SUMMARY
Disturbed sleep and on-the-job sleepiness are widespread problems among night shift workers. The pineal hormone melatonin may prove to be a useful treatment because it has both sleep-promoting and circadian phase-shifting effects. This study was designed to isolate melatonin’s sleep-promoting effects, and to determine whether melatonin could improve daytime sleep and thus improve night time alertness and performance during the night shift. The study utilized a placebo-controlled, double-blind, cross-over design. Subjects (n = 21, mean age = 27.0 ± 5.0 years) participated in two 6-day laboratory sessions. Each session included one adaptation night, two baseline nights, two consecutive 8-h night shifts followed by 8-h daytime sleep episodes and one recovery night. Subjects took 1.8 mg sustained-release melatonin 0.5 h before the two daytime sleep episodes during one session, and placebo before the daytime sleep episodes during the other session. Sleep was recorded using polysomnography. Sleepiness, performance, and mood during the night shifts were evaluated using the multiple sleep latency test (MSLT) and a computerized neurobehavioral testing battery. Melatonin prevented the decrease in sleep time during daytime sleep relative to baseline, but only on the first day of melatonin administration. Melatonin increased sleep time more in subjects who demonstrated difficulty in sleeping during the day. Melatonin had no effect on alertness on the MSLT, or performance and mood during the night shift. There were no hangover effects from melatonin administration. These findings suggest that although melatonin can help night workers obtain more sleep during the day, they are still likely to face difficulties working at night because of circadian rhythm misalignment. The possibility of tolerance to the sleep-promoting effects of melatonin across more than 1 day needs further investigation.
Keywords: circadian rhythms, human, melatonin, performance, shift work, sleep
INTRODUCTION
Shift work is associated with numerous negative effects, the most prominent of which is disturbed sleep (e.g. Akerstedt 1990; Folkard and Monk 1985; U.S. Congress and Office of Technology Assessment 1991). Night shift workers experience difficulty in sleeping in part because their internal circadian rhythms rarely phase shift to align with the sleep–wake schedule demanded by their jobs (e.g. Eastman et al. 1995; Quera-Salva et al. 1996; Roden et al. 1993). Because of this circadian misalignment, night workers are forced to work when their physiology is primed for sleep (e.g. Akerstedt 1988; Dinges 1995; Mitler et al. 1988) and must try to sleep when they are physiologically driven towards wakefulness (e.g. Akerstedt 1995; Tilley et al. 1982). The daytime sleep of night workers is shorter than that obtained at night, usually by about 2 h, although estimates as high as 4 h have also been reported (e.g. Akerstedt 1984; Tilley et al. 1982; Walsh et al. 1981). In addition, polysomnographic studies have demonstrated various differences in daytime sleep architecture compared with nocturnal sleep, including reductions in REM and Stage 2 sleep (e.g. Dahlgren 1981; Tilley et al. 1982; Walsh et al. 1981), increases in Stage 1 sleep (Weitzman et al. 1970), shorter REM latencies (Walsh et al. 1981; Weitzman et al. 1970) and premature awakenings (Akerstedt et al. 1982; Weitzman et al. 1970). The negative consequences of night workers’ sleep disruption can impinge on both the health of the workers (e.g. Moore-Ede and Richardson 1985) and on public safety (Akerstedt 1988; Lyznicki et al. 1998; Mitler et al. 1988).
Exogenous administration of the pineal hormone melatonin may be a promising treatment for improving daytime sleep in night workers. The soporific effects of melatonin have been described for decades (e.g. Lerner and Case 1960; Rollag and Niswender 1975). In addition, melatonin has low toxicity (Lerner and Nordlund 1978; Sugden 1983), and has not been associated with any serious negative physiological effects when administered to healthy humans (Arendt 1997; Sack et al. 1997). Some studies suggest that melatonin exerts a sleep-promoting effect only when endogenous melatonin levels are low (i.e. during the daytime), whereas when endogenous levels are high (i.e. at night), exogenous melatonin may not produce substantial effects (Dawson and Encel 1993; Stone et al. 2000). Indeed, melatonin has been shown to increase subjective estimates of daytime sleep or sleepiness consistently in doses ranging from 0.1 to 240 mg (e.g. Arendt et al. 1984; Dollins et al. 1994; French et al. 1993; Lieberman et al. 1984; Nickelsen et al. 1989), and has also been shown to increase sleep during evening naps (e.g. Nave et al. 1995; Stone et al. 2000; Zhdanova et al. 1995). Studies of melatonin administered to promote night-time sleep, however, have produced mostly negative results (e.g. Dawson and Encel 1993; James et al. 1987; Stone et al. 2000).
