Abstract
Background/Purpose:
The Myotonometer® is an electronic tissue compliance meter that has been used to quantify the compliance of soft tissues. The Myotonometer® may be a valuable tool to measure the effectiveness of interventions commonly used to increase tissue compliance in individuals with posterior shoulder tightness (PST). Limited data exist on reliability and responsiveness of the Myotonometer® for assessment of soft tissues about the shoulder; therefore, the purpose of this study is to determine the intra‐ and inter‐session reliability and responsiveness of the Myotonometer® in measuring tissue compliance of the posterior shoulder musculature in asymptomatic subjects with PST.
Methods:
Fifteen asymptomatic subjects with unilateral shoulder range of motion deficits attended two measurement sessions to assess the compliance of the tissues overlying the posterior deltoid, infraspinatus, and teres musculature. Analyses of reliability and responsiveness were conducted using intra‐class correlation coefficients (ICCs) and the determination of minimal detectible change (MDC).
Results:
Intra‐session ICC values ranged from 0.69 to 0.91 for all muscles with MDC never exceeding 1.0 mm. Inter‐session ICC values were best for the posterior deltoid, which averaged 0.82, compared to the infraspinatus and the teres complex, which averaged 0.42 and 0.5 respectively. Inter‐session MDC ranged from 0.55 to 1.20 mm across all muscles.
Conclusions:
Clinicians can reliably detect relatively small changes in tissue compliance within a single treatment session utilizing the Myotonometer®. The Myotonometer® can reliably detect changes between sessions for tissues overlying the posterior deltoid; however, observed change in the infraspinatus and teres musculature must be above 1 mm to achieve meaningful change and account for decreased inter‐session reliability.
Level of Evidence:
3
Keywords: Glenohumeral Internal Rotation Deficit, responsiveness, tissue compliance
INTRODUCTION
Overhead athletes commonly exhibit posterior shoulder pain and tightness.1‐4 The subjective complaint of “tightness” in the posterior shoulder or the inability to “get loose” is a sign that the athlete could be at risk for injuries to the glenohumeral (GH) joint.1,5‐7 Specifically, deficits in internal rotation (≥258) and total arc (sum of GH internal rotation + external rotation) motion (≥58) relative to the non‐dominant shoulder have been identified as risk factors for upper extremity injury.8,9 Debate exists as to exactly which structures of the posterior shoulder are responsible for the complaint of tightness—the posterior GH joint capsule and/or the posterior shoulder musculature.4
Traditional clinical measures of posterior shoulder tightness (PST) include passive GH internal rotation (IR) range of motion (ROM) measured in supine at 90° of shoulder abduction,10 as well as passive GH horizontal adduction (HADD) ROM measured in side‐lying or supine position.11 While these goniometric measures target the posterior shoulder, they are unable to delineate the source of the ROM restriction—capsular or muscular. Assessment of the muscular contribution to this motion restriction may help elucidate the primary tissue involved in this common clinical phenomenon.
Identifying the potential muscular contribution to PST requires a unique assessment technique. One device, the Myotonometer® (Neurogenic Technologies, Inc., Missoula, Montana, USA), measures a factor called tissue compliance and may prove useful for quantifying the muscular contribution to PST. Compliance can be defined as the displacement that occurs when a compressive force is applied to a surface, usually expressed in millimeters per Newton.12 The inverse of stiffness, tissue compliance can reflect various clinical factors such as muscle tone, edema, and skin elasticity.12 The ability to quantify tissue compliance may enable clinicians to objectively document the effectiveness of interventions commonly used to increase muscle compliance in individuals with PST.
