Abstract
Sleep has many inherent benefits, including an important role in memory consolidation. In this issue of Neuron, Ngo et al. demonstrate that appropriately timed sounds delivered during sleep can invigorate electrophysiological oscillations conducive to memory stabilization.
While many are inclined to devalue sleep as what Virginia Woolf called a deplorable curtailment of the joy of life, sleep deserves credit both for its major restorative properties and its clandestine benefits for memory consolidation. We are oblivious to this brain modification when it happens during sleep. We wake up none the wiser — but are we?
During sleep, new memories are reactivated, strengthened, reorganized, and integrated into existing networks (Stickgold and Walker, 2013). At the same time, synapses that have been strengthened during wake activity may be downscaled, which may be beneficial both for the fate of memory storage and for possibilities for new memory storage the following day (Tononi and Cirelli, 2006).
Slow-wave sleep may be particularly conducive to these memory and homeostatic processes. Cortical slow oscillations aren’t a sign of sleep so deep that nothing is happening; rather these oscillations set the stage for brain plasticity. Neuronal “up-states” and “down-states” take turns in repeating alternations of excitation and quiescence, each cycle lasting about a second. Widespread depolarization during up-states may be ideal for neural synchronization across brain regions; the depolarization orchestrates a flurry of neuronal activity as faster rhythms nested within the up-state also take hold. In particular, spindles and ripples can be observed as cortical and hippocampal networks interact so as to consolidate recently learned information (Mölle and Born, 2011).
Sleep, unfortunately, is not always optimal. In aging, slow-wave amplitudes tend to decline and sleep becomes dramatically less efficient (Ancoli-Israel, 2009). Sleep quality is also altered in many pathological conditions, including primary sleep disorders (e.g., sleep apnea) and many psychiatric disorders (e.g., depression).
Even in individuals with no sleep disturbances or other health issues, there is room for improving brain functioning during sleep. An interesting challenge for researchers would be to optimize our time asleep, and to thus produce improvements in memory. Pharmacological sleep aids that might seem up to this challenge (Mednick et al., 2013) usually bring unwanted side effects like drowsiness and nausea. Ultimately we need to understand the neural mechanisms of memory change during sleep. New neuroscientific understanding could lead to revolutionary ideas for mastering our sleep.
Rhythms in the brain matter. This insight has powerful implications; reinforcing rhythms in the right way could help sleep do its magic. For example, slowly rocking a bed can be sufficient to synchronize the brain, increasing the power of slow oscillations (Bayer et al., 2011). Applying an electric current on the scalp surface at a slow frequency potentiates both slow oscillations and memory (Marshall et al., 2006). Tone pips delivered at a constant rate of about one per second, starting prior to sleep onset, can also facilitate slow-wave activity (Ngo et al., 2012). A number of other strategies have been applied (Tononi et al., 2010).
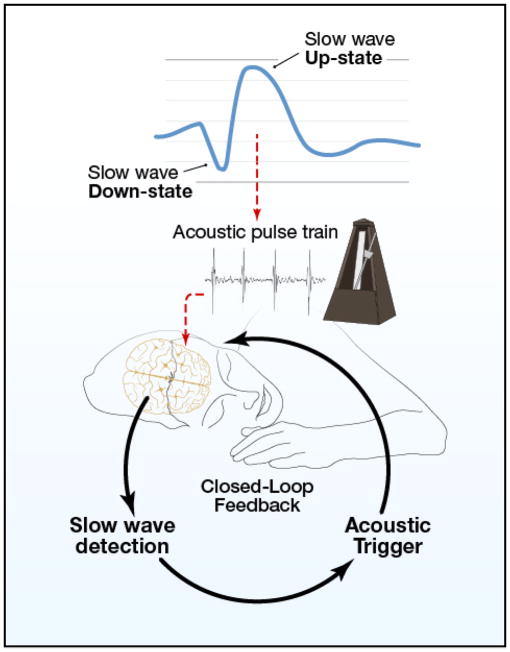
Yet, there may be even better ways to entrain the sleeping brain. In this issue of Neuron, Ngo and colleagues (2013) describe an innovative method to entrain slow oscillations during sleep by taking into account the specific phase of ongoing sleep oscillations. All prior methods to enhance slow-wave sleep disregarded the phase of concurrent slow oscillations.