A recent study specifically examined the circadian-dependent aspects of melatonin’s sleep promoting properties using a forced-desynchrony protocol (Wyatt et al. 1999). Preliminary data indicate that melatonin administration (0.3 and 5.0 mg) increased total sleep time (TST) when subjects slept out of circadian phase (i.e. when endogenous melatonin levels were low), but had no effect when subjects slept in phase with their circadian rhythms and thus had high sleep efficiencies and naturally elevated endogenous melatonin levels.
Very few studies have examined melatonin’s effects on daytime sleep using polysomnography (PSG). Two studies investigated melatonin’s effects on 4-h daytime sleep opportunities. In one, subjects had a 7-h nocturnal sleep episode before melatonin (1, 10 or 40 mg) was administered at 10:00 h, and daytime sleep was recorded from 12:00 to 16:00 h (Hughes and Badia 1997). Compared with placebo, all doses of melatonin decreased sleep latency and increased sleep efficiency. In addition, melatonin appeared to increase Stage 2% and decrease slow wave sleep (SWS) % relative to placebo. In the other investigation, subjects underwent a night of sleep restriction (4 h of nocturnal sleep) followed by a 4-h sleep recording from 13:00 to 17:00 h (Dijk et al. 1995). Melatonin (5 mg) administered at 12:30 h did not alter sleep duration or visually scored sleep stages compared with placebo, but did increase EEG power density in the sleep spindle frequency range and decrease power density in a higher frequency band typically associated with wakefulness. Although the results of these studies are encouraging, neither directly addressed the question of whether melatonin can help night shift workers, because the short (4 h) sleep opportunities were not long enough to provide adequate sleep as the major sleep episode of the day.
A recent study examined melatonin’s sleep promoting effects using a longer daytime sleep opportunity. Melatonin was administered before a single 8 h diurnal sleep opportunity after a full night of sleep. Compared with placebo, the 5 mg dose of melatonin increased PSG measures of total sleep time, REM % and Stage 2% (Matsumoto 1999).
In each of these polysomnographic studies, melatonin improved at least one measure of daytime sleep relative to placebo. Nevertheless, none of the studies assessed daytime sleep after a night of work and wakefulness – the situation of a real night worker. Furthermore, none of the studies assessed performance or alertness during the night shift.
The present study examined the effects of 1.8 mg sustained-release melatonin on sleep during two consecutive daytime sleep episodes. Each episode was the main sleep period of the day and followed a night of simulated night work. We also assessed the effects of melatonin administration on sleep architecture, and objectively evaluated sleepiness and performance during the night shifts.
METHODS
Participants
Twenty-one healthy adults (9 women, 12 men) between the ages of 18–37 (mean age ± SD = 27.0 ± 5.0 years) completed the study. Participants had no obvious medical, psychiatric or sleep disorders as assessed from telephone and in-person interviews, medical history, and several screening questionnaires. They were free from prescription medications, including oral contraceptives. To minimize any effect of irregular sleep schedules on entrained circadian phase, we excluded individuals who had worked a night shift job during the 3 months prior to the study or who had travelled across more than two time zones during the month prior to the study. We restricted the mg/kg dose of melatonin to within a relatively narrow range by excluding individuals who weighed >100 kg. We also excluded potential subjects with body mass indices >29 kg m−2. The protocol was approved by the Rush-Presbyterian-St Luke’s Medical Center Institutional Review Board. All subjects gave written informed consent and were paid for their participation.
