Abstract
Purpose/Background:
The semitendinosus‐gracilis tendon autograft is often used to reconstruct the anterior cruciate ligament. Tendon regeneration appears to occur for most individuals in the short term, but little is known about the long‐term effects of graft harvest. The purpose of this study was to describe the effect of semitendinosis‐gracilis tendon graft harvest on muscle and tendon morphology at least five years following reconstruction in a case series.
Methods:
Magnetic resonance images were taken of the knees of three subjects at least five years following anterior cruciate ligament reconstruction. These subjects represented the different regeneration patterns at the time of return‐to‐sport. Muscle and tendon morphology were analyzed by calculating the volume, peak cross‐sectional area, and length of the knee flexors. Muscle and tendon morphological changes were analyzed individually, and then in combination as defined as a knee flexor group.
Results:
Muscle and tendon regeneration continued in those tendons that had begun regeneration at the time of return‐to‐sports in two subjects. There was significant additional muscle degeneration in those muscles whose tendons had not regenerated at the time of return‐to‐sports, in the remaining subject. Compensatory hypertrophy of the remaining knee flexors restored the knee flexor group to near preoperative peak cross‐sectional area and volume across the each of the three case subjects.
Conclusions:
Knee flexor morphology at the time of return‐to‐sports foreshadowed the long‐term outcome in the three studied subjects. Preservation of the tendon sheath in situ may play a role in tendon regeneration. When tendon regeneration did not occur, fatty infiltration of the muscle may be a worst‐case outcome. Semitendinosus‐gracilis muscle synergists demonstrated hypertrophy, perhaps in an effort to compensate for knee flexor group morphology deficits that existed after Semitendinosus gracilis tendon graft harvest.
Clinical Relevance:
Semitendinosus and gracilis tendon harvest technique may play a role in regeneration. Additionally, knee flexor morphology at the time of return‐to‐sports may foreshadow the long‐term outcome.
Level of Evidence:
prospective (longitudinal) cohort ‐ level II
Keywords: Anterior cruciate ligament, magnetic resonance imaging, tendon regrowth
INTRODUCTION
Anterior cruciate ligament (ACL) reconstruction is one of the most commonly performed orthopedic surgeries in the United States, and the semitendinosus‐gracilis (STG) tendon autograft is a commonly used graft source.1‐3 Excellent functional outcomes (International Knee Documentation Survey, Lysholm and Tegner, Knee injury and Osteoarthritis Outcome Score) have been reported in patients who have undergone ACL reconstruction using this graft source, in long‐term follow‐up.4,5 However, while some report knee flexion strength is regained,5,6 others have found high knee flexion7‐9 and internal tibio‐femoral rotation strength deficits (in experiments that isolated internal rotation from knee flexion)10‐12 may remain, two years following graft harvest. Non‐regeneration of the STG tendons in an athlete that participates in running and cutting may have functional implications, as strength deficits in internal rotation may reduce the athlete's ability to control the three planes of tibio‐femoral motion during cutting and pivoting and set them up for additional injury.13 The physical therapist can play a key role in addressing these impairments prior to return‐to‐sports.
Varied knee flexor muscle and tendon morphology have been demonstrated less than three years post‐operatively, with patients demonstrating variable STG tendon regeneration and synergistic muscle hypertrophy.5,14‐20 Williams et al studied semitendinosus and gracilis muscle and tendon morphological changes from before surgery to the time the subjects returned to sport an average of 6 months after ACL reconstruction with an STG autograft in eight young, active athletes.21 At that time, six of eight subjects demonstrated substantial regeneration of both tendons. One had regeneration of only the gracilis tendon and one had no measureable regeneration of either tendon. The purpose of this study was to describe the effect of semitendinosis‐gracilis tendon graft harvest on muscle and tendon morphology at least five years following reconstruction in a case series.21 The authors of the current study repeated an analysis of one subject with early regeneration of both tendons, and two subjects with no regeneration of one or both tendons at the time of return‐to‐sports, at least five years after ACL reconstruction. All of the pre‐operative and return‐to‐sport measurements, from the previous study,21 were included in the analyses within the current study. The authors hypothesized that 1) regeneration would continue and produce a functional muscle tendon unit as manifested by an increased (operationally defined as increased to within 5% of pre‐surgical) muscle peak cross‐sectional area in those tendons that had begun regeneration at the time of return‐to‐sports, 2) there would be significant muscle degeneration, as manifested by a decreased (operationally defined as less than 80% of pre‐surgical) peak cross‐sectional area and the presence of fatty infiltration in those muscles whose tendons did not regenerate and 3) that compensatory hypertrophy of the remaining hamstrings would restore hamstring muscle bulk to near preoperative peak cross‐sectional area and volume.
METHODS
The current subjects were participants in a previous study. They were from a group that consisted of young (19.3 ± 4.3 years) regular participants (200 or more hours per year) in level I sports that required running and cutting (e.g. football, soccer, basketball) and level II activities that require running and jumping (e.g. racquet sports, skiing, manual labor occupations).22 All experienced an isolated rupture of their ACL within six months prior to enrollment in the original study (mean 2.7 ± 2.0 months; range, three weeks to six months). These patients were reconstructed with an STG autograft between 2001 and 2003. All of these patients returned to the same or similar sport or prior level of function after reconstruction (mean 6.1 ± 1.7 months; range, four to nine months) after completing a rigid criterion based rehabilitation program23 including a return to play battery of tests that included strength testing, functional performance measures and patient self‐reported outcomes using validated outcome questionnaires.
Three of the original eight patients were recruited for the current study. At the time of return‐to‐sport, prior to the present study, one had had no measureable regeneration of either semitendinosus or gracilis tendons (<10 cm), one had had considerable regeneration of both tendons (>20 cm), and one had had considerable regeneration of the gracilis (>18 cm) but not the semitendinosus (<2 cm). No medical or social history was obtained since the original follow‐up.
Two experienced fellowship trained sports orthopedic surgeons with considerable experience with ACL reconstruction (>50 cases/year using STG autograft) performed the surgeries. Case 1 injured his left ACL, and had his STG tendons stripped and fixed with a Washer‐loc and Bone mulch screw. Cases 2 and 3 injured their right ACLS, had their tendons stripped one cm proximal to the STG insertion, the tendon sheaths were preserved in situ and the graft fixed with endobutton and interference screw fixation. All three returned to full participation in their sport of basketball.
Imaging
Patients were evaluated using axial spin‐echo T1‐weighted magnetic resonance images (MRI) preoperatively, postoperatively at the time of return‐to‐sports, and at least 5 years (mean 6.5 years) after ACL reconstruction with a 1.5T Signa LX scanner (General Electric Medical Systems, Milwaukee, Wisconsin) from the level of the ankle mortise to the iliac crest while the subject lay supine in the scanner. The images were acquired in four sequences: lower leg, knee, thigh, and pelvis. Images of both limbs were acquired simultaneously with use of the scanner's body coil. The imaging protocol was as follows: repetition time, 350 msec; echo time, 9 msec; slice thickness, 10 mm (except in the knee section where it was 5 mm to acquire more detailed tendon data); gap between slices, 1.5 mm (except in the knee section where it was 1.0 mm), with a 256 × 160 matrix and a field of view that varied with the size of the subject.
