Abstract
Background
Technical innovations in corneal transplantation have now made it possible to replace only the diseased part of the cornea, rather than the entire cornea as in penetrating keratoplasty (PKP). Patients with endothelial insufficiency due to Fuchs endothelial dystrophy, bullous keratopathy, or endothelial failure after keratoplasty can be treated with the new methods of posterior lamellar corneal transplantation: Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK). It remains unclear which of these methods is better in the individual case.
Methods
We review the pertinent literature retrieved by a selective search in Medline and the Cochrane Library employing the terms “DMEK,” “DSAEK,” “DSEK,” and “posterior lamellar keratoplasty.” The publications considered in this article are those that contain important clinical information on the operative techniques.
Results
No randomized controlled trials of these techniques have been published to date. Numerous case series have shown that patients who undergo DSAEK (postoperative visual acuity ≥0.5 in 38–100%), and especially those who undergo it in early or intermediate stages of endothelial insufficiency, achieve a better functional result more rapidly than patients treated with PKP (postoperative visual acuity ≥0.5 in 47–61%). Only 23–47% of DSAEK patients achieve a visual acuity of 0.8 or more, compared to 36–79% of DMEK patients. Moreover, transplant rejection is seen in only 1–3% of cases of DMEK, compared to 0–8% after DSAEK and 1–23% after PKP.
Conclusion
Numerous case series show clear advantages of DMEK over DSAEK, which, in turn, has better results than PKP. Nonetheless, randomized controlled trials are needed to determine which operative method is best in each stage of corneal disease.
The most frequent indications for corneal grafting include diseases of the corneal endothelium, such as Fuchs endothelial dystrophy, bullous keratopathy, and endothelial failure following keratoplasty. Fuchs endothelial dystrophy is a hereditary disorder of the corneal endothelium that affects women more often than men and advances through various stages over a period of years (1). First, central asymptomatic thickening (guttae) appears on the Descemet membrane, the basal membrane of the endothelium, at the inner surface of the cornea. As the disease progresses there is increasing corneal edema, leading to light sensitivity and blurred vision. This is followed by subepithelial vesicle formation (bullous keratopathy); the patient experiences severe pain when the bullae burst. Finally the corneal stroma becomes fibrotic, with irreversible loss of transparency. Among persons over the age of 40 years, up to 3.8% have cornea guttata and 0.1% have bullous keratopathy (e1). There may be other causes of bullous keratopathy, including post-inflammatory, post-traumatic, or postoperative endothelial damage. Since these processes frequently involve inflammation with pronounced loss of endothelial cells, the prognosis for corneal transplantation is limited (2).
The first corneal grafting procedure was carried out by Eduard Zirn (3, 4) in 1905. This was the so-called penetrating keratoplasty (PKP), in which typically all five layers of the cornea (epithelium, Bowman layer, stroma, Descemet membrane, endothelium) are transplanted (Figure 1a). Because the only layer affected by the above-mentioned diseases is the endothelium, as early as 1956 Tillet (5) proposed replacing only the rear part of the cornea (posterior lamellar keratoplasty). This was intended to avoid some of the problems that can occur after PKP, such as postoperative astigmatism and wound healing disorders. Although the technical principle of the operation could be implemented, the visual results proved unsatisfactory for the patients.
Figure 1.
Figure 1: Techniques of corneal grafting
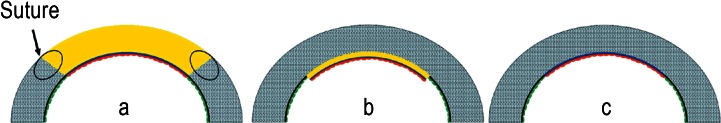
Penetrating keratoplasty: All layers of the central segment of the host cornea (cross-hatched) are replaced by a graft (yellow), held in place by a double-running cross-stitch suture.
Descemet stripping automated endothelial keratoplasty (DSAEK): The Descemet membrane (black) and the diseased endothelium (green) are the only layers of the host cornea removed. A graft comprising stroma (yellow), Descemet membrane (blue), and endothelium (red) is then applied to the posterior surface of the host cornea.
Descemet membrane endothelial keratoplasty (DMEK): The Descemet membrane (black) and the diseased endothelium (green) are removed and replaced by a graft of Descemet membrane (blue), and endothelium (red), restoring the original anatomical situation. Only the diseased part of the cornea is excised and replaced.