Protocol
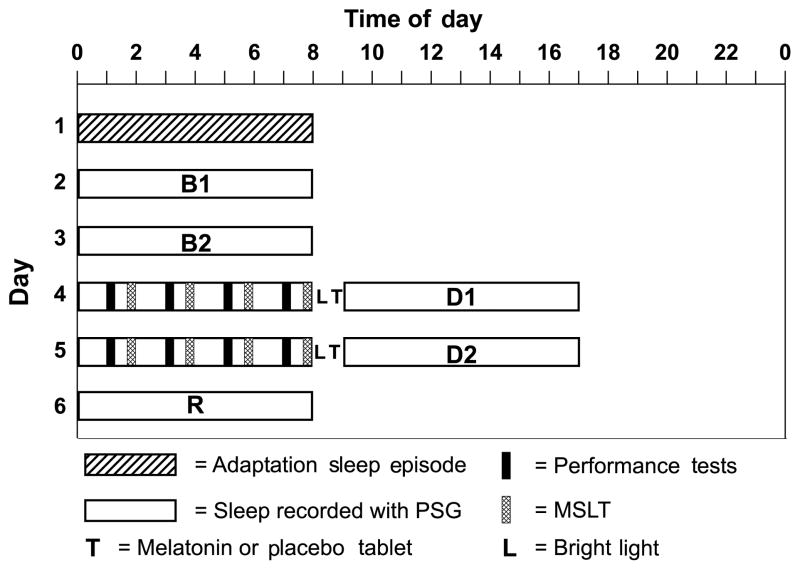
We used a within-subjects, double-blind, counterbalanced, cross-over design to examine the sleep-promoting effects of melatonin (1.8 mg sustained-release) compared with placebo on daytime sleep after simulated night work. Subjects completed two 6-day laboratory sessions (see Fig. 1). Each session consisted of one adaptation night for subjects to become accustomed to sleeping in the laboratory while wearing electrodes, two baseline nights, two night shifts followed by daytime sleep and one recovery night. Subjects were allowed to leave the laboratory during days 1–3 and after the daytime sleep episodes on days 4–5, and were instructed not to nap when they were outside the laboratory. All scheduled sleep episodes were 8 h long, and subjects were required to remain in bed and try to sleep for 8 h during all sleep episodes. The time of baseline sleep was customized for each subject on the basis of sleep logs kept before the study began. The average ±SD scheduled baseline bedtime was 23.27 ± 00.55 h. The night shifts occurred at the same clock time as the baseline sleep, and there was 1 h between the end of the night shifts and day sleep bedtime. Subjects took melatonin 0.5 h before both daytime sleep episodes in one session, and placebo 0.5 h before the daytime sleep episodes in the other session under double-blind conditions. The order of the sessions was counterbalanced, and there was at least 1 week between sessions (average time between sessions 4 ± 2 weeks).
Figure 1.
Sample protocol diagram for a participant with baseline sleep scheduled from 00:00 to 08:00 h. Each subject participated in the protocol twice; melatonin was administered during one session and placebo was administered during the other session. B1 = Baseline 1; B2 = Baseline 2; D1 = Day Sleep 1; D2 = Day Sleep 2; R = recovery.
Five of the female subjects were studied in the follicular phase of the menstrual cycle during both sessions; two were studied during the luteal phase during both sessions, and two were studied in the follicular phase during the melatonin session and in the luteal phase during the placebo session.
Polysomnography
Electroencephalograph (EEG) was recorded from C3/A2, C4/A1, O1/A2 and O2/A1 electrodes applied to the scalp with gauze and collodion. Left and right EOG were recorded by electrodes taped to the skin at the outer canthi of both eyes. EMG was recorded from electrodes taped to the mentalis/submentalis region. EKG was recorded from electrodes taped to the right and left shoulders. All recordings were made on Nihon Kohden polygraphs (Foothill Ranch, CA, USA) at a paper speed of 10 mm s−1. Sleep was scored visually in 30-s epochs using standard criteria (Rechtschaffen and Kales 1968) by the first author who was blind to treatment condition and who maintained >90% staging concordance with other trained sleep scorers as well as within-rater concordance of >90%. The following variables were included in the reduction and analyses of sleep data: TST, sleep efficiency (the percent of time in bed spent asleep; SE), sleep latency (time from lights out to the first epoch of any stage of sleep; SL), percent of total sleep time of Stages 1, 2, 3 and 4, REM sleep and SWS (Stage 3 + Stage 4), wake time during the sleep period (WASO), wake time after the final awakening (WAFA), transient arousal index (number of transient arousals per h of sleep; TAI) and body movement index (number of body movements per h of sleep; MI).