Determination of Muscle and Tendon Morphology
The morphologies (volume, peak cross‐sectional area, and length) of the semitendinosus, semimembranosus, gracilis, long head of the biceps femoris, short head of the biceps femoris, sartorius, vastus lateralis, vastus medialis, rectus femoris, vastus intermedius, medial head of the gastrocnemius, and lateral head of the gastrocnemius muscles were evaluated using digital reconstruction using the magnetic resonance images. This was achieved by tracing the contour of each muscle or tendon in each axial slice with use of a digitization tablet (Intuos2; Wacom Technology, Vancouver, Washington) and IMOD software (University of Colorado, Boulder, Colorado).24 A single rater who demonstrated a high degree of reproducibility in a preliminary test‐retest reliability and accuracy study that included a phantom of known dimensions (less than 5% difference from known dimensions and less than 1% difference across four tests) performed all digitization. The muscle and tendon components of the muscle tendon complex were traced as separate objects to differentiate the morphologic changes that occurred in either the muscle or the tendon. The contours from all of the axial slices in which each of the respective muscles or tendons were found were grouped and used to build patient‐specific triangle‐based mesh surface models of each muscle with use of Nuages software (INRIA, Sophia‐Antipolis, France).25 Muscle and tendon morphology/volumes were calculated with the use of subroutines, described as follows, from the Visualization Toolkit (Kitware, Clifton Park, New York).26 The peak cross‐sectional area of each muscle was calculated, in the first subroutine, by transforming the contours of each muscle from pixel coordinates to physical coordinates (mm) with use of parameters from the MRI header and then running a trapezoidal integration algorithm. The centroid of each muscle or tendon contour was calculated, in a second subroutine, from the enclosed plane area. Muscle and tendon length were calculated, in a third subroutine, by connecting the contour centroids of each muscle or tendon so that a line passing through the center of each contour spanned the entire length of each respective muscle or tendon. The intercentroid line segment lengths were then summed to produce the total length of each muscle or tendon. Finally, the quality of the muscle was qualitatively assessed by assessing abnormal signal intensity with regards to the normal anatomy of the leg to determine if fatty infiltrate, which appears bright, was present within the muscle.
Source of Funding
The funding source for this study was the National Institutes of Health (AR046386 and P30‐GM103333).
Statistical Methods
Within subject descriptive analysis was performed to compare the muscles and tendons of interest across time, as well as to compare between the injured and uninjured limbs.
RESULTS
Case 1: no tendon regeneration at the time of return‐to‐sport (basketball, soccer, softball)
A 26‐year‐old man had his left ACL reconstructed, but the tendon sheaths were not preserved in situ. At the time of return‐to‐sports, the injured limb demonstrated muscle atrophy and no tendon regeneration of either the semitendinosus and gracilis. Both heads of the biceps femoris were hypertrophied with regard to volume and peak cross‐sectional area, but the total knee flexor functional group was atrophic for volume (80%) and peak cross‐sectional area (93%) when compared to the same limb pre‐operatively (Table 1).
Table 1.
Case 1 knee flexor muscle and tendon morphology.
| Structure | Parameter | Injured Limb | Uninjured Limb | ||||
|---|---|---|---|---|---|---|---|
| Pre‐Surgery | Post‐Return to Sports | Follow‐Up | Pre‐Surgery | Post‐Return to Sports | Follow‐Up | ||
| Semitendinosus | |||||||
| Muscle | Volume | 271.83 | 115.75 | 56.08 | 314.08 | 296.28 | 301.47 |
| Peak cross‐sectional area | 15.74 | 11.88 | 4.84 | 17.49 | 16.13 | 14.90 | |
| Length | 36.30 | 24.85 | 22.86 | 34.57 | 33.80 | 35.70 | |
| Tendon | Volume | 1.31 | 1.24 | 0.00 | 1.29 | 1.59 | 3.68 |
| Peak cross‐sectional area | 0.16 | 0.13 | 0.00 | 0.19 | 0.19 | 0.35 | |
| Length | 27.63 | 8.14 | 0.00 | 27.19 | 18.23 | 17.80 | |
| Gracilis | |||||||
| Muscle | Volume | 96.35 | 71.40 | 43.76 | 87.16 | 71.40 | 86.94 |
| Peak cross‐sectional area | 4.83 | 3.19 | 2.88 | 3.80 | 3.19 | 3.60 | |
| Length | 33.27 | 24.72 | 19.00 | 33.36 | 33.73 | 32.49 | |
| Tendon | Volume | 1.15 | 0.61 | 0.00 | 1.33 | 1.30 | 2.74 |
| Peak cross‐sectional area | 0.16 | 0.10 | 0.00 | 0.22 | 0.19 | 0.40 | |
| Length | 11.35 | 8.20 | 0.00 | 12.21 | 11.86 | 14.52 | |
| Semimembranosus | |||||||
| Muscle | Volume | 173.64 | 165.86 | 215.65 | 198.83 | 177.85 | 187.76 |
| Peak cross‐sectional area | 9.77 | 9.49 | 12.15 | 11.46 | 10.75 | 11.08 | |
| Biceps femoris (long) | |||||||
| Muscle | Volume | 181.98 | 192.59 | 224.73 | 182.25 | 189.10 | 203.40 |
| Peak cross‐sectional area | 10.94 | 11.78 | 13.94 | 12.06 | 12.25 | 12.30 | |
| Biceps femoris (short) | |||||||
| Muscle | Volume | 104.95 | 116.19 | 142.36 | 106.11 | 106.92 | 112.89 |
| Peak cross‐sectional area | 7.46 | 8.06 | 8.34 | 7.45 | 7.60 | 8.12 | |
| Sartorius | |||||||
| Muscle | Volume | 133.15 | 133.59 | 182.54 | 142.12 | 132.21 | 170.41 |
| Peak cross‐sectional area | 3.87 | 4.30 | 4.93 | 4.35 | 4.03 | 4.48 | |
| Hamstrings+Gracilis+Sartorius | |||||||
| Muscle | Volume | 961.90 | 773.83 | 865.12 | 1030.55 | 973.76 | 1062.87 |
| Peak cross‐sectional area | 52.61 | 49.01 | 47.08 | 56.61 | 53.95 | 54.48 | |
Units: volume (cm3), peak cross‐sectional area (cm2), and length (cm).
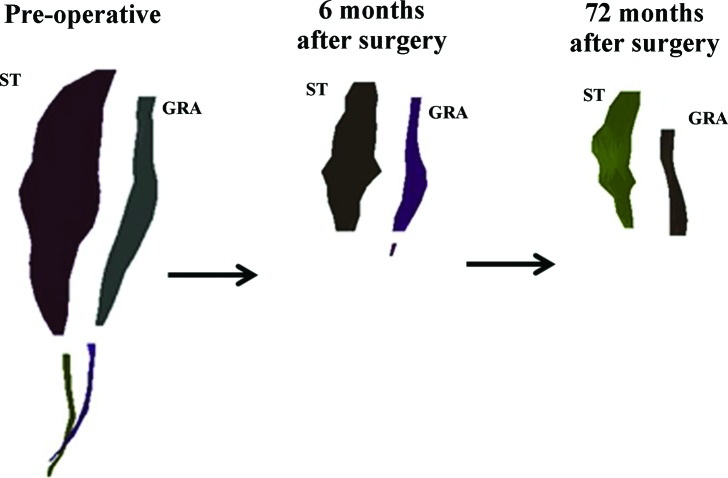
At follow‐up (72 months after surgery), there was no regeneration of the harvested tendons. The injured limb semitendinosus and gracilis muscles were almost entirely composed of fat distal to the mid‐femur (Figure 1). While the fascial plane could be distinguished for these muscles, the only muscle tissue found was proximal to the mid‐femur. All of the synergistic knee flexor muscles of the injured limb were hypertrophied in comparison to the same limb pre‐operatively, as well as to the uninjured limb at the time of follow‐up. However, the injured limb total knee flexor functional group was smaller in volume (90%) and peak cross‐sectional area (89%) than the same limb preoperatively and the uninjured limb (81% and 86%, respectively) at follow‐up (Figure 2).
