Abstract
There is accumulating evidence, that ischemic preconditioning - a non-damaging ischemic challenge to the brain - confers a transient protection to a subsequent damaging ischemic insult. We have established bilateral common carotid artery occlusion as a preconditioning stimulus to induce early ischemic tolerance to transient focal cerebral ischemia in C57Bl6/J mice. In this video, we will demonstrate the methodology used for this study.
Keywords: Medicine, Issue 75, Neurobiology, Anatomy, Physiology, Neuroscience, Immunology, Surgery, stroke, cerebral ischemia, ischemic preconditioning, ischemic tolerance, IT, ischemic stroke, middle cerebral artery occlusion, MCAO, bilateral common carotid artery occlusion, BCCAO, brain, ischemia, occlusion, reperfusion, mice, animal model, surgical techniques
Introduction
Ischemic stroke is a disease with a high mortality and an enormous socio-economic burden 1. Despite intensive experimental and clinical scientific efforts throughout recent decades, treatment options for acute ischemic stroke patients remain very limited 2. In contrast to that, increase in the percentage of elderly people in developed countries will dramatically increase incidences and prevalences of patients with ischemic stroke in the next decades 3. Therefore, there is a pressing need for new treatment strategies in patients with ischemic stroke.
One approach is to gain further understanding in mechanisms of endogenous adaptation of brain to cope with a damaging stimulus. This brain-derived neuroprotection is also known as ischemic preconditioning (PC) or ischemic tolerance (IT) and describes a phenomenon, in which a non-damaging noxious stimulus applied to the brain, induces a transient resistance against a subsequent damaging ischemic insult 4. IT occurs in two different time windows: early IT, which occurs within minutes to a few hours after PC and delayed IT, which needs a latency of a couple of hours to occur 4.
So far, research on IT in brain has focused on delayed IT. Much less is known on the mechanisms of early IT. The objective of this study is to establish bilateral common carotid artery occlusion (BCCAO) which is an established stimulus to induce delayed IT, as an adequate PC stimulus to induce early IT to transient focal cerebral ischemia (induced by middle cerebral artery occlusion, MCAO) in C57Bl6/J mice.
During both surgical procedures (BCCAO and MCAO), monitoring of cerebral blood flow (CBF) by laser Doppler flowmetry (LDF) was performed.
BCCAO was performed in anesthetized and spontaneously breathing mice. Both common carotid arteries (CCA) were exposed and occluded for 60 sec followed by 5 min of reperfusion. This BCCAO/reperfusion sequence was repeated twice. After surgery, mice recovered quickly and showed no signs of functional impairment. In order to rule out, that the BCCAO protocol described above did not result in delayed cell death in the brain, we performed a TdT-mediated dUTP-biotin nick end labelling (TUNEL) staining 72 hr after BCCAO (or sham surgery) in a separate group.
MCAO is a widely established model for induction of focal cerebral ischemia in rodents 5-7. The surgical procedures were carried out using standardized operating procedures (SOP) 8. After exposing left CCA, a silicon-covered monofilament was introduced into the distal CCA, advanced via the internal carotid artery (ICA) into the Circle of Willis until the anterior cerebral artery (ACA) and thereby, the origin of the middle cerebral artery (MCA) was occluded for a defined period of time. In this study, we used 45 min of MCAO, which typically results in an ischemic lesion in the MCA territory involving striatal and cortical brain areas.
In our study, we analyzed the temporal profile of early IT using BCCAO as the PC stimulus. Our data indicated, that the optimal time delay between BCCAO and MCAO to induce early IT is 30 min.
In this video, we will give a demonstration of both surgical procedures, i.e. BCCAO and MCAO.
Protocol
All procedures described in this article were performed in accordance with the guidelines and regulations of the Landesamt für Gesundheit und Soziales, Berlin, Germany.