The key innovation was to tune the auditory stimulation to the phase of the slow wave. Phase-dependent auditory stimulation was found to increase slow oscillations as well as phase-coupled spindle activity.
First, the researchers used online analysis of EEG activity to determine critical characteristics of each participant’s slow oscillations. The goal was to be able to estimate when the up-state would occur following detection of the prior negative half-wave. Brief pulses of auditory stimulation could then be delivered time-locked with the positive peak of ongoing slow oscillations. Compared with no stimulation, the auditory protocol successfully increased slow-oscillation power by about 9%. In addition, stimulation increased the probability of a train of successive slow-oscillation cycles consistently time-locked to the initial detection of a slow wave. Such increases must be evaluated according to whether slow waves increase in amplitude or only become better synchronized, and to whether increases are offset due to refractoriness following stimulation and homeostatic influences.
Were these induced oscillations simply by-products of the auditory stimulation or were they oscillations with the characteristics and functions of naturally occurring slow waves? A detailed analysis of time-locked activity revealed that induced oscillations exhibited a fronto-central topography, distinctive travelling patterns, and a decreasing slope of amplitude as the night unfolded — features typical of spontaneous slow oscillations.
Reinforcing slow oscillations during sleep may turn out to be beneficial in a variety of ways, but here the main focus was on whether memory functions would benefit. Indeed, Ngo and colleagues showed a striking enhancement in a cued-recall test.
Each of 11 adult participants was tested on two nights, one night with auditory stimulation and one night with the identical set-up except that auditory stimulation was withheld. At 9 PM, each participant tried to memorize 120 pairs of words, shown one at a time. The two words in each pair were semantically related (e.g., solution - problem).
Next came a memory test. The entire list was shown again (in a different order). After seeing the first word of a pair the participant tried to produce the second word, and then the correct answer was shown. The level of correct recall averaged 56 words. At 11 PM it was time for sleep.
The following morning, recall was better; this improvement was partially due to the feedback given during the evening test. Strikingly, the improvement was nearly two times greater when participants received auditory stimulation in-phase with slow oscillation up-states than when no sounds were presented during sleep (22 vs. 13 words better, respectively).
Interestingly, correlational analyses showed a greater stimulation-based gain in memory for participants with a greater percentage of slow-wave sleep during the stimulation period. Also, the memory gain was greater when greater fast spindle activity was registered. These fast spindles tend to be nested in the up-states of slow oscillations, and fast spindle power was increased by the stimulation. Spindles may signal an aspect of memory consolidation, although the precise connection with hippocampal-neocortical interactions remains to be specified. One might also question which neural events produced the memory boost and how they relate to slow-wave sleep, slow oscillations, spindle activity, and the details of timing. Follow-up studies will be needed to fully elucidate the neural mechanisms responsible for the stimulation effect on memory.
Was the elaborate emphasis on up-states necessary to reinforce slow oscillations and subsequent memory? To answer this question, Ngo and colleagues ran an additional experiment with auditory stimulation at a different phase. Instead of targeting up-states, stimulation was timed to coincide with the negative peaks of slow oscillations. This down-state stimulation disturbed slow oscillations, as EEG power in this frequency diminished and fewer trains of time-locked slow oscillations were observed. However, this disruption of slow oscillations was transient, as power recovered over the short intervals following stimulation. Importantly, auditory stimulation synchronized with down-states did not improve memory compared with sham stimulation. One can conclude that stimulation effects are phase-dependent. Applying sounds randomly during slow-wave sleep may be suboptimal — there are particular times when further oscillations are likely to be induced and lead to further gains.