Three of the 210 PSG recordings were not usable because of technical problems: the second placebo session baseline night for one subject, and the second placebo session day sleep and placebo session recovery night for one subject. In addition, proper sleep staging was not possible for two records because the incorrect EEG derivations were recorded: the second placebo session baseline night and the first placebo session day sleep for one subject; for these two recordings, only TST, REM, and NREM sleep were summarized. These subjects were not included in all analyses, and the degrees of freedom reflect where their data were omitted from the statistics.
Subjective sleep and sleepiness
Immediately prior to lights out time before each sleep episode, and immediately after waking, participants completed the Stanford sleepiness scale (SSS) (with 1 = ‘feeling active and vital; alert; wide awake’ and 7 = ‘almost in reverie; sleep onset soon; must struggle to remain awake’) (Hoddes et al. 1973), and a 100-mm visual analogue scale (VAS) with 0 = most sleepy and 100 = most alert. Immediately after waking, subjects completed a sleep log to estimate their latency to sleep onset, total sleep time and number of awakenings during the previous sleep episode.
Simulated night shifts
Subjects completed four bouts of the computerized neurobehavioral assessment battery (NAB) (Dinges et al. 1997) at 2 h intervals beginning 1 h after the start of the night shifts. The NAB takes about 20 min to complete and includes several tests which are sensitive to sleepiness/decreased alertness: the SSS (Hoddes et al. 1973), two visual analogue scales (physical and mental exhaustion), a probed recall memory task, a psychomotor vigilance task (PVT), the activation–deactivation adjective checklist (ADACL) (Thayer 1978), the digit symbol substitution test, a time estimation task and a subjective effort questionnaire. In order to familiarize the subjects with the testing protocol, subjects completed practice performance batteries after their electrodes were attached on nights 1–3 during the first session and on night 3 during the second session. Subjects were monitored by a research assistant during each test battery.
Four multiple sleep latency test (MSLT) (Carskadon and Dement 1987) naps were given at 2 h intervals beginning 1 h and 40 min after the beginning of the night shifts. For each nap, subjects were in bed in a darkened bedroom, and were asked to lie quietly with their eyes closed and try to fall asleep. If subjects fell asleep, they were awakened after three continuous epochs of unequivocal sleep so that they did not accumulate a significant amount of sleep during the MSLT. Sleep latency was defined as the first 30-s epoch with >15 s of sleep. If a subject remained awake, the nap ended after 20 min and a sleep latency of 20 min was used in data analysis.
During free time, subjects sat at a table and engaged in quiet activities, such as reading, playing board games, listening to music and watching television, under the supervision of a research assistant. Food and non-caffeinated drinks were available throughout the night shifts and were allowed ad libitum except between the performance battery and the sleep latency test. The average light intensity during the night shifts was 209 ± 43 lux measured at the level of the forehead using a light meter (Extech Instruments, Waltham, MA, USA).
Bright light exposure
The 9 h delay of the sleep schedule from baseline to daytime sleep and the timing of melatonin administration could have caused a phase delay of circadian rhythms, especially because this study was executed in a laboratory setting. In a real-world night work situation, circadian rhythm phase shifts are minimal, mostly because of the fact that the workers are exposed to morning bright light whilst travelling home. Morning light exposure usually occurs during the phase-advance portion of the phase response curve and prevents the circadian rhythms from phase delaying (Eastman et al. 1994; Eastman and Martin 1999). To mimic a real night work situation, subjects in the present study were exposed to morning bright light to inhibit phase delays in circadian rhythms. Thus, any changes we measured in sleep would be because of the sleep-promoting effects of melatonin rather than the phase-delaying effects of melatonin and/or the shift in the sleep schedule. Immediately after each night shift, subjects sat about 25 cm in front of a light box (65 cm wide, 43.5 cm tall, with 6 cool-white fluorescent lamps, Apollo Light Systems, Orem, UT, USA) for 0.5 h. The light intensity was 9145 ± 1011 lux measured at the level of the forehead.
Saliva samples
Subjects gave a 2-mL saliva sample immediately before bed time and at the conclusion of the 8 h daytime sleep episodes so that the levels of melatonin present after ingestion of the exogenous melatonin tablets could be determined. Saliva samples were collected with Salivette devices (Sardtsted, Newton, NC, USA) and were centrifuged and frozen at −9°C. The samples were later packed in dry ice and shipped overnight to DiagnosTech (Osceola, WI, USA) to be radioimmunoassayed for melatonin. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg mL−1 and the intra- and interassay variations were 7.5 and 9.6%, respectively.