The Myotonometer® is a patented, computerized, electronic tissue compliance meter. The underlying assumption related to the use of this device is that tissues with greater compliance have more displacement per unit of force and would therefore be more pliable to loads applied than tissues with less compliance. The device was originally developed for the evaluation of muscle compliance in individuals with upper motor neuron lesions to measure the efficacy of interventions meant to reduce muscle spasticity.13 The validity14,15 and reliability16,17 of the Myotonometer® has been previously assessed and reported in healthy normal populations13‐15,17 and in subjects with upper motor neuron lesion disorders.14‐17
The Myotonometer® has been used for assessment of posterior shoulder muscular compliance in two studies.18,19 The first study reported intra‐ and inter‐rater reliabilities with ICCs > 0.9 for the 14.70‐19.60N force levels.18 However, the authors did not report measures of responsiveness and reliability across days, nor did they detail the patient population and number of subjects used to determine reliability.18 The second study reported within‐session reliability pilot data on eight subjects with an ICC = 0.98, but also failed to report responsiveness measures.19 Reliability is only one component of establishing an instrument's clinical utility; responsiveness must also be determined in order for clinicians to identify and recognize meaningful change when measuring with a given device. Therefore, the purpose of this study is to determine the intra‐ and inter‐session reliability and responsiveness of the Myotonometer® in measuring tissue compliance of the posterior shoulder musculature in asymptomatic subjects with PST.
METHODS
Fifteen subjects (5 males, 10 females; group combined age 23±5 years; height 68±4 cm; weight 76±20 kg) participated in this study. This study was done as part of an ongoing intervention study that examined compliance and shoulder range of motion responses to an instrument‐assisted soft tissue mobilization intervention. This study was approved by the Institutional Review Board at the University of Kentucky. All subjects provided written informed consent prior to participation. Subjects were included if they presented with: (1) ≥158 deficit in glenohumeral internal rotation and ≥108 deficit in total arc of motion (TAM) in one arm compared to the contralateral limb,8,9 and (2) little to no shoulder pain or functional deficit as determined by a score of ≥26/30 on the pain subscale and ≥54/60 on the function subscale of the PENN shoulder score.20 Subjects were excluded if they had reported a history of shoulder surgery in the past year, a steroid injection in the past month, numbness and tingling in the upper extremity, or demonstrated signs consistent with cervical radiculopathy21 adhesive capsuliits,22 glenohumeral arthritis,23 or rotator cuff pathology24 as determined with a screening examination.
The Myotonometer® consists of a metal probe with an inner and outer cylinder. The outer cylinder remains stationary while the inner cylinder, which houses a force transducer, is pushed perpendicularly onto the underlying tissue by the examiner. Tissue displacement (mm), as measured by the distance between the outer and inner cylinders, is recorded by proprietary computer software (Neurogenic Technologies, Inc., Montana, USA) at eight predetermined loads (2.45—19.60N) to give an overall assessment of tissue compliance.
Testing was conducted with the subject prone, the test arm abducted to 90° and externally rotated to 45° (confirmed via inclinometer), and the neck positioned in neutral with the forehead supported by the opposite forearm. This test position was adapted from electromyographic methods to allow for proper identification of the muscle bellies of interest.25 To maintain proper alignment, the humerus was supported by a foam pillow (DonJoy Ultrasling 2, DJ Ortho, Vista, CA) (Figure 1). Measures of compliance were taken over the bellies of the posterior deltoid, infraspinatus, and teres musculature of the restricted shoulder with standardized probe placements (Table 1). The Myotonometer's software automatically averaged the displacement (mm) across five sequential probe depressions for each force load. These averages constituted one trial and were used for statistical analysis for each muscle.16
Figure 1.
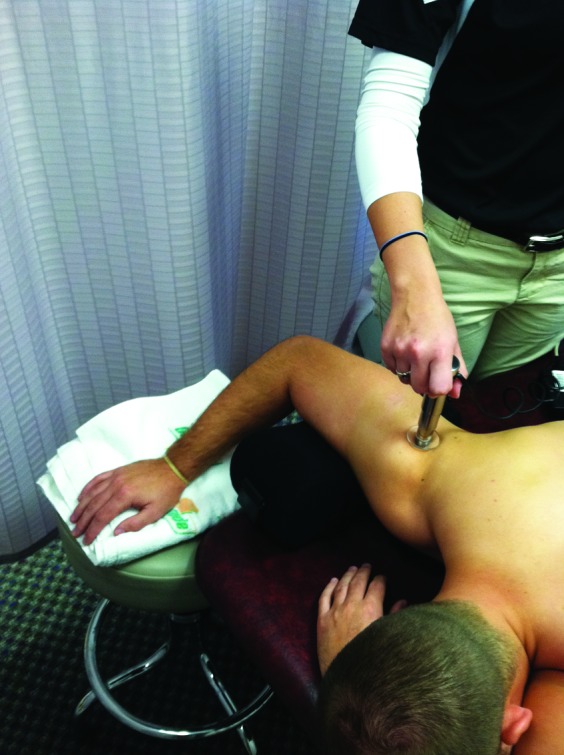
Position for Myotonometer® testing of the posterior deltoid.