Descemet stripping automated endothelial keratoplasty (DSAEK)
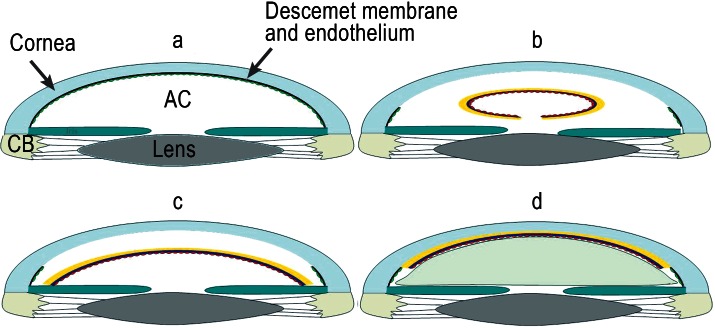
A crucial advance in surgical technique came with the introduction of descemetorhexis (6), in which the patient’s corneal stroma is left in place and only the Descemet membrane and the diseased endothelium are removed (7). The results improve on those of previous procedures because the graft can be laid onto a smooth surface. The graft material, comprising stroma, Descemet membrane, and endothelium, is introduced into the anterior chamber of the eye and pressed against the posterior surface of the host cornea by means of an air bubble, with no need for sutures (Figures 1b, 2). This technique is termed Descemet stripping endothelial keratoplasty (DSEK). A major problem with DSEK is the intricate manual preparation (e2) of the lamellar graft, often resulting in irreparable damage to the material intended for transplantation (8). Standardization of the graft preparation process was achieved with the introduction of the microkeratome, which is used to shave away a 400 to 450 µm layer from the anterior surface of the donor corneal stroma; the lamellar material for transplantation, 80 to 150 µm thick, can then be cut with a trephine (Descemet stripping automated endothelial keratoplasty, DSAEK). As a closed-system method, DSAEK can be performed under local anesthesia better than PKP (e3). Despite the advantages of DSAEK (suture-free transplantation, rapid improvement of vision with no change in refraction) the anticipated increase in visual acuity is not always attained and the results do not meet expectations (see Results).
Figure 2.
Figure 2: Descemet stripping automated endothelial keratoplasty (DSAEK)
Anterior part of the eye with diseased endothelium on Descemet membrane. CB = ciliary body, AC = anterior chamber
Introduction of a graft comprising a thin stromal layer (yellow), Descemet membrane (blue), and healthy donor endothelium (red) into the anterior chamber after removal of the diseased Descemet membrane and endothelium
Unfolding of the graft
Adaptation of the graft to the host cornea by gradual expansion of an air bubble (green) in the anterior chamber
Descemet membrane endothelial keratoplasty (DMEK)
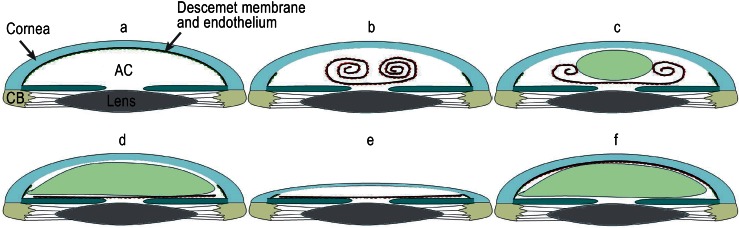
Descemet membrane endothelial keratoplasty (DMEK) is a modification of DSAEK in which the grafted material includes no corneal stroma at all (Figures 1c, 3), avoiding bothersome optical phenomena from the convergence of host and donor stromal fibers (9). The graft, comprising Descemet membrane and endothelium, is peeled off the donor cornea manually using fine tweezers. Macroscopically invisible collagen fibers extending from the donor stroma into the Descemet membrane may cause tears in the graft, which is only 15 µm thick (10). For example, Price et al. reported that 4.2% to 8% of grafts could not be prepared successfully (11, 12). Meanwhile, however, well-established preparation techniques achieve success rates of over 95% (13). The graft material rolls up after peeling, always with the endothelium on the outside. The graft can thus be introduced into the anterior chamber through a small incision and applied to the posterior stroma of the host cornea by means of an air bubble (Figure 3).
Figure 3.
Figure 3: Descemet membrane endothelial keratoplasty (DMEK)
Anterior part of the eye with diseased endothelium on Descemet membrane. CB = ciliary body, AC = anterior chamber
The rolled graft is introduced into the anterior chamber by means of conventional lens injection systems or pipettes after removal of the diseased Descemet membrane and endothelium; the endothelium (red) is always on the outside.
An air bubble is used to slowly unroll the graft until it lies against the iris.
The fully unrolled graft
Next, the bubble of air is removed from the anterior chamber; this causes the anterior chamber to collapse so that the graft cannot roll up again.
Finally the graft is carefully pressed against the posterior surface of the host cornea by inflation of a second air bubble, this time underneath the graft, until contact is complete.
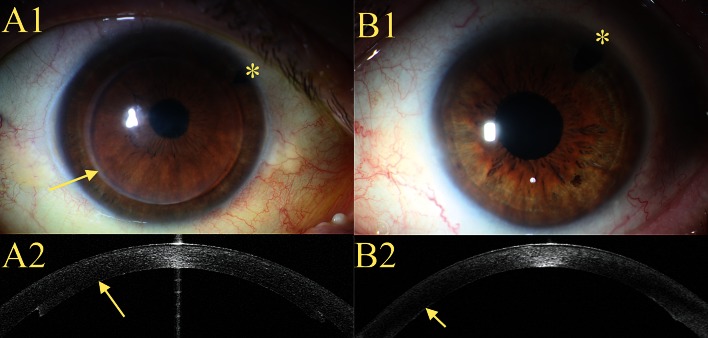
Because neither donor nor host stromal fibers are manipulated, DMEK can achieve optimal visual results (Figure 4, B2). However, neither the preparation nor the implantation and unfolding of the graft material is standardized to the same extent as with DSAEK (14).
Figure 4.