Other procedures
Subjects wore wrist activity monitors (BMA-32 Model, Ambulatory Monitoring Inc., Ardsley, NY, USA) on their nondominant wrists for 1 day before starting each session and during the 6-day laboratory sessions to confirm that they adhered to a regular schedule before starting the study and to verify that they did not nap when they were outside the laboratory. Actigraphy data were examined periodically to encourage compliance. A single subject reported napping for 1 h before the first night shift of the placebo session, and the nap was confirmed with actigraphy. This subject’s data were indistinguishable from data of subjects who did not nap, and thus her data were included in the final sample.
As part of the prestudy screening, each subject completed the Horne and Ostberg Morningness–Eveningness Questionnaire (MEQ) (Horne and Ostberg 1976). The average ± SD MEQ score was 50.6 ± 14.5. Five subjects were ‘morning’ types, three subjects were ‘evening’ types and the remaining 13 subjects were ‘neither’ types.
Caffeine intake was restricted such that subjects who regularly drank caffeinated beverages could consume caffeine during the study, but they were required to drink the same amount every day and only within the first hour after their scheduled wake time.
The study was conducted in January–May 1998, January–June 1999 and October–December 1999.
Data analysis
Data were analysed using SPSS for Windows (Ver. 8.0, Evanston, IL, USA). To assess the effects of melatonin administration on the various sleep parameters we ran 2 × 5 repeated measures ANOVAs with non-orthogonal planned contrasts. The within-subjects factors were drug (melatonin, placebo) and day (Baseline 1, Baseline 2, Day Sleep 1, Day Sleep 2, Recovery). The planned contrasts tested specific hypotheses about whether the sleep parameters from the two daytime sleep episodes and the recovery sleep episode differed from baseline as a function of melatonin administration; specifically, we compared Baseline 1 and 2 vs. Day Sleep 1, Baseline 1 and 2 vs. Day Sleep 2, and Baseline 1 and 2 vs. Recovery. Potentially significant effects of melatonin administration between baseline and the comparison periods (Day Sleep 1, Day Sleep 2, or Recovery) were indicated by significant drug by day interactions in the planned contrasts; for these interactions, we considered a probability value of 0.05 statistically significant. The significant drug by day interactions were then interpreted with paired-samples t-tests. To correct for type I error associated with multiple comparisons, we used Bonferroni corrections for the t-tests and set α = 0.025. To analyse the effects of melatonin on MSLT sleep latencies and mood and performance during the night shift, we used repeated-measures ANOVAs with factors drug (melatonin, placebo), night shift (first, second) and nap/test bout (first, second, third, fourth). A probability value of 0.05 was considered statistically significant. Mean data are presented ± SD unless otherwise specified.
