BA, Neely WC, Vodyanoy V. Specific and selective biosensor for Salmonella and its detection in the environment. J. Microbiol. Methods. 2003;53:273–285. doi: 10.1016/s0167-7012(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Pathirana ST, Barbaree J, Chin BA, Hartell MG, Neely WC, Vodyanoy V. Rapid and sensitive biosensor for Salmonella. Biosens. Bioelectron. 2000;15:135–141. doi: 10.1016/s0956-5663(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Sauerbrey G. The use of quartz oscillators for weighing thin layers and for microweighing. Z. Phys. 1959;155:206–222. [Google Scholar]
- Hook F, Rodahl M, Brzezinski P, Kasemo B. Energy Dissipation Kinetics for Protein and Antibody-Antigen Adsorption under Shear Oscillation on a Quartz Crystal Microbalance. Langmuir. 1998;14:729–734. [Google Scholar]
- Griffith J, Manning M, Dunn K. Filamentous bacteriophage contract into hollow spherical particles upon exposure to a chloroform-water interface. Cell. 1981;23:747–753. doi: 10.1016/0092-8674(81)90438-4. [DOI] [PubMed] [Google Scholar]
- Hosseinidoust Z, Van de Ven TGM, Tufenkji N. Bacterial Capture Efficiency and Antimicrobial Activity of Phage-Functionalized Model Surfaces. Langmuir. 2011;27:5472–5480. doi: 10.1021/la200102z. [DOI] [PubMed] [Google Scholar]
- Schofield DA, Molineux IJ, Westwater C. Bioluminescent' Reporter Phage for the Detection of Category A Bacterial Pathogens. J. Vis. Exp. 2011. p. e2740. [DOI] [PMC free article] [PubMed]
- Voinova MV, Jonson M, Kasemo B. Missing mass" effect in biosensor's QCM applications. Biosens. Bioelectron. 2002;17:835–841. doi: 10.1016/s0956-5663(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Gervals L, Gel M, et al. Immobilization of biotinylated bacteriophages on biosensor surfaces. Sensors and Actuators. 2007;125:615–621. [Google Scholar]
- Nanduri V, Sorokulova IB, Samoylov AM, Simonian AL, Petrenko VA, Vodyanoy V. Phage as a molecular recognition element in biosensors immobilized by physical adsorption. Biosens. Bioelectron. 2007;22:986–992. doi: 10.1016/j.bios.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Sorokulova I, Watt J, et al. Natural biopolymer for preservation of microorganisms during sampling and storage. J. Microbiol. Methods. 2012;88:140–146. doi: 10.1016/j.mimet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Sanders ER. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012. p. e3064. [DOI] [PMC free article] [PubMed]