Clinical images after DSAEK (A1–A2) and DMEK (B1–B2)
A1) The central cornea is optically clear; the margin of the graft is readily discernible (arrow)
A2) Optical coherence tomography of the anterior chamber of the eye after DSAEK; the graft of stroma, Descemet membrane, and endothelium can clearly be recognized on the posterior surface of the host cornea (arrow).
B1) The whole cornea is optically clear; the margin of the graft cannot be discerned.
B2) Optical coherence tomography of the anterior chamber of the eye after DMEK; the graft of Descemet membrane and endothelium can barely be distinguished from the host cornea (arrow).
*Peripheral iridectomy to avoid an episode of closed-angle glaucoma
Aim
Penetrating keratoplasty has recently given way to various posterior lamellar keratoplasty techniques in the treatment of patients with endothelial insufficiency. Our aim in writing this article was to provide a critical overview of the advantages and disadvantages of the current transplantation techniques. We reviewed all studies from Medline and the Cochrane Library that report relevant clinical data on the respective surgical procedures.
Methods
We conducted a selective literature search of Medline and the Cochrane Library using the following search terms:
Posterior lamellar keratoplasty (876 hits)
Descemet stripping automated endothelial keratoplasty (359 hits)
Descemet membrane endothelial keratoplasty (555 hits)
DSAEK (308 hits)
DMEK (71 hits)
Inspection of the headings reduced the total of 777 hits to 200 studies, of which 115 were excluded after perusal of the abstracts. The remaining 85 studies were analyzed more closely, and 70 were judged to contain important clinical information and therefore included in our review. These 70 studies included one Cochrane review and nine other reviews; the remaining 60 publications comprised nonrandomized cohort studies, some of them controlled, and individual case reports.
Results
Functional outcome
On average, improvement of visual acuity is achieved more quickly after DSAEK than after PKP (15– 17, e4). In a review by Anshu et al., visual acuity of ≥ 0.5 was attained by 38% to 100% of patients within 3 to 6 months, compared with 47% to 65% after 2 to 8 years for PKP. However, a higher proportion of patients eventually reach visual acuity of 1.0 following PKP (18). Only 12% to 23% of patients treated by DSAEK achieve visual acuity of ≥ 0.8 despite an optically clear cornea and an absence of vision-limiting disease (personal data, [19]), although this figure may rise as high as 47% with extended observation (20). The reason for this may be optically troublesome irregularities at the interface between graft and host cornea. These phenomena manifest as increased reflectivity of the interface on Scheimpflug images (Heinzelmann et al., under review), which may be caused by convergence of differently oriented host and donor collagen fibers. Finally, the disease stage could play a part: Fibrotic remodeling processes lead to decreased transparency of the cornea and do not regress after lamellar grafting. Nevertheless, no conclusive explanation for the reduced visual acuity in the presence of a clear cornea following DSAEK has yet been provided (21).
DMEK does not involve transplantation of the stromal layer, so the optical irregularities described above for DSAEK would not be expected to occur to the same extent. This is indeed reflected in the visual results. In the largest case series to date, 98% of 221 patients had achieved visual acuity of ≥ 0.5 with virtually unchanged refraction by 6 months after DMEK (79% ≥ 0.8, 46% ≥ 1.0, 14% ≥ 1.2) (22). In two case series from Erlangen, Germany, visual acuity of ≥ 0.8 was attained by 50% to 75% of patients within 6 months after DMEK (23), but the corresponding proportion after DSAEK was only 6% (24). At the University Eye Hospital in Freiburg, Germany, retrospective comparison revealed that 36% of patients achieved visual acuity of ≥ 0.8 within 3 months after DMEK, compared with 26% by 23 months after PKP and 12% by 8 months after DSAEK. The mean visual acuity of the last 100 patients treated with DMEK at the University Eye Hospital Cologne, Germany was 0.5 at 1 month after operation, 0.7 at 6 months, and 0.8 at 1 year (Heindl et al., submitted).
However, posterior lamellar keratoplasty is not indicated at all stages of endothelial insufficiency. For example, Sharma et al. preferred PKP in patients with advanced visual impairment (e.g., transplantation more than a year after cataract extraction or in patients with visual acuity of <0.06) (25). Moreover, PKP may be superior to the lamellar procedures in eyes with extremely complex anterior pathology (silicone oil-filled eyes, large defects of the iris, etc.) (26).
Patient satisfaction
Although the mean visual acuity after DSAEK is about 0.5 (27), contrast sensitivity is increased, so the patients have an impression of improved vision even if full acuity is not attained postoperatively (e5). A survey of patients treated by DSAEK showed high rates of overall satisfaction and satisfaction with the achieved postoperative visual acuity and the progress of the healing process (28). Furthermore, a retrospective survey of patients treated with PKP in one eye and DSAEK in the other showed that they would all opt for DSAEK if they needed surgery again (29). In contrast, 57% of 15 patients in Freiburg said they would prefer PKP, against 36% who would choose DSAEK, although subjective improvement in visual acuity occurred sooner after DSAEK than after PKP in 71% of cases in this survey (Gross et al., submitted).
The better functional results after DMEK are reflected in the subjective satisfaction rates. In a comparative retrospective case series, 85% of patients stated they were more satisfied with visual quality after DMEK than after DSAEK (19).