Figure 1.
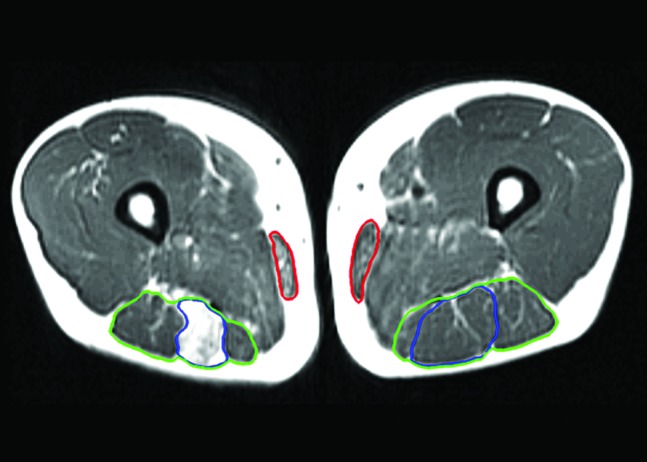
Axial T1‐weighted magnetic resonance image of the reconstructed and uninvolved limbs of case 1, taken 72 months following surgery displaying fatty infiltration of the semitendinosus muscle (blue), poor regeneration of the gracilis muscle (red) which became fatty as well approximately 3 cm more distal. The hamstring muscles are outlined in green.
Figure 2.
A three dimensional surface mesh of the semitendinosus (ST) and gracilis (GRA) muscles from the involved limb that was produced by digital reconstruction of the magnetic resonance images of case 1, taken over the three time points demonstrates the poor regeneration of these muscles. Muscle and tendon contours are separated to clearly distinguish the relative morphology differences over time.
Case 2: Substantial regeneration of both tendons at the time of return‐to‐sports (basketball and softball)
A 17‐year‐old man had his right ACL reconstructed, and the tendon sheaths were preserved in situ. At the time of return‐to‐sports, the injured limb demonstrated semitendinosus and gracilis muscle atrophy, but both tendons had regenerated in length and were hypertrophied in comparison to the same limb preoperatively (Table 2). The semimembranosus, biceps femoris (long head), and sartorius of the injured limb all demonstrated hypertrophy at the time of return‐to‐sports compared to the same limb preoperatively (Figure 3). The injured limb total knee flexor functional group volume was smaller (∼95%) in comparison to the preoperative volume and that of the uninjured limb, but the peak cross‐sectional area was greater (102% and 105%, respectively) (Figure 4).
Table 2.
Case 2 knee flexor muscle and tendon morphology.
| Structure | Parameter | Injured Limb | Uninjured Limb | ||||
|---|---|---|---|---|---|---|---|
| Pre‐Surgery | Post‐Return to Sports | Follow‐Up | Pre‐Surgery | Post‐Return to Sports | Follow‐Up | ||
| Semitendinosus | |||||||
| Muscle | Volume | 138.62 | 108.14 | 120.02 | 185.55 | 170.77 | 196.85 |
| Peak cross‐sectional area | 7.16 | 6.98 | 6.87 | 9.38 | 8.76 | 10.52 | |
| Length | 32.50 | 25.96 | 28.79 | 31.94 | 34.35 | 32.38 | |
| Tendon | Volume | 1.46 | 2.92 | 3.95 | 1.36 | 1.43 | 2.49 |
| Peak cross‐sectional area | 0.23 | 0.33 | 0.26 | 0.16 | 0.15 | 0.30 | |
| Length | 21.13 | 26.24 | 24.83 | 21.42 | 24.19 | 15.42 | |
| Gracilis | |||||||
| Muscle | Volume | 94.60 | 71.40 | 93.75 | 72.44 | 81.09 | 96.62 |
| Peak cross‐sectional area | 4.82 | 3.19 | 5.28 | 3.96 | 4.38 | 4.54 | |
| Length | 29.99 | 27.28 | 27.02 | 31.41 | 33.43 | 30.53 | |
| Tendon | Volume | 1.47 | 3.06 | 3.59 | 0.99 | 1.21 | 1.66 |
| Peak cross‐sectional area | 0.24 | 0.30 | 0.27 | 0.14 | 0.22 | 0.21 | |
| Length | 8.21 | 21.42 | 19.92 | 11.87 | 11.87 | 12.73 | |
| Semimembranosus | |||||||
| Muscle | Volume | 194.75 | 204.43 | 209.37 | 189.64 | 192.65 | 198.60 |
| Peak cross‐sectional area | 10.62 | 11.46 | 12.68 | 9.15 | 9.83 | 10.79 | |
| Biceps femoris (long) | |||||||
| Muscle | Volume | 174.56 | 184.50 | 231.10 | 177.19 | 188.95 | 209.69 |
| Peak cross‐sectional area | 10.85 | 12.01 | 15.34 | 10.88 | 10.57 | 12.63 | |
| Biceps femoris (short) | |||||||
| Muscle | Volume | 75.30 | 75.13 | 85.01 | 59.29 | 50.16 | 70.86 |
| Peak cross‐sectional area | 6.49 | 6.00 | 6.69 | 5.49 | 4.61 | 6.05 | |
| Sartorius | |||||||
| Muscle | Volume | 95.28 | 96.94 | 125.97 | 83.40 | 90.92 | 144.95 |
| Peak cross‐sectional area | 3.27 | 3.35 | 3.44 | 2.87 | 3.87 | 5.22 | |
| Hamstrings+Gracilis+Sartorius | |||||||
| Muscle | Volume | 773.11 | 737.94 | 865.24 | 767.51 | 774.54 | 917.58 |
| Peak cross‐sectional area | 43.21 | 43.92 | 50.30 | 41.73 | 42.02 | 49.75 | |
Units: volume (cm3), peak cross‐sectional area (cm2), and length (cm).
Figure 3.
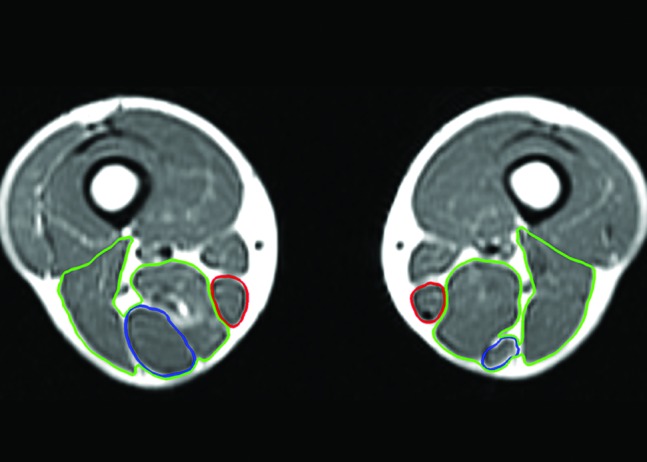
Axial T1‐weighted magnetic resonance image of the reconstructed and uninvolved limbs of case 2, taken 91 months following surgery, displaying semimembranosus and biceps femoris muscle (green) hypertrophy, and gracilis (red) and semitendinosus (blue) tendon and muscle regeneration.
Figure 4.