Table 1.
Probe placement for the Myotonometer®
| Muscle | Reference Mark 1 | Reference Mark 2 | Reference Mark 3 | Measurement Mark Placement |
|---|---|---|---|---|
| Posterior Deltoid | PLA* of acromion | Deltoid tuberosity | Proximal 1/3 distance between 1st and2ndRMf along the superior aspect of the arm | Superior 1/3 of the distance measured between 3rd RMf and axilla fold |
| Infraspinatus | PLA* of acromion | Inferior angle of scapula | •••••••••• | 1/2 distance between 1st and 2nd RMf 1/3 distance between 1st and 2nd RMf along lateral scapular border |
| Teres Complex | PLA* of acromion | Inferior angle of scapula | •••••••••• |
PLA, posterior‐lateral angle; †RM, reference mark
Subjects attended two measurement sessions. At the first session, compliance measures for the restricted shoulder were obtained twice to establish inter‐session reliability. For the purpose of the intervention study, shoulder internal rotation, external rotation, and horizontal adduction passive ROM measures were conducted between Trials 1 and 2.26 Compliance measures were obtained once at the second session (Trial 3), which occurred on average 26±7 (mean±SD) hours after the first session. Order of muscle testing between sessions was counterbalanced a priori.
STATISTICAL METHODS
An intraclass correlation coefficient (ICC2,5)27 was calculated to determine the intra‐ and intersession reliability of the average displacement at each increment of force for each site tested using SPSS (Version 20.0, IBM SPSS Inc, Armonk, NY). The resultant ICC values were interpreted with the scale described by Fleiss.28 Standard error of measurement (SEM) and minimal detectible change (MDC) were calculated in Excel (Microsoft Inc., Redwood, WA) using previously established formulas to determine responsiveness.29
RESULTS
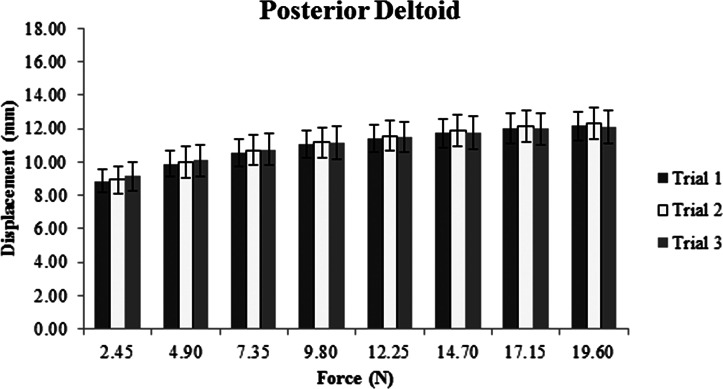
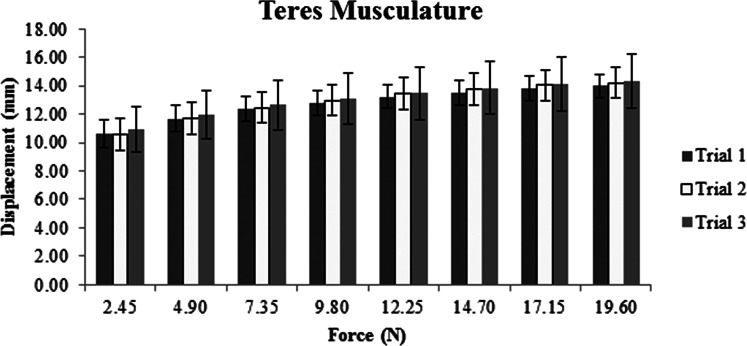
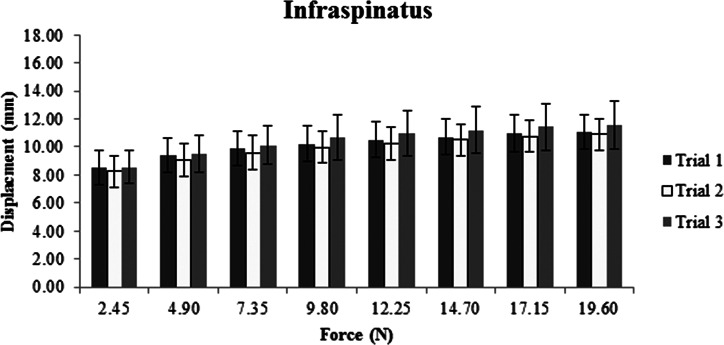
Descriptive data, represented as means and standard deviations in millimeters of displacement for each muscle, show increased displacement with increased force for all three sites (Figures 2‐4). The ICC, SEM, and MDC for both intra‐ and inter‐session reliability are presented in Table 2. Intra‐session ICC values were good to excellent for all muscles, ranging from 0.69 to 0.91. SEM ranged from 0.19 to 0.64 mm with MDC never exceeding 1.0 mm. Inter‐session ICC values varied greatly between muscles, ranging from 0.33 to 0.87. SEM increased compared to intra‐session SEM with values ranging from 0.39 to 0.85 mm. Inter‐session MDC ranged from 0.55 to 1.2 mm across all muscles.