Complications
Graft dislocation and renewed air injection into anterior chamber (rebubbling)
Secondary interventions are necessary more often after DSAEK and DMEK than after PKP (15, 30). Most of these cases merely involve repeat air instillation after dislocation of the graft. This may be required in 1% to 82% of cases following DSAEK (27). Rebubbling after graft dislocation is necessary more often after DMEK (33% to 81%) than after DSAEK (7% to 20%) (12, 24). The dislocation rate is lower with grafts from organ culture, as mostly used in Europe, than with grafts from short-term culture, as predominantly used in the USA (e6). With regard to postoperative supine positioning of the patient so that the air bubble in the anterior chamber presses the graft against the posterior stroma, it has been shown that the patient’s position (supine or upright) has no influence on the graft dislocation rate if the anterior chamber is completely filled with air for at least 2 hours (31). This is important in view of the risk of position-related deep vein thrombosis and resulting pulmonary embolism. Patients should therefore be mobilized soon after operation or should receive prophylactic anticoagulant medication if indicated.
Graft failure
The rates of primary graft failure (Table) are higher for DSAEK (0 to 29%) and DMEK (0 to 9%) than for PKP (0 to 3%). Five years after DSAEK—the longest period of postoperative observation to date—92% of the grafts were clear (32). In our own experience, the rate of regrafting in the first 12 months postoperatively was higher after DSAEK, at up to 10%, than after PKP. The reason is not always endothelial graft failure; sometimes patients are not satisfied with the achieved visual acuity. The rate of primary graft failure seems to be falling sharply, however, as more experience of these surgical techniques is gained. No case of primary graft failure was observed, for example, among the last 100 patients treated with DMEK at the University Eye Hospital Cologne.
Table. Summary of the complications after PKP. DSAEK. and DMEK: complication rates and references.
| PKP | DSAEK | DMEK | References | |
| Risk of rejection within 2 years | 0.5−23.3% | 0–14% | 1–3% | PKP: |
| (15) (30) (39) (e16– e22) | ||||
| DSAEK: | ||||
| (11) (15) (19) (30) (33) (39) (e13) (e16) (e21) (e22) | ||||
| DMEK: | ||||
| (11) (e22) (e23) | ||||
| Primary graft failure | 0–3% | 0–29% | 0–9% | PKP: |
| (15) (30) | ||||
| DSAEK: | ||||
| (15) (19) (27) (30) (33) (37) (39) (e4) (e12) (e13) (e24) | ||||
| DMEK | ||||
| (11) (12) (e23) (e25) (e26) | ||||
| Graft dislocation/rebubbling | n.a. | 0–82% | 31–81% | DSAEK: |
| (15) (19) (24) (33) (37) (39) (e4) (e12) (e13) (e23) | ||||
| (e27– e29) | ||||
| DMEK: (11) (24) (12) (e25) (e23) (e30) | ||||
| Suture complications | 6.9% | n.a. | n.a. | PKP: |
| (e9) | ||||
| Episode of glaucoma | n.d. | 0.1–9.5% | n.d. | DSAEK: |
| (e12) (e13) (27) | ||||
| Epithelial invasion | n.a. | 0.8–1.6% | n.d. | DSAEK: |
| (e12) (e13) | ||||
| Infections | 0.8–6.9% | 0.8–1.5% | n.d. | PKP: |
| (e17) (e9) | ||||
| DSAEK: | ||||
| (38) (e15) (e31) (e9) (e12) |
n.a., not applicable; n.d., no data; PKP, penetrating keratoplasty; DSAEK, Descemet stripping automated endothelial keratoplasty; DMEK, Descemet membrane endothelial keratoplasty
Immune reaction
Endothelial immune reaction remains the most frequent cause of graft failure after PKP (e7). Recent studies seem to show that the risk of rejection within 2 years of grafting is 0 to 23% after PKP, 0 to 14% after DSAEK, and 1 to 3% after DMEK (Table). Apparently the risk of rejection increases with the amount of tissue transplanted. The postoperative treatment also plays an important part. The one single endothelial rejection reaction we have observed at the University Eye Hospital Freiburg among nearly 200 DMEK procedures to date occurred in a patient in whom topical steroids were discontinued much too soon. No endothelial immune reactions have yet been observed in the last 150 patients undergoing DMEK at the University Eye Hospital Cologne, where topical steroids are prescribed for a year after operation.
Endothelial cell loss
The loss of endothelial cells in the early postoperative period is much higher after DSAEK/DMEK than after PKP, owing to the intraoperative manipulation of the graft. The extent of primary endothelial cell loss seems to depend on the experience of the surgeon (e8). The mean endothelial cell loss 6 months after DSAEK is 36% (33). Three years after surgery, however, the mean endothelial cell loss was 39% to 46% for DSAEK and 47% to 51% for PKP (34, e9), so the chronic endothelial cell loss seems lower after DSAEK, and the endothelial cell density values converge 5 years after operation (30, 35, e10). Comparison of endothelial cell loss 6 months after operation showed rates of 41% for DMEK and 39% for DSAEK (24). The endothelial cell loss also seems slower after DMEK than after PKP, so that the endothelial cell densities are almost identical by 2 years after surgery (23). The longest postoperative observation period reported to date is 5 years; loss of endothelial cells was high early after DMEK but around 7% per year thereafter (36).