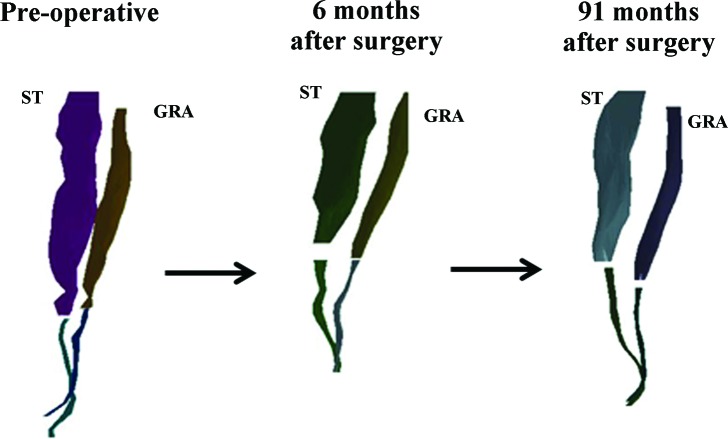
A three dimensional surface mesh of the semitendinosus (ST) and gracilis (GRA) muscles from the involved limb that was produced by digital reconstruction of the magnetic resonance images of case 2, taken over the three time points demonstrates the regeneration of these muscles over time. Muscle and tendon contours are separated to clearly distinguish the relative morphology differences over time.
At follow‐up (91 months after surgery), the injured limb semitendinosus and gracilis muscles and tendons demonstrated nearly complete regeneration across all measures in comparison to the same limb preoperatively. Both tendons regenerated at least to the level of the distal insertions on the tibia before surgery. All of the synergistic knee flexors, with the exception of the sartorius (87%), were hypertrophied compared to the contralateral muscles (105–120%). All of the synergistic knee flexors were hypertrophied as compared to the same limb before surgery. The total knee flexor functional group at follow‐up demonstrated hypertrophy in both muscle volume (112%) and peak cross‐sectional area (116%), in comparison to the same limb preoperatively. Further, the injured and uninjured limb total knee flexor functional group was nearly symmetrical in volume (94%) and peak cross‐sectional area (101%).
Case 3: Gracilis but not semitendinosus tendon regeneration at the time of return‐to‐sport (wrestling and softball)
An 18‐year‐old man had his right ACL reconstructed, and the tendon sheaths were preserved in situ. At the time of return‐to‐sports the injured limb primarily demonstrated semitendinosus muscle atrophy to the same limb preoperatively, and almost no tendon regeneration (Table 3). The gracilis muscle however was almost completely regenerated with volumetric hypertrophy, and the tendon hypertrophied for all measures (Figure 5). All of the synergistic knee flexor muscles hypertrophied at the time of return‐to‐sports in comparison to the injured limb preoperatively, and to the uninjured limb at the same time point. The total knee flexor functional group volume and peak cross‐sectional area were nearly identical to the same limb preoperatively (98% and 107%, respectively), and to the uninjured limb at the time of post‐return‐to‐sports (98% and 106%, respectively) (Figure 6).
Table 3.
Case 3 knee flexor muscle and tendon morphology.
| Structure | Parameter | Injured Limb | Jninjured Limb | ||||
|---|---|---|---|---|---|---|---|
| Pre‐Surgery | Post‐Return to Sports | Follow‐Up | Pre‐Surgery | Post‐Return to Sports | Follow‐Up | ||
| Semitendinosus | |||||||
| Muscle | Volume | 121.85 | 84.12 | 58.50 | 126.64 | 130.76 | 172.75 |
| Peak cross‐sectional area | 8.56 | 8.60 | 7.00 | 8.71 | 8.46 | 10.42 | |
| Length | 25.86 | 18.67 | 16.80 | 24.71 | 26.54 | 27.89 | |
| Tendon | Volume | 1.47 | 0.11 | 2.35 | 1.24 | 0.94 | 2.81 |
| Peak cross‐sectional area | 0.16 | 0.13 | 0.21 | 0.13 | 0.20 | 0.41 | |
| Length | 20.98 | 1.15 | 19.65 | 20.43 | 19.89 | 18.94 | |
| Gracilis | |||||||
| Muscle | Volume | 66.50 | 71.40 | 98.90 | 62.14 | 60.86 | 111.68 |
| Peak cross‐sectional area | 4.88 | 3.19 | 5.67 | 4.07 | 4.14 | 6.37 | |
| Length | 27.59 | 26.17 | 25.46 | 27.54 | 30.18 | 28.04 | |
| Tendon | Volume | 0.61 | 1.01 | 3.85 | 0.94 | 0.61 | 2.89 |
| Peak cross‐sectional area | 0.14 | 0.14 | 0.47 | 0.20 | 0.10 | 0.48 | |
| Length | 11.19 | 18.94 | 21.25 | 9.45 | 12.72 | 14.79 | |
| Semimembranosus | |||||||
| Muscle | Volume | 170.12 | 185.12 | 254.02 | 159.48 | 169.54 | 236.85 |
| Peak cross‐sectional area | 9.93 | 11.77 | 16.79 | 10.17 | 11.44 | 15.01 | |
| Biceps femoris (long) | |||||||
| Muscle | Volume | 153.97 | 164.70 | 214.47 | 161.60 | 169.07 | 216.70 |
| Peak cross‐sectional area | 12.62 | 14.13 | 17.80 | 12.34 | 13.30 | 15.39 | |
| Biceps femoris (short) | |||||||
| Muscle | Volume | 60.36 | 65.69 | 87.14 | 51.20 | 56.52 | 82.90 |
| Peak cross‐sectional area | 6.17 | 6.98 | 7.75 | 5.42 | 5.82 | 7.30 | |
| Sartorius | |||||||
| Muscle | Volume | 99.60 | 107.90 | 189.84 | 83.22 | 85.49 | 166.60 |
| Peak cross‐sectional area | 3.47 | 3.46 | 5.03 | 2.75 | 2.91 | 4.76 | |
| Hamstrings+Gracilis+Sartorius | |||||||
| Muscle | Volume | 672.40 | 661.60 | 902.87 | 644.28 | 672.24 | 987.47 |
| Peak cross‐sectional area | 45.63 | 48.62 | 60.04 | 43.46 | 46.07 | 59.25 | |
Units: volume (cm3), peak cross‐sectional area (cm2), and length (cm).
Figure 5.
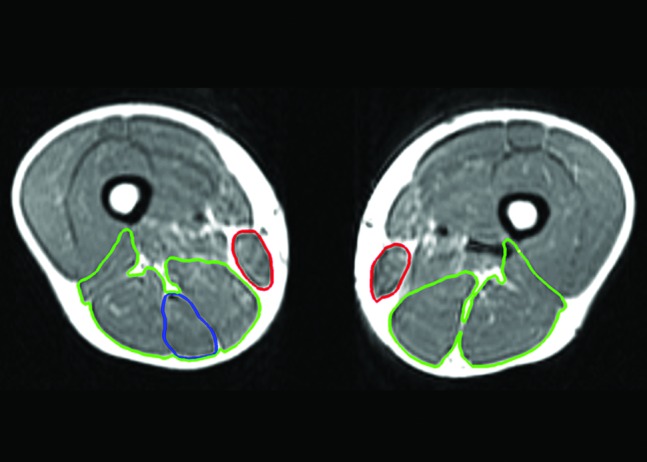
Axial T1‐weighted magnetic resonance image of the reconstructed and uninvolved limbs of case 3, taken 77 months following surgery, displaying semimembranosus and biceps femoris muscle hypertrophy (green), regeneration of the gracilis (red), but poor regeneration of the semitendinosus (blue).
Figure 6.