Pre-surgical procedures
1. Preparation of Monofilaments
For preparation of intraluminal filaments, 8-0 nylon monofilament sutures were used and were cut to a length of 13 mm each.
For coating of the monofilaments, Xantopren M mucosa, (Heraeus Kulzer GmbH, Hanau, Germany) was used activated by adding a drop of activator solution (Activator, Universal plus, Heraeus Kulzer GmbH, Hanau, Germany).
The monofilament was slowly pulled through the silicone until it was completely covered with it. By doing so, the monofilament would be evenly covered with silicone. Avoid waiting too long because the activated coating material will become firm and dry quickly.
Before the silicone starts to solidify, it is recommended to strip off excessive silicone from the filament by pulling it over a sheet of paper. By doing so, the silicone layer can be thinned out to an approximate thickness of 200 μm.
The base of the silicone-covered monofilament was stuck into modeling clay in order to let it dry overnight.
Comment: Ideally, only sterilized or at least disinfected monofilaments should be used to ensure sterility at the surgical site. In practice, sterilization or disinfection of the hand-made monofilaments is very difficult because the quality of the monofilaments may worsen 9. Therefore, it might be preferable to use commercially available sterile monofilaments instead.
2. Preparation of Surgical Instruments and of the Animals
All surgical instruments were kept in 70% ethyl alcohol. Additionally, prior to each new surgical procedure, all surgical instruments were carefully cleaned and disinfected using 70% ethyl alcohol.
The surgical area was thoroughly disinfected using 70% ethyl alcohol in all animals before transection. The disinfected sites were allowed to completely dry before performing the incisions. Additional clipping of the fur was not performed as this may cause microabrasions and subsequent skin inflammation 9.
Surgical procedures
3. Positioning of the LDF Probe and LDF Monitoring
For induction of anesthesia, mice were exposed to a gaseous mixture consisting of 30% oxygen, 70% N2O and 2.5% isoflurane using a vaporizer. For maintenance of anesthesia, isoflurane concentration was reduced to 1.5%. Mice were breathing spontaneously via breathing mask throughout the surgical procedure.
A rectal temperature probe was inserted and mice were fixed to the stereotactic frame exposing the parietal head. During surgery, mice were resting on a thermostat-controlled heating pad, ensuring a constant core temperature of 37.0 ± 0.5 °C. A sagittal midline incision (~1 cm length) was performed to expose the parietal skull.
The left side of the skull was carefully dissected and a fiberoptic probe was fixed directly to the skull 2 mm caudal and 5 mm lateral from bregma using instant glue. Afterwards, wound edges were carefully adapted to avoid brain hypothermia as well as infections.
The mouse was turned around to expose the ventral side of the neck. The fiberoptic probe was connected to the LDF device (PeriFLUX System 5000, Perimed, Järfälla, Sweden) via connecting probe adaptor (Master Probe 418-1, Perimed; Järfälla, Sweden).
Comment: While turning the mouse around, the fiberoptic probe should be bent with care in order to avoid that it breaks off from the skull.
The LDF device was connected to a laptop computer. Continuous recording of LDF values was initiated using PeriSoft for Windows (Version 2.50, Järfälla, Sweden). Figure 1 demonstrates the mean CBF course obtained from ten LDF recordings during BCCAO and reperfusion.
4. Bilateral Common Carotid Artery Occlusion
Anesthesia was maintained as described in 3.1. Mouse was placed on its back. The animal's tail and paws were fixed to the heating pad using adhesive tape. A sagittal ventral midline incision (~ 1cm length) was performed.
Both salivatory glands were carefully separated and mobilized to visualize the underlying CCA's.
Both CCA's were carefully separated from the respective vagal nerves and accompanying veins without harming these structures. Manipulations of the vagal nerves might lead to transient or permanent dysfunction of the parasympathetic nerve system, which has the potential for the occurrence of significant cardiac arrhythmia or even irreversible cardiac arrest. Therefore, it is crucial to avoid any manipulations of the vagal nerves. A loose 5-0 silk suture loop was made around each CCA.