Rare complications
DSAEK and DMEK are associated with other characteristic intra- and postoperative problems. For instance, Afshari et al. reported eight cases in which the graft dislocated into the vitreous cavity during DSAEK, necessitating retransplantation (e11). In 0.8% to 9.5% of cases filling of the anterior chamber with air displaces the pupil and thus gives rise to an acute episode of glaucoma (27, e12, e13); this can usually be avoided by sufficiently extensive iridectomy. There are also case reports of Urrets–Zavalia syndrome after DSAEK, with irreversible postoperative widening of the pupil (37, e14). Furthermore, in 0.8% to 1.7% of cases DSAEK is followed by epithelial invasion of the interface between donor lamella and host cornea, frequently necessitating regrafting (27, e12, e13). Finally, a number of cases of microbial infection at the interface have been reported, most of which could be cured only by means of PKP (38, e15).
Perspective
Numerous case series show that good visual results are achieved more quickly after posterior lamellar keratoplasty than after PKP and without any change in refraction. Over the past few years the high degree of standardization of DSAEK has made it the standard procedure worldwide for patients with endothelial insufficiency, despite the not always satisfactory visual results. Although DMEK yields better visual results and involves a lower risk of rejection, the lack of technical standardization and the possibility of preparation-related graft loss mean that it cannot compete with DSAEK; DMEK is currently offered at only a small number of corneal surgery centers. It seems only a matter of time, however, before DMEK is routinely offered at all centers, because commercially available instrument sets, pre-prepared grafts, and numerous training courses are constantly increasing the degree of standardization (26). However, DSAEK will remain the procedure of choice in certain situations (e.g., in aphakia, aniridia, or pronounced anterior synechiae) (18). In comparison with PKP, DSAEK and DMEK have higher rates of reoperation (mostly repeat air instillation) and primary transplant failure. PKP also seems superior to the lamellar techniques in advanced disease with fibrotic remodeling of the corneal stroma. To date there have been no long-term studies to permit sufficiently confident estimates of the overall survival rate of lamellar grafts compared with PKP. For these reasons Nanavaty and Shortt (39) conclude that randomized controlled trials are required to decide which operation is best for which individual patient in the long term.
Key Messages.
In recent years conventional penetrating keratoplasty has given way to new posterior lamellar keratoplasty techniques for selective replacement of the diseased posterior layers of the cornea in patients with endothelial insufficiency.
Descemet stripping automated endothelial keratoplasty (DSAEK) has established itself as the standard technique worldwide, because patients achieve satisfactory visual acuity more quickly than after penetrating keratoplasty and without changes in refraction.
Descemet membrane endothelial keratoplasty (DMEK) is a more demanding surgical technique but can yield functional results much better than those attained with DSAEK or penetrating keratoplasty.
DMEK seems to be followed by immune reactions much less frequently than DSAEK or penetrating keratoplasty, but the rate of early graft failure can be higher.
It can be assumed that increasing standardization of surgical technique will lead to DMEK becoming the standard procedure in the near future; only nonrandomized cohort studies have yet been published, however, and randomized controlled trials seem necessary to establish which procedure is best at which stage of disease.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Prof. Reinhard has received payment from med update for the preparation of scientific seminars.
Prof. Cursiefen has received payment from Santen for acting as a consultant and for preparing scientific seminars.
PD Dr. Maier declares that no conflict of interest exists.
References
- 1.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 2.Böhringer D, Böhringer S, Poxleitner K, et al. Long-term graft survival in penetrating keratoplasty: the biexponential model of chronic endothelial cell loss revisited. Cornea. 2010;29:1113–1117. doi: 10.1097/ICO.0b013e3181d21d07. [DOI] [PubMed] [Google Scholar]
- 3.Zirm EK. A successful total keratoplasty. Refract Corneal Surg. 1989;5:258–261. [PubMed] [Google Scholar]
- 4.Cursiefen C, Seitz B, Kruse F. Hornhauttransplantation: Glänzende Bilanz und viele Perspektiven. Dtsch Arztebl. 2005;102(45) [Google Scholar]
- 5.Tillet C. Posterior lamellar keratoplasty. Am J Ophthalmol. 1956;41:530–533. doi: 10.1016/0002-9394(56)91269-7. [DOI] [PubMed] [Google Scholar]