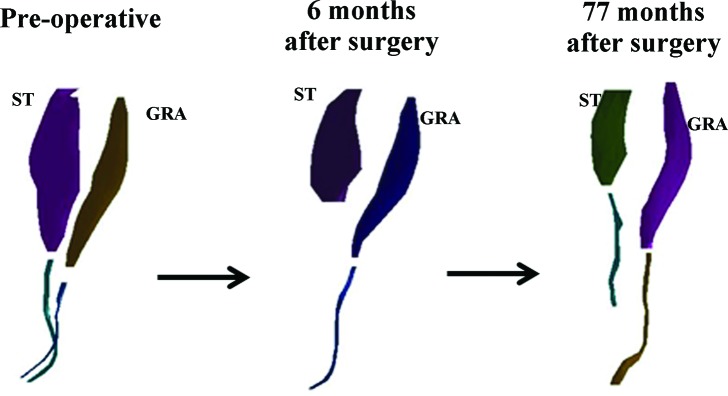
A three dimensional surface mesh of the semitendinosus (ST) and gracilis (GRA) muscles from the involved limb that was produced by digital reconstruction of the magnetic resonance images of case 3, taken over the three time points demonstrates the continued ST regeneration over time and hypertrophy of the GRA neotendon. Muscle and tendon contours are separated to clearly distinguish the relative morphology differences over time.
At follow‐up (77 months after surgery), the injured limb semitendinosus muscle had atrophied further, but the tendon demonstrated considerable regeneration (36.5 of the original 46.8 cm) but was 10.4 cm shy of the original insertion on the tibia. Despite poor musculotendinous length regeneration, the semitendinosus tendon volume and peak cross‐sectional area hypertrophied in comparison to the same limb preoperatively. The gracilis tendon had regenerated to the level of its tibial insertion prior to surgery. The gracilis muscle and tendon both hypertrophied from the return to play time frame (Table 3). All of the synergistic knee flexor muscles continued to hypertrophy in comparison to both the injured and uninjured limbs across time points. The total knee flexor functional group at follow‐up hypertrophied in comparison to the same limb for both time points, and was nearly symmetrical for both volume and peak cross‐sectional area (91% and 101%, respectively) to the uninjured limb.
DISCUSSION
This is the first long‐term follow‐up study of the morphological changes that result from STG tendon graft harvest. The authors' hypotheses that regeneration would continue and produce a functional muscle tendon unit as manifested by increased muscle peak cross‐sectional area in those tendons that had begun regeneration by the time of return‐to‐sports, that there would be significant muscle degeneration, manifested by lower peak cross‐sectional area and fatty infiltration in those muscles whose tendons did not regenerate and that compensatory hypertrophy of the remaining hamstrings would restore hamstring muscle bulk to near preoperative peak cross‐sectional area and volume Table 4 were supported by the results from these three cases. Muscle and tendon regeneration at the time of return‐to‐sports seems to dictate morphology in the long‐term. Synergistic muscle hypertrophy appears to be the common outcome regardless of the regeneration of the harvested STG tendons, among the three studied patients. As reflected in Table 5, the musculature of the uninvolved limb retained relatively normal volume and peak cross‐sectional area over time (81‐112%) as related to pre‐surgical values.
Table 4.
Cases knee flexor muscle and tendon morphology of the injured limb across timepoints.
| Structure | Parameter | % Pre‐Surgical at Return to Sports | % Pre‐Surgical at Return to Follow‐Up | ||||
|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 1 | Case 2 | Case 3 | ||
| Semitendinosus | |||||||
| Muscle | Volume | 42.58 | 78.01 | 69.04 | 20.63 | 86.58 | 48.01 |
| Peak cross‐sectional area | 75.48 | 97.49 | 100.47 | 30.75 | 95.95 | 81.78 | |
| Length | 68.46 | 79.88 | 72.20 | 62.98 | 88.58 | 64.97 | |
| Tendon | Volume | 94.66 | 200.00 | 7.48 | 0.00 | 270.55 | 159.86 |
| Peak cross‐sectional area | 81.25 | 143.48 | 81.25 | 0.00 | 113.04 | 131.25 | |
| Length | 29.46 | 124.18 | 5.48 | 0.00 | 117.51 | 93.66 | |
| Gracilis | |||||||
| Muscle | Volume | 74.10 | 75.48 | 107.37 | 45.42 | 99.11 | 148.72 |
| Peak cross‐sectional area | 66.05 | 66.18 | 65.37 | 59.63 | 109.54 | 116.19 | |
| Length | 74.30 | 90.96 | 94.85 | 57.11 | 90.10 | 92.28 | |
| Tendon | Volume | 53.04 | 208.16 | 165.57 | 0.00 | 244.22 | 631.15 |
| Peak cross‐sectional area | 62.50 | 125.00 | 100.00 | 0.00 | 112.50 | 335.71 | |
| Length | 72.25 | 260.90 | 169.26 | 0.00 | 242.63 | 189.90 | |
| Semimembranosus | |||||||
| Muscle | Volume | 95.52 | 104.97 | 108.82 | 124.19 | 107.51 | 149.32 |
| Peak cross‐sectional area | 97.13 | 107.91 | 118.53 | 124.36 | 119.40 | 169.08 | |
| Biceps femoris (long) | |||||||
| Muscle | Volume | 105.83 | 105.69 | 106.97 | 123.49 | 132.39 | 139.30 |
| Peak cross‐sectional area | 107.68 | 110.69 | 111.97 | 127.42 | 141.38 | 141.05 | |
| Biceps femoris (short) | |||||||
| Muscle | Volume | 110.71 | 99.77 | 108.83 | 135.64 | 112.90 | 144.36 |
| Peak cross‐sectional area | 108.04 | 92.45 | 113.13 | 111.80 | 103.08 | 125.61 | |
| Sartorius | |||||||
| Muscle | Volume | 100.33 | 101.74 | 108.33 | 137.09 | 132.21 | 190.60 |
| Peak cross‐sectional area | 111.11 | 102.45 | 99.71 | 127.39 | 105.20 | 144.96 | |
| Hamstrings+Gracilis+Sartorius | |||||||
| Muscle | Volume | 80.45 | 95.45 | 98.39 | 89.94 | 111.92 | 134.28 |
| Peak cross‐sectional area | 93.16 | 101.64 | 106.55 | 89.49 | 116.41 | 131.58 | |
Units: volume (cm3), peak cross‐sectional area (cm2), and length (cm).
Table 5.
Measurements of uninvolved limb musculature across time points.
| Structure | Parameter | Uninjured Limb | |||||
|---|---|---|---|---|---|---|---|
| % Pre‐Surgical at Return to Sports | % Pre‐Surgical at Return to Follow‐Up | ||||||
| Case 1 | Case 2 | Case 3 | Case 1 | Case 2 | Case 3 | ||
| Semitendinosus | |||||||
| Muscle | Volume | 94.33 | 92.03 | 103.25 | 95.99 | 92.03 | 103.25 |
| Peak cross‐sectional area | 92.22 | 93.39 | 97.13 | 85.19 | 93.39 | 97.13 | |
| Gracilis | |||||||
| Muscle | Volume | 81.92 | 111.94 | 97.94 | 99.75 | 111.94 | 97.94 |
| Peak cross‐sectional area | 83.95 | 110.61 | 101.72 | 94.74 | 110.61 | 101.72 | |
| Semimembranosus | |||||||
| Muscle | Volume | 89.45 | 101.59 | 106.31 | 94.43 | 101.59 | 106.31 |
| Peak cross‐sectional area | 93.80 | 107.43 | 112.49 | 96.68 | 107.43 | 112.49 | |
| Biceps femoris (long) | |||||||
| Muscle | Volume | 103.76 | 106.64 | 104.62 | 111.60 | 106.64 | 104.62 |
| Peak cross‐sectional area | 101.58 | 97.15 | 107.78 | 101.99 | 97.15 | 107.78 | |
| Biceps femoris (short) | |||||||
| Muscle | Volume | 100.76 | 84.60 | 110.39 | 106.39 | 84.60 | 110.39 |
| Peak cross‐sectional area | 102.01 | 83.97 | 107.38 | 108.99 | 83.97 | 107.38 | |
| Sartorius | |||||||
| Muscle | Volume | 93.03 | 109.02 | 102.73 | 119.91 | 109.02 | 102.73 |
| Peak cross‐sectional area | 92.64 | 134.84 | 105.82 | 102.99 | 134.84 | 105.82 | |
| Hamstrings+Gracilis+Sartorius | |||||||
| Muscle | Volume | 94.49 | 100.92 | 104.34 | 103.14 | 100.92 | 104.34 |
| Peak cross‐sectional area | 95.30 | 100.69 | 106.01 | 96.24 | 100.69 | 106.01 | |
Units: volume (cm3), peak cross‐sectional area (cm2), and length (cm).