A small silicon tubing (diameter 2 mm) was inserted in between the CCA and the silk sutures on each side. This tubing was used as a splinting of the CCA's in order to avoid damaging of the arterial walls when the sutures were tightened (Figure 4).
Both CCA's were occluded for one minute by tightening the silk sutures. Only mice, in which the LDF signal was reduced to <10% of the baseline signal were included in the study. After reopening of both CCA's, a five minutes reperfusion period was initiated. By LDF a complete reperfusion was ensured. Animals with incomplete reperfusion (i.e. <90 % recovery of CBF compared to baseline CBF) were excluded. The sequence of one minute of BCCAO and five minutes of reperfusion was repeated twice for a total BCCAO duration of 3 min. Figure 1 shows the mean CBF course obtained from ten LDF recordings during BCCAO and reperfusion. During the procedure, it is recommended to keep the neck wound wet using normal saline.
After the last reperfusion, all tubing and sutures as well as the fiberoptic probe attached to the skull were removed, the wounds were sutured and lidocaine ointment (Xylocain 2%; AstraZeneca GmbH, Wedel, Germany) was repeatedly applied to the wounds as needed for analgetic management.
Mice were transferred to a pre-heated (30 °C) recovery box (MediHEAT. PecoServives Ltd. Brough, Cumbria, UK) until they had regained complete consciousness. Thereafter, mice were returned to their animal cages.
5. Middle Cerebral Artery Occlusion
Mice were re-anesthetized, a rectal temperature probe was inserted and mice were positioned on a heating pad to expose the ventral side of the neck. Afterwards, they were fixed as described in the BCCAO section.
A permanent ligature using a long 7-0 silk suture was made around the left proximal CCA and the thread was gently pulled caudally using a mosquito clamp (Figure 4).
Another permanent ligature was made around the left external carotid artery (ECA) and the thread was pulled towards the right side of the animal (Figure 4).
Connective tissue surrounding the left ICA was carefully mobilized and the hypoglossal nerve was gently lifted to get a good overview of the distal course of ICA. The left ICA bends to the left while the pterygopalatine artery (PPA) follows the course of the proximal ICA to the right (Figure 4).
Comment: There is another artery to be identified which turns to the right in caudal direction. This is the occipital artery, which variably originates either from the CCA bifurcation or from the proximal part of the ECA (Figure 4).
A loose suture loop was made around the proximal ICA using a 5-0 silk thread.
Using a small microvascular clamp, the ICA was temporarily occluded distally to the 5-0 loose suture loop (Figure 4).
A small incision was made in the distal part of CCA just before the CCA bifurcation using microvascular scissors (Figure 4).
A preselected silicone-covered monofilament was introduced into the CCA via the small incision and pushed into the ICA until it stopped at the microvascular clamp (Figure 4).
The microclamp was carefully removed from the ICA, while the monofilament was advanced deeper into the ICA until it stopped (Figure 4). Comment: At this point, flip the forceps in the left hand so that you do not hold it like a pen. This helps to grab the filament with the forceps more easily. The vascular clamp-applicator should be held in the right hand. It might be necessary to nearly tighten the loose 5-0 suture around the ICA in order to prevent bleeding from the incision side.
The exact location of the monofilament tip was identified to check if it was advanced into the ICA and not into the PPA. If the monofilament tip was introduced into the proximal PPA, the filament was gently pulled back just before the ICA/PPA bifurcation. It was attempted to advance the monofilament into the ICA again, while gently pulling the proximal ICA a bit to the right side. By doing so, the natural course of the distal ICA was simulated making it easier to access the ICA.
After the monofilament was advanced into the ICA almost completely, the loose 5-0 suture loop around the proximal ICA was tightened in order to hold the filament in place. Afterwards, all silk threads were shortened and the skin wound was sutured.