- 6.Melles GRJ, Wijdh RHJ, Nieuwendaal CP. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis) Cornea. 2004;23:286–288. doi: 10.1097/00003226-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Heindl LM, Hofmann-Rummelt C, Schlötzer-Schrehardt U, Kruse FE, Cursiefen C. Histologic analysis of descemet stripping in posterior lamellar keratoplasty. Arch Ophthalmol. 2008;126:461–464. doi: 10.1001/archophthalmol.2007.75. [DOI] [PubMed] [Google Scholar]
- 8.Price MO, Price FW., Jr Descemet’s stripping with endothelial keratoplasty: comparative outcomes with microkeratome-dissected and manually dissected donor tissue. Ophthalmology. 2006;113:1936–1942. doi: 10.1016/j.ophtha.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Melles GRJ, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK) Cornea. 2006;25:987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 10.Schlötzer-Schrehardt U, Bachmann BO, Laaser K, Cursiefen C, Kruse FE. Characterization of the cleavage plane in DESCemet’s membrane endothelial keratoplasty. Ophthalmology. 2011;118:1950–1957. doi: 10.1016/j.ophtha.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Price MO, Giebel AW, Fairchild KM, Price FW Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118:2368–2373. doi: 10.1016/j.ophtha.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Kruse FE, Laaser K, Cursiefen C, et al. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea. 2011;30:580–587. doi: 10.1097/ico.0b013e3182000e2e. [DOI] [PubMed] [Google Scholar]
- 14.Heinzelmann S, Maier P, Reinhard T. Perspectives of posterior lamellar keratoplasty. In search of the perfect lamella. Ophthalmologe. 2011;108:825–832. doi: 10.1007/s00347-011-2330-0. [DOI] [PubMed] [Google Scholar]
- 15.Bahar I, Kaiserman I, McAllum P, Slomovic A, Rootman D. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115:1525–1533. doi: 10.1016/j.ophtha.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Cursiefen C, Kruse FE. Descemet’s stripping with automated endothelial keratoplasty (DSAEK) Ophthalmologe. 2008;105:183–190. doi: 10.1007/s00347-007-1680-0. [DOI] [PubMed] [Google Scholar]
- 17.Busin M. DSAEK for the treatment of endothelial disease: results in the initial 100 cases. Klin Monbl Augenheilkd. 2009;226:757–760. doi: 10.1055/s-0028-1109545. [DOI] [PubMed] [Google Scholar]
- 18.Anshu A, Price MO, Tan DTH, Price FW., Jr Endothelial keratoplasty: a revolution in evolution. Surv Ophthalmol. 2012;57:236–252. doi: 10.1016/j.survophthal.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2011;30:1382–1386. doi: 10.1097/ICO.0b013e31821ddd25. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Terry MA, Goshe J, Davis-Boozer D, Shamie N. Three-year visual acuity outcomes after Descemet’s stripping automated endothelial keratoplasty. Ophthalmology. 2012;119:1126–1129. doi: 10.1016/j.ophtha.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Maier P, Reinhard T. Keratoplasty: laminate or penetrate? Part 2: lamellar keratoplasty. Ophthalmologe. 2009;106:649–662. doi: 10.1007/s00347-009-1943-z. quiz 663. [DOI] [PubMed] [Google Scholar]
- 22.van Dijk K, Ham L, Tse WHW, Liarakos VS, Quilendrino R, Yeh RY, et al. Near complete visual recovery and refractive stability in modern corneal transplantation: Descemet membrane endothelial keratoplasty (DMEK) Cont Lens Anterior Eye. 2013;36:13–21. doi: 10.1016/j.clae.2012.10.066. [DOI] [PubMed] [Google Scholar]
- 23.Cursiefen C, Kruse FE. DMEK: Descemet membrane endothelial keratoplasty. Ophthalmologe. 2010;107:370–376. doi: 10.1007/s00347-010-2155-2. [DOI] [PubMed] [Google Scholar]
- 24.Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153:1082–1090e2. doi: 10.1016/j.ajo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Sharma N, Sachdev R, Pandey RM, Titiyal JS, Sinha R, Tandon R, et al. Study of factors for unsuitability of DSAEK in cases of corneal decompensation following cataract surgery. Int Ophthalmol. 2012;32:313–319. doi: 10.1007/s10792-012-9521-9. [DOI] [PubMed] [Google Scholar]
- 26.Cursiefen C. Taming of the Shrew. JAMA Ophthalmol. 2013;131:88–89. doi: 10.1001/jamaophthalmol.2013.609. [DOI] [PubMed] [Google Scholar]
- 27.Arenas E, Esquenazi S, Anwar M, Terry M. Lamellar corneal transplantation. Surv Ophthalmol. 2012;57:510–529. doi: 10.1016/j.survophthal.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann BO, Pogorelov P, Kruse FE, Cursiefen C. Patient satisfaction after posterior lamellar keratoplasty (DSAEK) Klin Monbl Augenheilkd. 2008;225:577–581. doi: 10.1055/s-2008-1027499. [DOI] [PubMed] [Google Scholar]
- 29.Bahar I, Kaiserman I, Levinger E, Sansanayudh W, Slomovic AR, Rootman DS. Retrospective contralateral study comparing descemet stripping automated endothelial keratoplasty with penetrating keratoplasty. Cornea. 2009;28:485–488. doi: 10.1097/ICO.0b013e3181901df4. [DOI] [PubMed] [Google Scholar]