Ultimately STG regeneration is only important if the muscle tendon units regain their function. This unique longitudinal case‐cohort allowed the authors to investigate recovery using imaging. At the time of return‐to‐sports none of the musculotendinous units were morphologically normal, but some had substantial regeneration. In the ensuing years the muscles of the tendons that regenerated, even partially, showed a hypertrophic response. Following tenotomy muscle atrophy and force production continues to decline until re‐attachment to surrounding connective tissue or the tendon stump when the muscle rapidly increases tension production and hypertrophy.27 Those whose tendons reached the tibial insertion level recovered to near or full pre‐injury muscle volume and peak cross‐sectional area levels. The authors' believe that muscle hypertrophy following tendon graft harvest to mean that these muscles are under tension, however this was not measured directly.
Initial fibroblastic activity is important for structural development,28 but loading of the tendon and muscle are also important for the healing process especially during the repair and remodeling phase. Tendon healing and regeneration has been reported to take several years.28 Takeda et al reported signs of both the semitendinosus and gracilis muscles function, using T2 relaxation time as a quantitative index of activation, during active contractions between 7 and 32 months after following surgery,29 likely meaning that both the muscle and tendons are being loaded. Data from the present study support these findings and uniquely demonstrate the qualitative appearance, and lack of fatty infiltrates, in cross‐section were indistinguishable from its contralateral counterpart. Tensile loading of the tendon has been hypothesized to aid in the regeneration,30,31 so these findings are promising, as early regeneration seems to foreshadow long‐term reestablishment of the functioning musculotendinous unit, in these three patient cases.
For the physical therapist the implications appear to be that hamstring strengthening activities may be beneficial at the appropriate time after surgery. To limit tissue irritation, the recommendation within the literature is to wait until weeks 6‐8 weeks following STG tendon graft harvest to begin hamstring strengthening.32,33 Non‐weight‐bearing isometric knee flexion exercises may occur as early as 6 weeks post‐operatively, progressing to dynamic knee flexion exercises at week 8, both of which should be performed in a pain‐free range of motion between approximately 0 to 90 degrees of knee flexion. In the current study tendon regeneration at the time of return‐to‐sports foreshadows morphology at long‐term follow‐up suggests that rehabilitation interventions aimed at loading the hamstrings are important to include prior to the time of return‐to‐sports. However, further study is required to determine dosage and timing of such loading in order to optimize tendon regeneration through exercise (for STG tendons or others e.g. supraspinatus), and these recommendations are made solely on the basis of expert opinion.
Graft harvest technique may explain the variations in STG muscle and tendon morphology found between the three subjects in this study. Regardless of the harvest technique, several steps appear to be required for muscle and tendon regeneration to occur. Regeneration has been postulated to occur from an extrasynovial postharvest hematoma in the tendon canal.18 Bundles of collagen with fibroblastic proliferation were found after performing a biopsy on the neotendons. Eriksson et al postulated that this bruising may act as a scaffold for fibroblasts to invade and acts as a mechanism for collagen production and tendon regeneration. This hypothesis was recently supported in an animal model,34 where a hematoma progressively became more tendon like over time. Additionally, the regenerated tendons are thought to grow in a proximal to distal fashion,14,31,34 in a manner similar to nerve regrowth. The regenerating tendon must have good facial coverage in order for regrowth to occur.35 Perhaps for tendon there is a sequence for these mechanisms at work, where the hematoma is initially required for fibroblastic activity, but ultimately a clear path along a fascial plane is required for the tendon to regrow to a similar insertion point. This would explain how Case 1, that did not have the tendon sheaths preserved in situ at the time of surgery, had almost no tendon regeneration initially and ultimately did not regenerate at all while Cases 2 and 3 demonstrated moderate to almost complete regeneration. The differential morphological response gives insight into how regeneration may be enhanced or retarded. These findings warrant further study with larger numbers of subjects.
STG muscle synergists appear to hypertrophy following graft harvest, regardless of the regeneration of the harvested tendons. Hypertrophy of the synergistic knee flexors was found in nearly all muscles for all patients in response to STG graft harvest at the time of this follow‐up. The total knee flexor functional group was nearly symmetrical (less than 10% difference) at the time of follow‐up for cases 2 and 3, and within 20% for case 1. Both heads of the biceps femoris, sartorius, and semimembranosus muscle volumes hypertrophied in all patients suggesting that hypertrophy of synergists may be a compensatory strategy following STG tendon graft harvest. Others have also found the synergistic muscle hypertrophy following STG graft harvest,17,21,36 but not all authors agree.5 Total knee flexor volume and peak cross‐sectional area were nearly symmetrical at the time of follow‐up in this study. Other authors have reported an almost full recovery of peak knee flexor torque,6,37‐41 and hypothesize that this may be attributed to hamstring tendon regeneration.18,20,21,28,42 Alternatively, the hypertrophy of synergistic knee flexors may explain the recovery of peak torque values, however strength or functional outcomes were not collected in the present study.17,21,36 However, knee flexor strength deficits of up to 20% have been found,7,43 that are more pronounced (up to 30%) at greater knee flexion angles.7,8,35,44,45 These findings may be explained because peak knee flexion strength occurs near full knee extension, and may be more readily compensated for by biceps femoris and semimembranosus. Ultimately, STG muscle synergistic hypertrophy does not appear to make up for the tibial internal rotator role of the STG muscles,10,12 and this deficit may lead to rotational instability when the muscle is fatigued.46 However, the long‐term clinical impact of STG graft harvest is still under debate.47,48
Hamstring morphology at the time of return‐to‐sports may be indicative of the long‐term outcome. Previously,21 it was not known if the atrophic tendons observed from this patient sample would regenerate to form normal tendons. This study demonstrates in case 1 that the previously atrophic tendon may not be capable of regenerating. In case 1, the fatty infiltration that was found in the semitendinosus and gracilis muscles is similar to that seen in the supraspinatus of patients with chronic rotator cuff tear.49 Determining the mechanisms behind such a poor outcome for regeneration requires further study. Most patients demonstrate a positive outcome, as nearly 90% of published cases (146 out of the 164 studied patients) regenerate their hamstring tendons, although “regeneration” was not clearly defined.19 Perhaps the optimal outcome following STG tendon graft harvest is a return to prior level of function and the ability to functionally utilize the STG muscles. Both cases 2 and 3 demonstrated tendon regeneration at the time of return‐to‐sports, and had continued regrowth in the long‐term. Varied regeneration may occur commonly following STG tendon harvest.35 Improving the consistency of regrowth must be examined.