Figure 2.
Average displacement in millimeters across three trials for the posterior deltoid and its overlying tissues.
Figure 4.
Average displacement in millimeters across three trials for the teres complex and its overlying tissues.
Table 2.
Intra‐ and inter‐session reliability with responsiveness values reported in millimeters of displacement. Responsiveness reported to the nearest tenth of a millimeter as per measurement accuracy indicated in the Myotonometer's manual.
| Force (N) | Intra‐session | Inter‐session | |||||
|---|---|---|---|---|---|---|---|
| ICC* | SEM† | MDC‡ | ICC* | SEM† | MDC‡ | ||
| Infraspinatus | 2.45 | 0.95 | 0.2 | 0.3 | 0.77 | 0.5 | 0.6 |
| 4.90 | 0.94 | 0.2 | 0.3 | 0.66 | 0.6 | 0.8 | |
| 7.35 | 0.93 | 0.2 | 0.3 | 0.59 | 0.6 | 0.9 | |
| 9.80 | 0.94 | 0.2 | 0.3 | 0.34 | 0.8 | 1.1 | |
| 12.25 | 0.93 | 0.2 | 0.3 | 0.35 | 0.8 | 1.1 | |
| 14.70 | 0.94 | 0.2 | 0.3 | 0.33 | 0.8 | 1.1 | |
| 17.15 | 0.94 | 0.2 | 0.3 | 0.35 | 0.8 | 1.1 | |
| 19.60 | 0.94 | 0.2 | 0.3 | 0.38 | 0.8 | 1.1 | |
| Teres Complex | 2.45 | 0.69 | 0.6 | 0.9 | 0.64 | 0.7 | 1.0 |
| 4.90 | 0.72 | 0.6 | 0.9 | 0.58 | 0.7 | 1.0 | |
| 7.35 | 0.75 | 0.6 | 0.8 | 0.49 | 0.8 | 1.1 | |
| 9.80 | 0.77 | 0.6 | 0.8 | 0.49 | 0.8 | 1.1 | |
| 12.25 | 0.76 | 0.6 | 0.8 | 0.49 | 0.8 | 1.1 | |
| 14.70 | 0.76 | 0.6 | 0.8 | 0.47 | 0.8 | 1.2 | |
| 17.15 | 0.76 | 0.6 | 0.8 | 0.44 | 0.8 | 1.2 | |
| 19.60 | 0.78 | 0.5 | 0.8 | 0.43 | 0.9 | 1.2 | |
| Posterior Deltoid | |||||||
| 2.45 | 0.73 | 0.6 | 0.8 | 0.65 | 0.6 | 0.9 | |
| 4.90 | 0.80 | 0.5 | 0.7 | 0.78 | 0.5 | 0.7 | |
| 7.35 | 0.81 | 0.5 | 0.7 | 0.85 | 0.4 | 0.6 | |
| 9.80 | 0.83 | 0.5 | 0.7 | 0.86 | 0.4 | 0.6 | |
| 12.25 | 0.88 | 0.4 | 0.6 | 0.86 | 0.4 | 0.6 | |
| 14.70 | 0.88 | 0.4 | 0.5 | 0.87 | 0.4 | 0.5 | |
| 17.15 | 0.91 | 0.3 | 0.5 | 0.86 | 0.4 | 0.6 | |
| 19.60 | 0.91 | 0.3 | 0.5 | 0.86 | 0.4 | 0.6 | |
ICC. Intraclass correlations coefficient: †SEM. standard erroi of measure: ‡MDC. minimal detectible change
Figure 3.