- 30.Price MO, Gorovoy M, Benetz BA, et al. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117:438–444. doi: 10.1016/j.ophtha.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saethre M, Drolsum L. The role of postoperative positioning after DSAEK in preventing graft dislocation. Acta Ophthalmol. 2012;19 doi: 10.1111/j.1755-3768.2012.02560.x. Doi: 10.1111/j.1755-3768.2012.02560.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Ratanasit A, Gorovoy MS. Long-term results of Descemet stripping automated endothelial keratoplasty. Cornea. 2011;30:1414–1418. doi: 10.1097/ICO.0b013e31820ca34b. [DOI] [PubMed] [Google Scholar]
- 33.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–1830. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Price MO, Gorovoy M, Price FW, Jr, Benetz BA, Menegay HJ, Lass JH. Descemet’s Stripping automated endothelial keratoplasty: Three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology. 2013;120(2):246–251. doi: 10.1016/j.ophtha.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SV. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp Eye Res. 2012;95:40–47. doi: 10.1016/j.exer.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baydoun L, Tong CM, Tse WW, et al. Endothelial cell density after descemet membrane endothelial keratoplasty: 1 to 5-year follow-up. Am J Ophthalmol. 2012;154:762–763. doi: 10.1016/j.ajo.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Heindl LM, Kruse FE, Cursiefen C. Complications after posterior lamellar keratoplasty (DSAEK): prevention, detection and treatment. Klin Monbl Augenheilkd. 2010;227:478–482. doi: 10.1055/s-0029-1245447. [DOI] [PubMed] [Google Scholar]
- 38.Kymionis GD, Plaka AD, Limnopoulou AN, Rallis KI, Grentzelos MA, Ziakas N. Interface lamellar keratitis induced by a post-descemet stripping automated endothelial keratoplasty. Cornea. 2013;32:362–364. doi: 10.1097/ICO.0b013e3182656866. [DOI] [PubMed] [Google Scholar]
- 39.Nanavaty MA, Shortt AJ. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD008420.pub2. CD008420. [DOI] [PubMed] [Google Scholar]
- e1.Lorenzetti DW, Uotila MH, Parikh N, Kaufman HE. Central cornea guttata. Incidence in the general population. Am J Ophthalmol. 1967;64:1155–1158. [PubMed] [Google Scholar]
- e2.Price FW, Jr, Price MO. Descemet’s stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J Refract Surg. 2005;21:339–345. doi: 10.3928/1081-597X-20050701-07. [DOI] [PubMed] [Google Scholar]
- e3.Fang JP, Hamill MB. Descemet’s stripping endothelial keratoplasty under topical anesthesia. J Cataract Refract Surg. 2007;33:187–188. doi: 10.1016/j.jcrs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- e4.Chen ES, Terry MA, Shamie N, Hoar KL, Friend DJ. Descemet-stripping automated endothelial keratoplasty: six-month results in a prospective study of 100 eyes. Cornea. 2008;27:514–520. doi: 10.1097/ICO.0b013e3181611c50. [DOI] [PubMed] [Google Scholar]
- e5.Nielsen E, Hjortdal J. Visual acuity and contrast sensitivity after posterior lamellar keratoplasty. Acta Ophthalmol. 2012;90:756–760. doi: 10.1111/j.1755-3768.2011.02218.x. [DOI] [PubMed] [Google Scholar]
- e6.Laaser K, Bachmann BO, Horn FK, Schlötzer-Schrehardt U, Cursiefen C, Kruse FE. Donor tissue culture conditions and outcome after descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2011;151:1007–1018e2. doi: 10.1016/j.ajo.2010.11.027. [DOI] [PubMed] [Google Scholar]
- e7.Claesson M, Armitage WJ, Fagerholm P, Stenevi U. Visual outcome in corneal grafts: a preliminary analysis of the Swedish Corneal Transplant Register. Br J Ophthalmol. 2002;86:174–180. doi: 10.1136/bjo.86.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Koenig SB, Covert DJ, Dupps WJ, Jr, Meisler DM. Visual acuity, refractive error, and endothelial cell density six months after Descemet stripping and automated endothelial keratoplasty (DSAEK) Cornea. 2007;26:670–674. doi: 10.1097/ICO.0b013e3180544902. [DOI] [PubMed] [Google Scholar]
- e9.Ang M, Mehta JS, Lim F, Bose S, Htoon HM, Tan D. Endothelial cell loss and graft survival after descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2012;119:2239–2244. doi: 10.1016/j.ophtha.2012.06.012. [DOI] [PubMed] [Google Scholar]
- e10.Dooren BTHV, Saelens IEY, Bleyen I, Mulder PGH, Bartels MC, Rij GV. Endothelial cell decay after descemet’s stripping automated endothelial keratoplasty and top hat penetrating keratoplasty. Invest Ophthalmol Vis Sci. 2011;52:9226–9231. doi: 10.1167/iovs.11-8107. [DOI] [PubMed] [Google Scholar]
- e11.Afshari NA, Gorovoy MS, et al. Dislocation of the donor graft to the posterior segment in descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153:638–642. doi: 10.1016/j.ajo.2011.09.006. 642.e1-2. [DOI] [PubMed] [Google Scholar]
- e12.Shih CY, Ritterband DC, Rubino S, et al. Visually significant and nonsignificant complications arising from Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2009;148:837–843. doi: 10.1016/j.ajo.2009.06.034. [DOI] [PubMed] [Google Scholar]