The fluid loss during surgery was replenished by injecting 1.0 ml of normal saline solution i.p. Afterwards, the mouse was transferred to the pre-heated recovery box until it regained complete consciousness.
For reperfusion, mice were re-anaesthetized and the monofilament was removed after carefully loosening the 5-0 silk suture around the proximal ICA. Afterwards, the 5-0 suture was tightened again carefully in order to prevent bleeding.
After confirmation of complete hemostasis, the neck wound was sutured.
Lidocaine ointment was repeatedly applied locally to the skin as needed for pain relief and the mouse was transferred to the pre-heated (30 °C) recovery box until it regained complete consciousness.
As a first hint for a successful MCAO procedure, a clockwise circling of the animal was observed. The severity of the functional deficit was quantified using the Bederson score modified for mice 10. According to this modified Bederson score, neurological scores were graded as follows: 0 (normal motor function), 1 (flexion of torso and contralateral forelimb when mouse was lifted by the tail), 2 (circling to the contralateral side when mouse was held by the tail on a flat surface, but normal posture at rest), 3 (leaning to the contralateral side at rest), and 4 (no spontaneous motor activity or dead).
6. BCCAO Sham Surgery
For BCCAO sham procedures, mice were anaesthetized for the duration of a real BCCAO PC procedure, the salivation glands were mobilized and the CCA's were exposed. It is crucial that direct manipulations to the carotid arteries or vagal nerves are avoided during sham procedure. Manipulations of the CCA can damage its endothelial layer, which can result in a release of vasoactive compounds from endothelium or induce local thrombosis within the arterial lumen. As a consequence, thromboembolic events might occur after sham surgery. This would be a major confounder of the procedure. On the other hand, manipulations of the vagal nerves might lead to transient or permanent dysfunction of the parasympathetic nerve system, which has the potential for the occurrence of significant cardiac arrhythmia or even irreversible cardiac arrest. Therefore, it is crucial to avoid any manipulations of the vagal nerves during BCCAO.
Post-surgical procedures
7. Infarct Volume Measurements
For assessment of infarct sizes, mice survived for 72 hr after MCAO. Afterwards animals were deeply anesthetized, brains were removed, snap-frozen in methylbutan, sectioned and stained with hematoxylin (using Papanicolau's protocol) for infarct volume measurements. Brain sections were collected at 600 μm-intervals and infarct volumes were determined using an image analyzer (MCID core 7.0 Rev.2.0, GE Healthcare Niagara Inc) and corrected for edema according to the method of Lin et al. 7,11.
8. Inclusion Criteria
We included only animals which had a LDF-measured CBF reduction by more than 90% during each of the three BCCAO episodes. Ideally, LDF monitoring should be performed in both cerebral hemispheres during BCCAO. For practicability, we performed LDF monitoring only in the left hemispheres, which will be subjected to MCAO later on. This approach has been described before 6,12. Reperfusion after each BCCAO episode was required to be complete (i.e. return to baseline values). For reasons of reducing time duration mice were kept under anesthesia, we did not monitor CBF during MCAO surgery. In a pilot study with a separate group of animals however, we monitored CBF by LDF during MCAO surgery. In this group of mice, we consistently measured a reduction in CBF by 90% compared to baseline before CCA occlusion. Additionally, after removal of monofilaments CBF recovered to >90% of the respective baseline values after CCA occlusion. These LDF criteria are established in the literature and result in consistent infarct volumes 7. We only included animals which had a Bederson score of at least of 1 after recovery from anesthesia after MCAO. Furthermore, only animals which survived for 72 hr after MCAO and which showed no signs of intracranial hemorrhage were included in the study.
Representative Results
Our BCCAO protocol resulted in immediate and profound (>90%) CBF reductions when both CCA's were occluded (Figure 1). Reopening of the CCA's led to complete reperfusion as monitored by LDF (Figure 1). After PC, animals showed no signs of functional disability. Furthermore, there was no mortality. TUNEL staining performed 72 hr after BCCAO in a separate group of animals showed no signs of delayed cell death in the brain after BCCAO (data not shown).