RESULTS
Daytime sleep
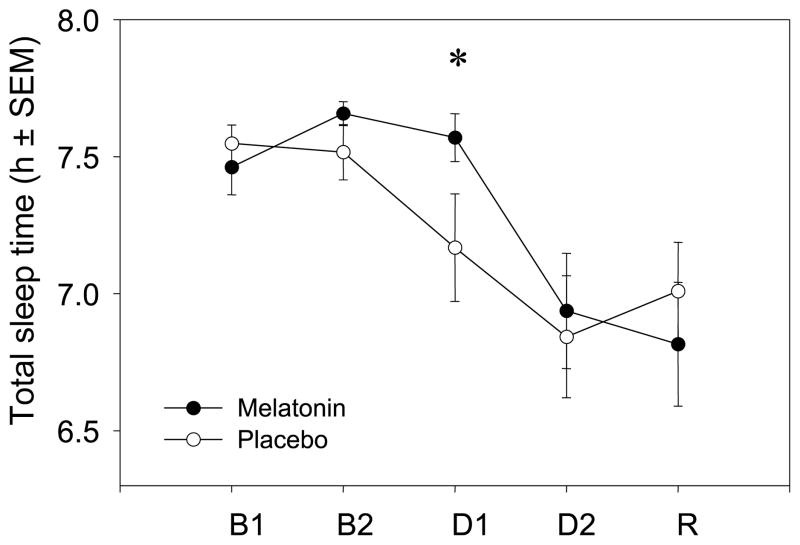
Melatonin administration increased sleep time only during the first daytime sleep episode. As illustrated in Fig. 2, PSG recordings demonstrated that subjects had longer TST with melatonin compared with placebo during Day Sleep 1, but that melatonin administration had no effect on TST during Day Sleep 2. Similar results were obtained for subjective sleep times recorded on sleep logs. Sleep parameters from PSG and sleep logs are listed in Tables 1 and 2, respectively. On Day Sleep 1, subjects had 24 min more sleep after melatonin compared with placebo according to PSG and 26 min more sleep according to sleep logs. There were four significant drug by Day interactions in the Baseline vs. Day Sleep 1 planned contrasts – PSG TST (F1,19 = 6.05, P = 0.024), sleep log TST (F1,19 = 7.09, P = 0.015), PSG sleep efficiency (F1,19 = 5.97, P = 0.024) and PSG WASO (F1,19 = 5.06, P = 0.036). These interactions indicated that the sleep parameters differed between Baseline and Day Sleep 1 during only one session, i.e, during either the melatonin or placebo session. To interpret the interactions, we performed paired-samples t-tests between the placebo and melatonin sessions for each sleep parameter. The t-tests showed that on Day Sleep 1 after melatonin, subjects had longer sleep durations (PSG: t1,20 = 2.658, P = 0.015; Sleep Log: t1,19 = 2.585, P = 0.018), higher sleep efficiencies (t1,20 = 2.67, P = 0.015) and less WASO (t1,20 = −2.595, P = 0.017), compared with after placebo. There were no differences in these sleep parameters between the melatonin and placebo sessions at baseline. Furthermore, there were no drug by day interactions for the sleep parameters examined in the planned contrasts between Baseline and Day Sleep 2; melatonin administration did not affect sleep on Day Sleep 2.
Figure 2.
Total sleep times (mean ± SEM) recorded with polysomnography during the melatonin session (●) and placebo session (○). B1 = Baseline 1; B2 = Baseline 2; D1 = Day Sleep 1; D2 = Day Sleep 2; R = Recovery. Melatonin or placebo was administered 0.5 h before sleep on D1 and D2. Time in bed was 8 h. n = 21, *P < 0.05 between melatonin and placebo.
Table 1.
Sleep parameters from polysomnography (mean ± SD)
| Total sleep time (min) | Sleep efficiency (%) | Sleep latency (min) | WASO (min) | WAFA (min) | Movement index | Transient arousal index |