- e13.Suh LH, Yoo SH, Deobhakta A, et al. Complications of Descemet’s stripping with automated endothelial keratoplasty: survey of 118 eyes at One Institute. Ophthalmology. 2008;115:1517–1524. doi: 10.1016/j.ophtha.2008.01.024. [DOI] [PubMed] [Google Scholar]
- e14.Fournié P, Ponchel C, Malecaze F, Arné JL. Fixed dilated pupil (urrets-zavalia syndrome) and anterior subcapsular cataract formation after descemet stripping endothelial keratoplasty. Cornea. 2009;28:1184–1186. doi: 10.1097/ICO.0b013e31819aaa13. [DOI] [PubMed] [Google Scholar]
- e15.Yamazoe K, Den S, Yamaguchi T, Tanaka Y, Shimazaki J. Severe donor-related Candida keratitis after Descemet’s stripping automated endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2011;249:1579–1582. doi: 10.1007/s00417-011-1710-0. [DOI] [PubMed] [Google Scholar]
- e16.Allan BDS, Terry MA, Price FW, Jr, Price MO, Griffin NB, Claesson M. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26:1039–1042. doi: 10.1097/ICO.0b013e31812f66e5. [DOI] [PubMed] [Google Scholar]
- e17.Pineros O, Cohen EJ, Rapuano CJ, Laibson PR. Long-term results after penetrating keratoplasty for Fuchs’ endothelial dystrophy. Arch Ophthalmol. 1996;114:15–18. doi: 10.1001/archopht.1996.01100130013002. [DOI] [PubMed] [Google Scholar]
- e18.Reinhard T, Böhringer D, Hüschen D, Sundmacher R. Chronic endothelial cell loss of the graft after penetrating keratoplasty: influence of endothelial cell migration from graft to host. Klin Monbl Augenheilkd. 2002;219:410–416. doi: 10.1055/s-2002-32876. [DOI] [PubMed] [Google Scholar]
- e19.Seitz B, Langenbucher A, Diamantis A, Cursiefen C, Küchle M, Naumann GO. Immunological graft reactions after penetrating keratoplasty—A prospective randomized trial comparing corneal excimer laser and motor trephination. Klin Monbl Augenheilkd. 2001;218:710–719. doi: 10.1055/s-2001-18662. [DOI] [PubMed] [Google Scholar]
- e20.Thompson RW, Jr, Price MO, Bowers PJ, Price FW., Jr Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- e21.Hjortdal J, Pedersen IB, Bak-Nielsen S, Ivarsen A. Graft rejection and graft failure after penetrating keratoplasty or posterior lamellar keratoplasty for Fuchs endothelial dystrophy. Cornea. 2012;18 doi: 10.1097/ICO.0b013e3182687ff3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- e22.Anshu A, Price MO, Price FW., Jr Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119:536–540. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- e23.Ham L, Dapena I, van Luijk C, van der Wees J, Melles GRJ. Descemet membrane endothelial keratoplasty (DMEK) for Fuchs endothelial dystrophy: review of the first 50 consecutive cases. Eye. 2009;23:1990–1998. doi: 10.1038/eye.2008.393. [DOI] [PubMed] [Google Scholar]
- e24.Foster JB, Vasan R, Walter KA. Three-millimeter incision descemet stripping endothelial keratoplasty using sodium hyaluronate (healon): a survey of 105 eyes. Cornea. 2011;30:150–153. doi: 10.1097/ICO.0b013e3181e3f07e. [DOI] [PubMed] [Google Scholar]
- e25.Yoeruek E, Bayyoud T, Röck D, Szurman P, Bartz-Schmidt K-U. Clinical results after Descemet membrane endothelial keratoplasty. Klin Monbl Augenheilkd. 2012;229:615–620. doi: 10.1055/s-0032-1312913. [DOI] [PubMed] [Google Scholar]
- e26.Dirisamer M, Ham L, Dapena I, et al. Efficacy of descemet membrane endothelial keratoplasty: clinical outcome of 200 consecutive cases after a learning curve of 25 cases. Arch Ophthalmol. 2011;129:1435–1443. doi: 10.1001/archophthalmol.2011.195. [DOI] [PubMed] [Google Scholar]
- e27.Hjortdal J, Ehlers N. Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty for Fuchs’ endothelial dystrophy. Acta Ophthalmol. 2009;87:310–314. doi: 10.1111/j.1755-3768.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- e28.Droutsas K, Ham L, Dapena I, Geerling G, Oellerich S, Melles G. Visual acuity following Descemet-membrane endothelial keratoplasty (DMEK): first 100 cases operated on for Fuchs endothelial dystrophy. Klin Monbl Augenheilkd. 2010;227:467–477. doi: 10.1055/s-0029-1245446. [DOI] [PubMed] [Google Scholar]
- e29.Chen ES, Terry MA, Shamie N, Hoar KL, Phillips PM, Friend DJ. Endothelial keratoplasty: vision, endothelial survival, and complications in a comparative case series of fellows vs attending surgeons. Am J Ophthalmol. 2009;148:26–31e2. doi: 10.1016/j.ajo.2009.01.022. [DOI] [PubMed] [Google Scholar]
- e30.Dirisamer M, van Dijk K, Dapena I, et al. Prevention and management of graft detachment in descemet membrane endothelial keratoplasty. Arch Ophthalmol. 2012;130:280–291. doi: 10.1001/archophthalmol.2011.343. [DOI] [PubMed] [Google Scholar]
- e31.Anshu A, Chee S-P, Mehta JS, Tan DTH. Cytomegalovirus endotheliitis in Descemet’s stripping endothelial keratoplasty. Ophthalmology. 2009;116:624–630. doi: 10.1016/j.ophtha.2008.10.031. [DOI] [PubMed] [Google Scholar]