When analyzing the temporal profile of the optimal time delay between BCCAO and MCAO for early IT (Figure 2), we found a significant reduction in the infarct volumes (reduction by 65%, n=10) compared to Sham group (n=10) when BCCAO was performed 30 min before MCAO. Furthermore, infarct volumes were reduced when BCCAO was performed 2 hr and 24 hr before MCAO (n=10). 24 hr represents the second (delayed) time window of IT 6.
Thus, it can be concluded that the optimal time window for early IT using BCCAO as a PC stimulus is 30 min before induction of transient focal cerebral ischemia (MCAO).
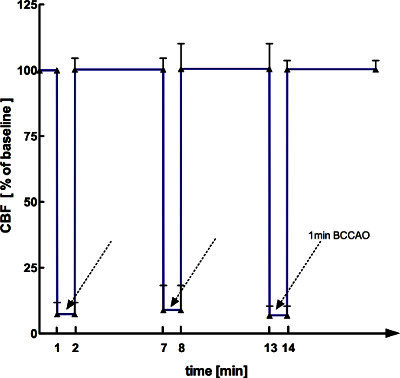 Figure 1. Course of mean CBF values measured by LDF during BCCAO and reperfusion (n=10).
Figure 1. Course of mean CBF values measured by LDF during BCCAO and reperfusion (n=10).
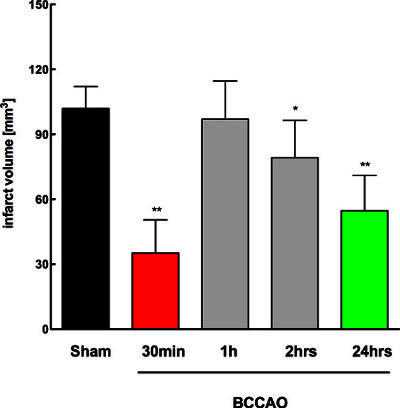 Figure 2. Temporal profile of infarct volumes at different time delays of MCAO after BCCAO n=10/group, graphs display mean ± standard deviation, * p< 0,05 from sham, ** p< 0,01 from sham (One-way ANOVA with Bonferroni's posthoc test).
Figure 2. Temporal profile of infarct volumes at different time delays of MCAO after BCCAO n=10/group, graphs display mean ± standard deviation, * p< 0,05 from sham, ** p< 0,01 from sham (One-way ANOVA with Bonferroni's posthoc test).
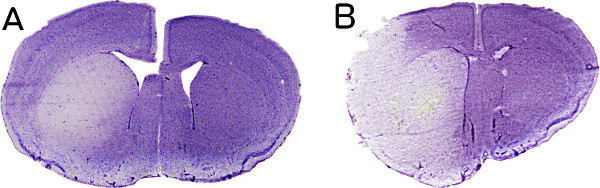 Figure 3. Representative hematoxylin-stained brain sections derived from a BCCAO-preconditioned (A) and a sham-operated animal (B), which were subjected to 45 min MCAO 30 min after BCCAO/sham. Brains were removed and processed 72 hr after MCAO.
Figure 3. Representative hematoxylin-stained brain sections derived from a BCCAO-preconditioned (A) and a sham-operated animal (B), which were subjected to 45 min MCAO 30 min after BCCAO/sham. Brains were removed and processed 72 hr after MCAO.