Stage 1 (%) | Stage 2 (%) | Stage 3 (%) | Stage 4 (%) | SWS (%) | REM (%) | REM latency | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline 1 | ||||||||||||||
| Melatonin | 448 ± 28 | 93.3 ± 5.8 | 11 ± 9 | 20 ± 21 | 1 ± 6 | 5.9 ± 2.3 | 5.7 ± 2.1 | 5.0 ± 2.7 | 57.6 ± 7.5 | 7.8 ± 2.9 | 7.5 ± 6.7 | 15.4 ± 7.7 | 22.0 ± 5.5 | 93 ± 38 |
| Placebo | 453 ± 19 | 94.4 ± 3.9 | 8 ± 5 | 17 ± 19 | 1 ± 2 | 6.8 ± 3.5 | 6.5 ± 3.2 | 5.0 ± 2.0 | 57.2 ± 8.5 | 7.8 ± 3.1 | 7.4 ± 6.2 | 15.2 ± 7.5 | 22.6 ± 3.9 | 75 ± 13 |
| Baseline 2 | ||||||||||||||
| Melatonin | 459 ± 12 | 95.7 ± 2.5 | 6 ± 4 | 12 ± 10 | 1 ± 3 | 6.7 ± 2.5 | 6.4 ± 2.0 | 4.3 ± 2.4 | 55.2 ± 7.4 | 8.3 ± 3.1 | 7.2 ± 5.7 | 15.5 ± 6.7 | 24.9 ± 3.3 | 82 ± 26 |
| Placebo | 456 ± 18 | 94.8 ± 3.6 | 8 ± 5 | 15 ± 17 | 1 ± 2 | 6.7 ± 2.9 | 6.3 ± 2.8 | 3.8 ± 2.0 | 55.3 ± 7.2 | 8.2 ± 3.2 | 8.6 ± 6.1 | 16.7 ± 7.4 | 24.1 ± 3.2 | 72 ± 13 |
| Day sleep 1 | ||||||||||||||
| Melatonin | 454 ± 24* | 94.5 ± 5.0* | 2 ± 1 | 19 ± 19* | 4 ± 16 | 5.6 ± 2.8 | 5.2 ± 1.9 | 4.0 ± 2.1 | 56.1 ± 7.4 | 8.6 ± 3.3 | 11.1 ± 7.5 | 19.7 ± 7.8 | 20.2 ± 4.8 | 60 ± 24 |
| Placebo | 430 ± 54 | 89.5 ± 11.2 | 2 ± 2 | 40 ± 47 | 7 ± 16 | 6.0 ± 2.8 | 5.9 ± 2.3 | 4.1 ± 2.6 | 53.0 ± 8.4 | 10.4 ± 2.9 | 12.5 ± 7.5 | 22.9 ± 8.1 | 20.3 ± 5.0 | 64 ± 13 |
| Day sleep 2 | ||||||||||||||
| Melatonin | 416 ± 58 | 86.6 ± 2.0 | 4 ± 3 | 43 ± 45 | 16 ± 43 | 5.8 ± 3.1 | 6.1 ± 2.5 | 5.9 ± 3.0 | 53.3 ± 8.2 | 9.5 ± 3.6 | 9.2 ± 7.5 | 18.7 ± 7.8 | 22.0 ± 5.2 | 60 ± 11 |
| Placebo | 411 ± 63 | 85.4 ± 13.0 | 3 ± 2 | 22 ± 5 | 35 ± 37 | 5.7 ± 2.9 | 5.3 ± 2.2 | 4.8 ± 2.6 | 53.9 ± 9.1 | 9.2 ± 3.2 | 9.9 ± 6.5 | 19.1 ± 8.6 | 22.3 ± 4.7 | 60 ± 14 |
| Recovery | ||||||||||||||
| Melatonin | 409 ± 62 | 85.1 ± 2.9 | 23 ± 31 | 32 ± 36 | 15 ± 37 | 6.4 ± 3.3 | 6.7 ± 3.2 | 5.5 ± 2.4 | 59.5 ± 8.9 | 7.5 ± 3.3 | 5.6 ± 5.0 | 13.1 ± 6.3 | 21.8 ± 5.0 | 73 ± 23 |
| Placebo | 421 ± 50 | 87.5 ± 10.4 | 21 ± 19 | 31 ± 42 | 6 ± 13 | 6.5 ± 3.0 | 6.5 ± 2.5 | 4.6 ± 1.6 | 59.7 ± 6.9 | 6.8 ± 2.7 | 5.9 ± 4.9 | 12.7 ± 6.6 | 23.0 ± 3.3 | 68 ± 13 |
P < 0.05, melatonin vs. placebo.
Table 2.
Sleep parameters from subjects’ sleep logs (mean ± SD)
| Total sleep time (min) | Sleep latency (min) | Number of wakes | |
|---|---|---|---|
| Baseline 1 | |||
| Melatonin | 432 ± 46 | 16 ± 10 | 2.2 ± 1.2 |
| Placebo | 451 ± 24 | 13 ± 7 | 1.7 ± 1.6 |
| Baseline 2 | |||
| Melatonin | 454 ± 23 | 14 ± 6 | 2.0 ± 1.6 |
| Placebo | 453 ± 33 | 11 ± 7 | 1.7 ± 1.6 |
| Day sleep 1 | |||
| Melatonin | 461 ± 40* | 7 ± 7 | 1.6 ± 1.6 |
| Placebo | 435 ± 53 | 6 ± 4 | 1.4 ± 1.6 |
| Day sleep 2 | |||
| Melatonin | 425 ± 49 | 9 ± 7 | 1.8 ± 1.6 |
| Placebo | 421 ± 52 | 7 ± 5 | 2.1 ± 1.6 |
| Recovery | |||
| Melatonin | 413 ± 80 | 35 ± 52 | 1.8 ± 1.6 |
| Placebo | 405 ± 96 | 17 ± 11 | 1.6 ± 1.6 |
P < 0.05, melatonin vs. placebo.