Average displacement in millimeters across three trials for the infraspinatus and its overlying tissues.
DISCUSSION
This study demonstrated good to excellent intra‐session reliability for the three posterior shoulder tissues tested. While inter‐session reliability remained good to excellent for the tissues overlying the posterior deltoid, it was poor to moderate for those overlying the infraspinatus and teres complex. This is the first study to investigate inter‐session reliability and report responsiveness values of the Myotonometer® in the shoulder region on subjects with asymptomatic posterior shoulder tightness.
Previous studies13,16‐18 have reported moderate to excellent intra‐session reliability of the Myotonometer® (ICCs 0.54 to 0.99) on healthy subjects, subjects with spastic‐type disorders, and subjects with stiff shoulders that correspond with the current study's findings. Specifically, a previous study18 reported intra‐rater reliability exceeding 0.9 for the posterior deltoid, infraspinatus, and teres musculature at 14.70 to 19.60 N of force with the subjects seated with the arm at their side. Using the reported ICC and standard deviations for healthy subjects from the previous study, SEM and MDC were calculated for comparison with the current study. The precision of the instrument determined by SEM and MDC were very similar between the two studies, with the present study demonstrating slightly smaller values for both SEM (average difference of 0.3 mm) and MDC (average difference of 0.4 mm). The current and previous study18 indicate that the Myotonometer® can reliably evaluate soft tissue compliance over the posterior shoulder musculature and could be a useful tool to evaluate the effect of soft‐tissue treatments on compliance of the posterior deltoid, infraspinatus, and teres musculature within a single session.
This is the first study to examine inter‐session reliability of the Myotonometer®; therefore, direct comparison with existing results is not feasible. However, the results of the current study follow the typical pattern observed when measuring joint mobility and muscular strength in that inter‐session reliability is somewhat lower than intra‐session reliability.11,30 According to the inter‐session MDC values obtained in the current study, the observed change in the infraspiantus and teres complex must be above 1 mm in order to identify a meaningful difference and account for decreased inter‐session reliability. Previous data reported differences in average compliance between healthy and stiff shoulders of 2.0 to 6.0 mm in the infraspinatus and teres minor18, indicating that it is feasible to observe a change above 1 mm.
There are a few limitations of the current study. First, the Myotonometer®'s manual indicates that the probe should be depressed at an angle perpendicular to the test muscle. Consistent perpendicular depression of the probe was difficult to obtain for the tissues overlying the infraspinatus and teres complex due to the angled orientation of the scapula relative to the table. Consistent perpendicular probe depression is important to maintain because muscle is less compliant when compressed in the cross‐fiber direction (perpendicular to the muscle fibers) compared to a 45 degree angle.31 If precise perpendicular probe depression was not achieved, then the displacement collar, meant to stay flush with the skin, may have been wedged away or forced up on one side, resulting in inconsistent displacement values.12 Although the investigators carefully attempted to maintain perpendicular orientation during depression, small orientation errors of the probe likely occurred. Another limitation is that the rate of probe depression was not standardized. This is a common limitation among studies utilizing the Myotonometer®.13,16 However, this limitation should be addressed in future studies because rate of compression has been reported to affect compliance. Muscles compressed at a faster rater are less compliant than those compressed at a slower rate.32 One way to potentially standardize the rate of probe depression is to use a metronome. The final limitation concerns the state of muscle relaxation during testing. Although subjects were instructed to relax, it is unknown if they were completely relaxed during testing. Future studies utilizing the Myotonometer® in the relaxed state may consider using electromyography (EMG) in order to objectively monitor muscle activity.