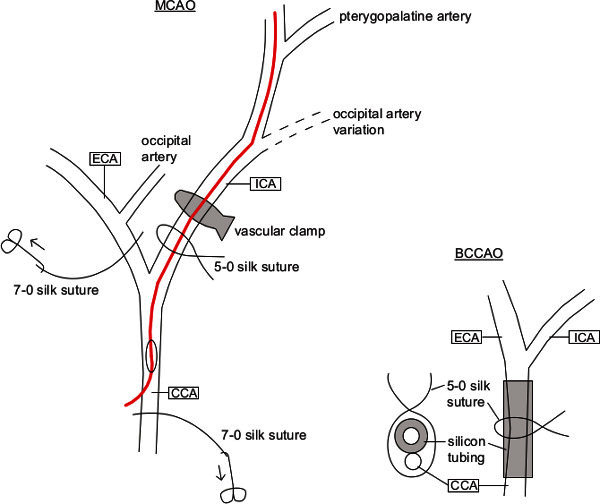 Figure 4. Scheme of the left carotid artery's vascular anatomy including the procedures performed for BCCAO and MCAO.
Figure 4. Scheme of the left carotid artery's vascular anatomy including the procedures performed for BCCAO and MCAO.
Discussion
In our study, we have shown that three sequences of BCCAO, each lasting for one minute and followed by reperfusion for five minutes, are an adequate ischemic PC stimulus to induce early IT.
Furthermore, we have demonstrated that the preconditioning protocol used is a safe and minor invasive procedure with no noteworthy postoperative morbidity, no signs of apoptosis in brain and no mortality. Several different preconditioning protocols have been described previously 6-7,12. Others have used 6 min of BCCAO as a preconditioning stimulus 13-14. However, due to the fact that one minute of BCCAO for three times resulted in a protective effect in the early time window of IT without any detectable ischemic brain injury, we decided to use this protocol.
For consistent results, duration of anesthesia and surgical procedures as well as other variables such as animal weights, brain temperatures during surgery, postsurgical fluid replenishment, postsurgical pain management as well as pre- and postsurgical cage enrichment should be standardized 15. Particular attention requires maintenance of normothermic conditions of the mice throughout the complete period of anesthesia because hypothermia itself has a preconditioning effect and can therefore interfere with the BCCAO effect 16. In awake and freely moving rodents, core temperatures are approximately between 0.5 and 1 degree Celsius higher than the corresponding brain temperatures 17. Resting brain temperatures in awake and freely moving rodents are approximately 36.5 degree Celsius 18. Since we did not measure brain temperatures in our experiments, we adjusted mice' core temperatures to 37.0 ± 0.5 degree Celsius to achieve brain temperatures which resemble the above mentioned resting brain temperatures.
Theoretically, use of volatile anesthetics itself can be an additional confounder because it has been demonstrated that isoflurane itself can induce IT. However, isoflurane anesthesia becomes a relevant PC stimulus only when administered repetitively for several hours which was not the case in the procedures described in the current study 19-21. Furthermore, we also analyzed Sham-operated animals to control for the potential confounding effect of isoflurane anesthesia.
The size and shape of the monofilament used for induction of MCAO plays a critical role. Evenly silicone covered filaments improve the success rate of MCAO and decrease the rate of complications such as subarachnoid hemorrhages and should therefore be preferred compared to heat blunted filaments 22. Another crucial point is the filament length. Optimal filament lengths between 9-11 mm are reported 15. Due to the fact that our incisions were made proximal to the CCA bifurcation, we used a monofilament length of 13 mm and a thickness of approximately 200 μm.
Taken together, the type and quality of monofilament's coating and its length are crucial to ensure a sufficient occlusion of the MCA and to avoid significant crossflow via anterior communicating artery. Substantial crossflow from the right side of the Circle of Willis results in an unwanted reduction of the infarct volume and thereby increases the variability of the results 23. Additional procedural standardizations with the intention to further reduce outcome variability include LDF-based CBF monitoring during MCAO and reperfusion and the use of commercially available industrial made, standardized monofilaments. By the use of commercially available monofilaments also their sterility can be ensured.