There are some limitations to this study. This study represents only three cases, however, the longitudinal quantification of volume, length, and cross section area following STG tendon graft harvest is a novel and valuable addition to the literature. Additionally, only one of the 6 patients who had substantial regeneration of both tendons at the time of return to play was included. It is unknown whether deterioration occurred in the 5 cases that were not recruited. The authors believe presenting one of the 6 who had the most common early response and another 2 patients who demonstrated a different pattern (no regeneration of one or both of the tendons) provides a meaningful presentation of the possible range of regeneration after ACL reconstruction using an STG autograft. While hamstring muscle strength was not measured in this study and isolated individual muscle strength is hard to determine, the total knee flexor functional group cross‐sectional area and volume (that are directly related to strength) are related to regeneration. No attempt was made to determine actual physical function and/or muscular torque production or endurance. Finally, there were no attempts made to control for activity of the athletes following their return‐to‐sport, or to address the difference in time between original surgery and return to sport and long‐term follow‐up.
CONCLUSIONS
These long‐term case‐control results suggest that knee flexor morphology at the time of return‐to‐sports may foreshadow the long‐term outcome. Graft harvest technique may play a role in tendon regeneration. When tendon regeneration does not occur, fatty infiltration of the muscle in the fascial plane may be a worst‐case outcome. STG muscle synergist hypertrophy was demonstrated in all three cases, perhaps in an effort to compensate for knee flexor morphology deficits that existed after reconstructive surgery using the STG tendons.
REFERENCES
- 1.Francis A, Thomas RD, McGregor A. Anterior cruciate ligament rupture: reconstruction surgery and rehabilitation. A nation‐wide survey of current practice. The Knee. Mar 2001;8(1):13–18 [DOI] [PubMed] [Google Scholar]
- 2.Delay BS, Smolinski RJ, Wind WM, Bowman DS. Current practices and opinions in ACL reconstruction and rehabilitation: results of a survey of the American Orthopaedic Society for Sports Medicine. The American journal of knee surgery. Spring 2001;14(2):85–91 [PubMed] [Google Scholar]
- 3.Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. Oct 1997;79(10):1556–1576 [DOI] [PubMed] [Google Scholar]
- 4.Spindler KP, Huston LJ, Wright RW, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. The American journal of sports medicine. Feb 2011;39(2):348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonian PT, Harrison SD, Cooley VJ, Escabedo EM, Deneka DA, Larson RV. Assessment of morbidity of semitendinosus and gracilis tendon harvest for ACL reconstruction. The American journal of knee surgery. Spring 1997;10(2):54–59 [PubMed] [Google Scholar]
- 6.Yasuda K, Tsujino J, Ohkoshi Y, Tanabe Y, Kaneda K. Graft site morbidity with autogenous semitendinosus and gracilis tendons. The American journal of sports medicine. Nov‐Dec 1995;23(6):706–714 [DOI] [PubMed] [Google Scholar]
- 7.Nakamura N, Horibe S, Sasaki S, et al. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. Jul‐Aug 2002;18(6):598–602 [DOI] [PubMed] [Google Scholar]
- 8.Tashiro T, Kurosawa H, Kawakami A, Hikita A, Fukui N. Influence of medial hamstring tendon harvest on knee flexor strength after anterior cruciate ligament reconstruction. A detailed evaluation with comparison of single‐ and double‐tendon harvest. The American journal of sports medicine. Jul‐Aug 2003;31(4):522–529 [DOI] [PubMed] [Google Scholar]
- 9.Ahlen M, Liden M, Bovaller A, Sernert N, Kartus J. Bilateral magnetic resonance imaging and functional assessment of the semitendinosus and gracilis tendons a minimum of 6 years after ipsilateral harvest for anterior cruciate ligament reconstruction. The American journal of sports medicine. Aug 2012;40(8):1735–1741 [DOI] [PubMed] [Google Scholar]
- 10.Armour T, Forwell L, Litchfield R, Kirkley A, Amendola N, Fowler PJ. Isokinetic evaluation of internal/external tibial rotation strength after the use of hamstring tendons for anterior cruciate ligament reconstruction. The American journal of sports medicine. Oct‐Nov 2004;32(7):1639–1643 [DOI] [PubMed] [Google Scholar]
- 11.Segawa H, Omori G, Koga Y, Kameo T, Iida S, Tanaka M. Rotational muscle strength of the limb after anterior cruciate ligament reconstruction using semitendinosus and gracilis tendon. Arthroscopy. Feb 2002;18(2):177–182 [DOI] [PubMed] [Google Scholar]
- 12.Viola RW, Sterett WI, Newfield D, Steadman JR, Torry MR. Internal and external tibial rotation strength after anterior cruciate ligament reconstruction using ipsilateral semitendinosus and gracilis tendon autografts. The American journal of sports medicine. Jul‐Aug 2000;28(4):552–555 [DOI] [PubMed] [Google Scholar]
- 13.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. The American journal of sports medicine. Feb 2006;34(2):299–311 [DOI] [PubMed] [Google Scholar]
- 14.Rispoli DM, Sanders TG, Miller MD, Morrison WB. Magnetic resonance imaging at different time periods following hamstring harvest for anterior cruciate ligament reconstruction. Arthroscopy. Jan 2001;17(1):2–8 [DOI] [PubMed] [Google Scholar]
- 15.Papandrea P, Vulpiani MC, Ferretti A, Conteduca F. Regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Evaluation using ultrasonography. The American journal of sports medicine. Jul‐Aug 2000;28(4):556–561 [DOI] [PubMed] [Google Scholar]
- 16.Irie K, Tomatsu T. Level of the semitedinosus and gracilis muscle stumps following tendon harvesting: a cadaveric study. Archives of orthopaedic and trauma surgery. Nov 2002;122(8):459–461 [DOI] [PubMed] [Google Scholar]
- 17.Eriksson K, Hamberg P, Jansson E, Larsson H, Shalabi A, Wredmark T. Semitendinosus muscle in anterior cruciate ligament surgery: Morphology and function. Arthroscopy. Oct 2001;17(8):808–817 [DOI] [PubMed] [Google Scholar]
- 18.Eriksson K, Kindblom LG, Hamberg P, Larsson H, Wredmark T. The semitendinosus tendon regenerates after resection: a morphologic and MRI analysis in 6 patients after resection for anterior cruciate ligament reconstruction. Acta orthopaedica Scandinavica. Aug 2001;72(4):379–384 [DOI] [PubMed] [Google Scholar]
- 19.Nikolaou VS, Efstathopoulos N, Wredmark T. Hamstring tendons regeneration after ACL reconstruction: an overview. Knee Surg Sports Traumatol Arthrosc. Feb 2007;15(2):153–160 [DOI] [PubMed] [Google Scholar]
- 20.Cross MJ, Roger G, Kujawa P, Anderson IF. Regeneration of the semitendinosus and gracilis tendons following their transection for repair of the anterior cruciate ligament. The American journal of sports medicine. Mar‐Apr 1992;20(2):221–223 [DOI] [PubMed] [Google Scholar]
- 21.Williams GN, Snyder‐Mackler L, Barrance PJ, Axe MJ, Buchanan TS. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus‐gracilis graft. J Bone Joint Surg Am. Sep 2004;86‐A(9):1936–1946 [DOI] [PubMed] [Google Scholar]