CONCLUSION
The Myotonometer® reliably measured intra‐session posterior shoulder tissue compliance with MDC values less than 0.9 mm; thus, the Myotonometer® can be used confidently within a single intervention session to assess the immediate effect of treatments targeting compliance of tissue overlying the posterior shoulder musculature. The device's inter‐session reliability for the posterior deltoid was excellent with MDC values less than 0.9 mm, indicating that it can be used to measure tissue compliance changes across days of treatment. However, observed change in the infraspinatus and teres musculature must be above 1 mm to identify a meaningful difference and account for decreased inter‐session reliability.
REFERENCES
- 1.Tyler TF, Nicholas SJ, Roy T, et al. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. American Journal of Sports Medicine. 2000;28(5):668–673 [DOI] [PubMed] [Google Scholar]
- 2.Tyler TF, Roy T, Nicholas SJ, et al. Reliability and validity of a new method of measuring posterior shoulder tightness. Journal of Orthopaedic & Sports Physical Therapy. 1999;29(5):262–274 [DOI] [PubMed] [Google Scholar]
- 3.Burkhart SS, Morgan CD, Kibler B. Shoulder injuries in overhead athletes: the “dead arm” revisited. Clinics in Sports Medicine. 2000;19(1):125–158 [DOI] [PubMed] [Google Scholar]
- 4.Borsa PA, Laudner KG, Sauers EL. Mobility and Stability Adaptations in the Shoulder of the Overhead Athlete: A Theoretical and Evidence‐Based Perspective. Sports Medicine. 2008;38(1):17–36 [DOI] [PubMed] [Google Scholar]
- 5.Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology Part III: The SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641–661 [DOI] [PubMed] [Google Scholar]
- 6.Warner JJP, Micheli LJ, Arslanian LE, et al. Patterns of flexibility, laxity, and strength in normal shoulders and shoulders with instability and impingement. American Journal of Sports Medicine. 1990;18(4):366–375 [DOI] [PubMed] [Google Scholar]
- 7.Myers JB, Laudner KG, Pasquale MR, et al. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. American Journal of Sports Medicine. 2006;34(3):385–391 [DOI] [PubMed] [Google Scholar]
- 8.Shanley E, Rauh MJ, Michener LA, et al. Shoulder Range of Motion Measures as Risk Factors for Shoulder and Elbow Injuries in High School Softball and Baseball Players. American Journal of Sports Medicine. 2011;39(9):1997–2006 [DOI] [PubMed] [Google Scholar]
- 9.Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of Glenohumeral Internal Rotation Deficit and Total Rotational Motion to Shoulder Injuries in Professional Baseball Pitchers. American Journal of Sports Medicine. 2011;39(2):329–335 [DOI] [PubMed] [Google Scholar]
- 10.McClure P, Balaicuis J, Heiland D, et al. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. Journal of Orthopaedic & Sports Physical Therapy. 2007;37(3):108–114 [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Oyama S, Wassinger C, et al. Reliability, precision, accuracy, and validity of posterior shoulder tightness assessment in overhead athletes. American Journal of Sports Medicine. 2007;35(11): 1922–1930 [DOI] [PubMed] [Google Scholar]
- 12.Kawchuk G, Herzog W. The reliability and accuracy of a standard method of tissue compliance assessment. Journal of Manipulative & Physiological Therapeutics. 1995;18(5):298–301 [PubMed] [Google Scholar]
- 13.Leonard CT, Deshner WP, Romo JW, et al. Myotonometer intra‐ and interrater reliabilities. Archives of Physical Medicine & Rehabilitation. 2003;84(6):928–932 [DOI] [PubMed] [Google Scholar]
- 14.Leonard CT, Stephens JU, Stroppel SL. Assessing the spastic condition of individuals with upper motoneuron involvement: validity of the myotonometer. Archives of Physical Medicine & Rehabilitation. 2001;82(10):1416–1420 [DOI] [PubMed] [Google Scholar]