In addition, animals which do not have a >90% CBF reduction during BCCAO or MCAO, or which do not have a >90% reperfusion after re-opening of the arteries should be excluded from the study. This approach will help to further reduce variability in infarct volumes, which mainly occurs as a result of common variants in cerebral collateral circulation.
In summary, BCCAO is an established and easily applicable model to investigate effects of IT in early and delayed time windows. Studying the phenomenon of IT helps to get a better understanding of the mechanisms underlying endogenous protection in the brain. In perspective, this may help to develop alternative treatment opportunities for patients with acute ischemic stroke.
Disclosures
We have nothing to disclose.
References
- Roger VL, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaja AM, Grotta JC. Established treatments for acute ischaemic stroke. Lancet. 2007;369:319–330. doi: 10.1016/S0140-6736(07)60154-8. [DOI] [PubMed] [Google Scholar]
- Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T. Future demographic trends decrease the proportion of ischemic stroke patients receiving thrombolytic therapy: a call to set-up therapeutic studies in the very old. Stroke. 2009;40:1900–1902. doi: 10.1161/STROKEAHA.108.531061. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003;34:1299–1303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- Cho S, et al. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J. Cereb. Blood. Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- Kunz A, et al. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J. Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, et al. Standard operating procedures (SOP) in experimental stroke research: SOP for middle cerebral artery occlusion in the mouse. Nature Precedings. 2010.
- Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling stroke in mice - middle cerebral artery occlusion with the filament model. J. Vis. Exp. 2011. p. e2423. [DOI] [PMC free article] [PubMed]
- Bederson JB, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Kawano T, et al. iNOS-derived NO and nox2-derived superoxide confer tolerance to excitotoxic brain injury through peroxynitrite. J. Cereb. Blood Flow Metab. 2007;27:1453–1462. doi: 10.1038/sj.jcbfm.9600449. [DOI] [PubMed] [Google Scholar]
- Qi S, et al. Sublethal cerebral ischemia inhibits caspase-3 activation induced by subsequent prolonged ischemia in the C57Black/Crj6 strain mouse. Neurosci. Lett. 2001;315:133–136. doi: 10.1016/s0304-3940(01)02368-0. [DOI] [PubMed] [Google Scholar]
- Wu C, et al. A forebrain ischemic preconditioning model established in C57Black/Crj6 mice. J. Neurosci. Methods. 2001;107:101–106. doi: 10.1016/s0165-0270(01)00356-9. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhen G, Meloni BP, Campbell K, Winn HR. Rodent Stroke Model Guidelines for Preclinical Stroke Trials (1st Edition) J. Exp. Stroke Transl. Med. 2009;2:2–27. doi: 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio S, et al. Hypothermia-induced ischemic tolerance. Ann. N.Y. Acad. Sci. 1999;890:26–41. doi: 10.1111/j.1749-6632.1999.tb07978.x. [DOI] [PubMed] [Google Scholar]
- DeBow S, Colbourne F. Brain temperature measurement and regulation in awake and freely moving rodents. Methods. 2003;30:167–171. doi: 10.1016/s1046-2023(03)00080-x. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Sutherland GR, Auer RN. An automated system for regulating brain temperature in awake and freely moving rodents. J. Neurosci. Methods. 1996;67:185–190. [PubMed] [Google Scholar]
- Kapinya KJ, Prass K, Dirnagl U. Isoflurane induced prolonged protection against cerebral ischemia in mice: a redox sensitive mechanism? Neuroreport. 2002;13:1431–1435. doi: 10.1097/00001756-200208070-00017. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, et al. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth. Analg. 2003;96:233–237. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J. Neurosci. Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Zarow GJ, Karibe H, States BA, Graham SH, Weinstein PR. Endovascular suture occlusion of the middle cerebral artery in rats: effect of suture insertion distance on cerebral blood flow, infarct distribution and infarct volume. Neurol. Res. 1997;19:409–416. doi: 10.1080/01616412.1997.11740834. [DOI] [PubMed] [Google Scholar]