Given that sensory processing is reduced during sleep, it is remarkable that sleep stimulation is effective at all. Moreover, previous studies demonstrated weakened sensory processing at particular times — during spindles and during the negative slope of slow oscillations (Schabus et al., 2012). One could hypothesize that auditory stimulation failed to potentiate slow oscillations during down-states because environmental input channels were shut down. However, analyses of event-related potentials suggested comparable auditory processing for up-state and down-state stimulation. Phase specificity is conceivably due to how auditory processing interacts with ongoing slow oscillations.
The present findings open the door to new research directions as well as novel applications. Because this technique is relatively straightforward and inexpensive to implement, it could be used to improve sleep quality in people with inefficient slow-wave sleep, especially older adults whose sleep disturbances accompany memory decline (Westerberg et al., 2012). It might also boost the benefits of a power nap in sleep-deprived individuals.
In keeping with previous links between slow-wave sleep and memory consolidation, the new findings not only lend support to mechanistic accounts involving slow oscillations, they also provide a new tool for investigating these mechanisms. A major actor responsible for memory consolidation could be the reactivation of recently acquired information. Accordingly, further studies should seek to link the observed boost of slow waves to increased reactivation.
Additionally, accounting for the phase of slow oscillations could be very useful for scientists using targeted memory reactivation techniques (Oudiette and Paller, 2013). In a typical protocol, an auditory or olfactory stimulus is associated with learning and then re-applied during slow-wave sleep. The corresponding memory storage can thereby be strengthened. This method successfully improved spatial learning (Rudoy et al., 2009) and a complex motor skill (Antony et al., 2012). Perhaps the efficiency of targeted memory reactivation could be enhanced by applying auditory stimuli precisely during up-states. Beyond mere memory improvements aligned with individual goals, targeted memory reactivation, under a highly efficient protocol, might also help to counteract maladaptive learning or phobic fears or to revive long-forgotten memories.
Understanding the functional significance of slow waves remains a particularly promising research agenda. Rhythmic stimulation to drive neural networks constitutes a valuable manipulation, going well beyond correlative strategies that used to dominate work in this area. More generally, phase-guided stimulation and closed-loop methods could be applied in other neuroscience contexts.
At present, slow oscillations during sleep can be conceived as a good way for the brain to organize neural synchrony in the service of consolidation. Working out the details of these brain rhythms and associated mechanisms could be particularly informative, with neural principles that apply widely (such as to waking-state cross-frequency coupling between brain oscillations of two different frequencies). With a closed-loop brain-computer interface that can produce resonance and enhance these brain rhythms (Figure 1), we are well positioned both to experimentally elucidate the value of brain dynamics during sleep, and to discover ways to drive these rhythms advantageously.
Figure 1.
A nighttime metronome for neuronal synchrony and memory enhancement.
Acknowledgments
We thank the National Science Foundation (grant BCS1025697), NIH (P01 AG11412), the AXA Research Fund, the BrightFocus Foundation, and the Bettencourt Schueller Foundation for support.
This is a commentary on article Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013 May 8;78(3):545-53.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ancoli-Israel S. Sleep Med. 2009;10:S7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Antony JW, Gobel EW, O’Hare JK, Reber PJ, Paller KA. Nat Neurosci. 2012;15:1114–1116. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer L, Constantinescu I, Perrig S, Vienne J, Vidal PP, Muhlethaler M, Schwartz S. Curr Biol. 2011;21:R461–462. doi: 10.1016/j.cub.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Mölle M, Born J. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Born J. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Ngo HV, Claussen JC, Born J, Mölle M. J Sleep Res. 2012;22:22–31. doi: 10.1111/j.1365-2869.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, Mölle M. Neuron. 2013 doi: 10.1016/j.neuron.2013.03.006. this issue. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Paller KA. Trends Cogn Sci. 2013;17:142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Heib DP, Boly M, Desseilles M, Vandewalle G, Schmidt C, Albouy G, Darsaud A, Gais S, et al. Front Neurol. 2012;3:40. doi: 10.3389/fneur.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Nat Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Riedner BA, Hulse BK, Ferrarelli F, Sarasso S. Medica Mundi. 2010;54:82–88. [Google Scholar]
- Tononi G, Cirelli C. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, Paller KA. J Int Neuropsychol Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]