- 15.Rydahl SJ, Brouwer BJ. Ankle stiffness and tissue compliance in stroke survivors: a validation of myotonometer measurements. Archives of Physical Medicine & Rehabilitation. 2004;85(10):1631–1637 [DOI] [PubMed] [Google Scholar]
- 16.Aarrestad DD, Williams MD, Fehrer SC, et al. Intra‐ and interrater reliabilities of the Myotonometer when assessing the spastic condition of children with cerebral palsy. Journal Of Child Neurology. 2004;19(11):894–901 [DOI] [PubMed] [Google Scholar]
- 17.Lidström A, Ahlsten G, Hirchfeld H, et al. Intrarater and interrater reliability of Myotonometer measurements of muscle tone in children. Journal Of Child Neurology. 2009;24(3):267–274 [DOI] [PubMed] [Google Scholar]
- 18.Hung C‐J, Hsieh C‐L, Yang P‐L, et al. Relationships between posterior shoulder muscle stiffness and rotation in patients with stiff shoulder. Journal Of Rehabilitation Medicine: Official Journal Of The UEMS European Board Of Physical And Rehabilitation Medicine. 2010;42(3):216–220 [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Chen S, Hsieh C, et al. Effects and predictors of shoulder muscle massage for patients with posterior shoulder tightness. Musculoskeletal Disorders. 2012;13(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leggin B, Michener L, Schaffer M, et al. The Penn Shoulder Score: Reliability and Validity. JOSPT. 2006;36(3):138–151 [DOI] [PubMed] [Google Scholar]
- 21.Wainner RS, Fritz JM, Irrgang JJ, et al. Reliability and diagnostic accuracy of the clinical examination and patient self‐report measures for cervical radiculopathy. Spine. Jan 1 2003;28(1):52–62 [DOI] [PubMed] [Google Scholar]
- 22.Griggs SM, Ahn A, Green A. Idiopathic adhesive capsulitis. A prospective functional outcome study of nonoperative treatment. J Bone Joint Surg Am. Oct 2000;82‐A(10):1398–1407 [PubMed] [Google Scholar]
- 23.Kelley MJ, Ramsey ML. Osteoarthritis and traumatic arthritis of the shoulder. J Hand Ther. Apr‐Jun 2000;13(2):148–162 [DOI] [PubMed] [Google Scholar]
- 24.Michener LA, Seitz AL, McClure PW, et al. Is scapular dyskinesis relevant in patients with impingement syndrome? Journal of Orthopaedic & Sports Physical Therapy. 2009 [Google Scholar]
- 25.Perotto A. Anatomical Guide for the Electromyographer: the Limbs and Trunk. Springfield, Illinois: Charles C Thomas; 1994 [Google Scholar]
- 26.Laudner KG, Sipes RC, Wilson JT. The Acute Effects of Sleeper Stretches on Shoulder Range of Motion. Journal of Athletic Training. 2008;43(4):359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denegar CR, Ball DW. Assessing reliability and precision of measurement: an introduction to intraclass correlation and standard error of measurement. Journal of Sport Rehabilitation. 1993;2(1):35–42 [Google Scholar]
- 28.Fleiss J, ed The Design and Analysis of Clinical Experiments. New York: Wiley; 1986 [Google Scholar]
- 29.Beaton DE. Understanding the relevance of measured change through studies of responsiveness. Spine. 2000;25(24):3192–3199 [DOI] [PubMed] [Google Scholar]
- 30.Kollock RO, Jr., Onate JA, Van Lunen B. The reliability of portable fixed dynamometry during hip and knee strength assessments. Journal of Athletic Training. 2010;45(4):349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Loocke M, Lyons CG, Simms CK. A validated model of passive muscle in compression. Journal of Biomechanics. 2006;39(16):2999–3009 [DOI] [PubMed] [Google Scholar]
- 32.Van Loocke M, Lyons CG, Simms CK. Viscoelastic properties of passive skeletal muscle in compression: Stress‐relaxation behaviour and constitutive modelling. Journal of Biomechanics. 2008;41(7):1555–1566 [DOI] [PubMed] [Google Scholar]