- 22.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL‐injured patient. A prospective outcome study. The American journal of sports medicine. Sep‐Oct 1994;22(5):632–644 [DOI] [PubMed] [Google Scholar]
- 23.Manal TJ, Snyder‐Mackler L. Practice Guidelines for Anterior Cruciate Ligament Rehabilitation: A criterion‐based rehabilitation progression. Op Tech Orthop. 1996;6(3):190–196 [Google Scholar]
- 24.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three‐dimensional image data using IMOD. Journal of structural biology. Jan‐Feb 1996;116(1):71–76 [DOI] [PubMed] [Google Scholar]
- 25.Geiger B. Three‐dimensional modeling of human organs and its application to diagnosis and surgical planning., Institut National de Recherche en Informatique et Automatique; 1993 [Google Scholar]
- 26.The Visualization Toolkit [computer program]. Upper Saddle River, NJ: Prentice Hall, Inc.; 1997 [Google Scholar]
- 27.Jamali AA, Afshar P, Abrams RA, Lieber RL. Skeletal muscle response to tenotomy. Muscle & nerve. Jun 2000;23(6):851–862 [DOI] [PubMed] [Google Scholar]
- 28.Ferretti A, Conteduca F, Morelli F, Masi V. Regeneration of the semitendinosus tendon after its use in anterior cruciate ligament reconstruction: a histologic study of three cases. The American journal of sports medicine. Mar‐Apr 2002;30(2):204–207 [DOI] [PubMed] [Google Scholar]
- 29.Takeda Y, Kashiwaguchi S, Matsuura T, Higashida T, Minato A. Hamstring muscle function after tendon harvest for anterior cruciate ligament reconstruction: evaluation with T2 relaxation time of magnetic resonance imaging. The American journal of sports medicine. Feb 2006;34(2):281–288 [DOI] [PubMed] [Google Scholar]
- 30.Gill SS, Turner MA, Battaglia TC, Leis HT, Balian G, Miller MD. Semitendinosus regrowth: biochemical, ultrastructural, and physiological characterization of the regenerate tendon. The American journal of sports medicine. Jul‐Aug 2004;32(5):1173–1181 [DOI] [PubMed] [Google Scholar]
- 31.Leis HT, Sanders TG, Larsen KM, Lancaster‐Weiss KJ, Miller MD. Hamstring regrowth following harvesting for ACL reconstruction: The lizard tail phenomenon. The journal of knee surgery. Jul 2003;16(3):159–164 [PubMed] [Google Scholar]
- 32.Adams D, Logerstedt DS, Hunter‐Giordano A, Axe MJ, Snyder‐Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion‐based rehabilitation progression. The Journal of orthopaedic and sports physical therapy. 2012;42(7):601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escamilla RF, Macleod TD, Wilk KE, Paulos L, Andrews JR. Anterior cruciate ligament strain and tensile forces for weight‐bearing and non‐weight‐bearing exercises: a guide to exercise selection. The Journal of orthopaedic and sports physical therapy. 2012;42(3):208–220 [DOI] [PubMed] [Google Scholar]
- 34.Otoshi K, Kikuchi S, Ohi G, Numazaki H, Sekiguchi M, Konno S. The process of tendon regeneration in an achilles tendon resection rat model as a model for hamstring regeneration after harvesting for anterior cruciate ligament reconstruction. Arthroscopy. Feb 2010;27(2):218–227 [DOI] [PubMed] [Google Scholar]
- 35.Tadokoro K, Matsui N, Yagi M, Kuroda R, Kurosaka M, Yoshiya S. Evaluation of hamstring strength and tendon regrowth after harvesting for anterior cruciate ligament reconstruction. The American journal of sports medicine. Oct‐Nov 2004;32(7):1644–1650 [DOI] [PubMed] [Google Scholar]
- 36.Irie K, Tomatsu T. Atrophy of semitzendinosus and gracilis and flexor mechanism function after hamstring tendon harvest for anterior cruciate ligament reconstruction. Orthopedics. May 2002;25(5):491–495 [DOI] [PubMed] [Google Scholar]
- 37.Carter TR, Edinger S. Isokinetic evaluation of anterior cruciate ligament reconstruction: hamstring versus patellar tendon. Arthroscopy. Mar 1999;15(2):169–172 [DOI] [PubMed] [Google Scholar]
- 38.Kramer J, Nusca D, Fowler P, Webster‐Bogaert S. Knee flexor and extensor strength during concentric and eccentric muscle actions after anterior cruciate ligament reconstruction using the semitendinosus tendon and ligament augmentation device. The American journal of sports medicine. Mar‐Apr 1993;21(2):285–291 [DOI] [PubMed] [Google Scholar]
- 39.Lipscomb AB, Johnston RK, Snyder RB, Warburton MJ, Gilbert PP. Evaluation of hamstring strength following use of semitendinosus and gracilis tendons to reconstruct the anterior cruciate ligament. The American journal of sports medicine. Nov‐Dec 1982;10(6):340–342 [DOI] [PubMed] [Google Scholar]
- 40.Maeda A, Shino K, Horibe S, Nakata K, Buccafusca G. Anterior cruciate ligament reconstruction with multistranded autogenous semitendinosus tendon. 1996 [DOI] [PubMed]
- 41.Adachi N, Ochi M, Uchio Y, Sakai Y, Kuriwaka M, Fujihara A. Harvesting hamstring tendons for ACL reconstruction influences postoperative hamstring muscle performance. Archives of orthopaedic and trauma surgery. Nov 2003;123(9):460–465 [DOI] [PubMed] [Google Scholar]
- 42.Eriksson K, Larsson H, Wredmark T, Hamberg P. Semitendinosus tendon regeneration after harvesting for ACL reconstruction. A prospective MRI study. Knee Surg Sports Traumatol Arthrosc. 1999;7(4):220–225 [DOI] [PubMed] [Google Scholar]
- 43.Aune AK, Holm I, Risberg MA, Jensen HK, Steen H. Four‐strand hamstring tendon autograft compared with patellar tendon‐bone autograft for anterior cruciate ligament reconstruction. A randomized study with two‐year follow‐up. The American journal of sports medicine. Nov‐Dec 2001;29(6):722–728 [DOI] [PubMed] [Google Scholar]
- 44.Ohkoshi Y, Inoue C, Yamane S, Hashimoto T, Ishida R. Changes in muscle strength properties caused by harvesting of autogenous semitendinosus tendon for reconstruction of contralateral anterior cruciate ligament. Arthroscopy. Sep 1998;14(6):580–584 [DOI] [PubMed] [Google Scholar]
- 45.Ardern CL, Webster KE, Taylor NF, Feller JA. Hamstring strength recovery after hamstring tendon harvest for anterior cruciate ligament reconstruction: a comparison between graft types. Arthroscopy. Apr 2010;26(4):462–469 [DOI] [PubMed] [Google Scholar]
- 46.Hantes ME, Tsarouhas A, Giakas G, et al. Effect of fatigue on tibial rotation after single‐ and double‐bundle anterior cruciate ligament reconstruction: a 3‐dimensional kinematic and kinetic matched‐group analysis. The American journal of sports medicine. Sep 2012;40(9):2045–2051 [DOI] [PubMed] [Google Scholar]
- 47.Koh JL. Anatomic muscular deficits persist in the medial hamstrings nine to eleven years following harvest, but the clinical consequences remain unclear: Commentary on an article by Brian J. Snow, MD, et al.: “Evaluation of muscle size and fatty infiltration with MRI nine to eleven years following hamstring harvest for ACL reconstruction”. J Bone Joint Surg Am. Jul 18 2012;94(14):e108. [DOI] [PubMed] [Google Scholar]
- 48.Snow BJ, Wilcox JJ, Burks RT, Greis PE. Evaluation of muscle size and fatty infiltration with MRI nine to eleven years following hamstring harvest for ACL reconstruction. J Bone Joint Surg Am. Jul 18 2012;94(14):1274–1282 [DOI] [PubMed] [Google Scholar]
- 49.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons … [et al. Nov‐Dec 2007;16(6):691–696 [DOI] [PubMed] [Google Scholar]