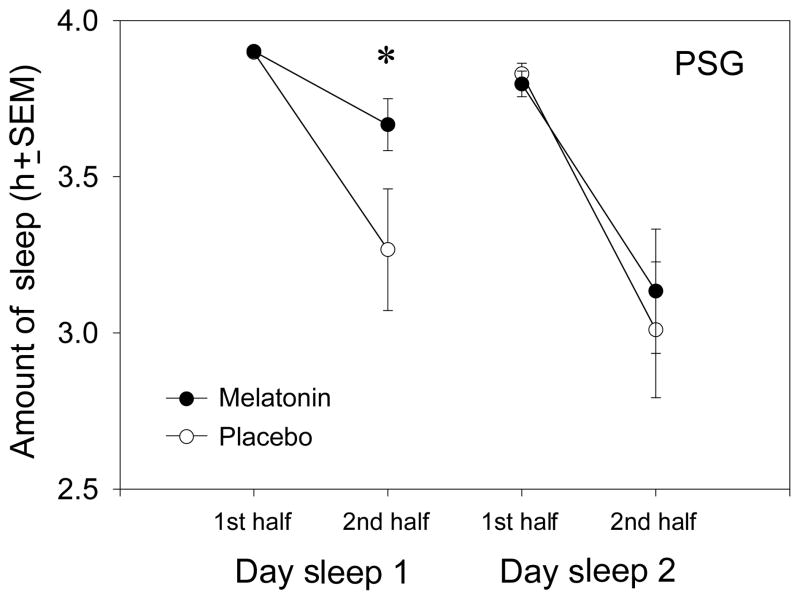
Further analysis of the daytime sleep episodes by PSG showed that melatonin exerted its effect on sleep during Day Sleep 1 mainly by increasing sleep duration during the second half of the sleep episode (see Fig. 3). Subjects had an average of 24 ± 42 min more sleep during the second half of Day Sleep 1 after melatonin compared with after placebo, and repeated measures ANOVA with factors drug, day and half showed a significant drug by half interaction (F1,18 = 4.99, P = 0.038). Figure 3 shows that subjects slept for nearly the entire 4 h during the first half of all daytime sleep episodes. The decrease in daytime sleep occurred during the second half of the daytime sleep episodes. Again, melatonin significantly attenuated the decrease only during day Sleep 1.
Figure 3.
Amount of polysomnographic sleep (mean ± SEM) during the first and second halves of the day sleep episodes (first and last 4 h) during the melatonin session (●) and the placebo session (○). *P < 0.05 between melatonin and placebo.
Recovery sleep
Subjects slept less during the recovery night than during baseline (see Fig. 2), most probably because they had only been awake for 7 h before recovery bedtime. Subjects had average PSG TSTs ranging from 3.9 to 7.9 h (mean TST = 7.0 ± 0.8 h) during the recovery night after placebo and from 5.0 to 7.8 h (mean TST = 6.8 ± 1.0 h) during the recovery night after melatonin. There were no significant effects of melatonin administration on any PSG or sleep log recovery sleep parameters, i.e. no significant drug by day interactions in the Baseline vs. Recovery planned contrasts. Total sleep time on the recovery nights was not correlated with the amount of sleep subjects obtained during the two daytime sleep episodes, nor was it correlated with the level of sleepiness exhibited during the night shift (average sleep latencies measured during the MSLTs, see below).
Salivary melatonin levels
After taking the placebo pill, subjects’ melatonin levels at bedtime were 16.9 ± 26.0 pg mL−1 and 14.7 ± 16.1 pg mL−1 on Day 1 and Day 2, and at wake time were 2.1 ± 2.0 and 1.7 ± 1.7 pg mL−1 on Day 1 and Day 2. Bedtime melatonin levels 0.5 h after taking the melatonin pill were 145.3 ± 103.8 and 142.0 ± 104 pg mL−1 on Days 1 and 2, respectively; at wake time, 8.5 h after taking the melatonin pill, melatonin levels were 24.2 ± 25.9 and 20.5 ± 15.8 pg mL−1 on Days 1 and 2. Repeated measures ANOVA with factor drug (melatonin, placebo), day (Day Sleep 1, Day Sleep 2) and time (bedtime, wake time) showed a significant main effect of drug. Thus, administration of the melatonin tablets produced significantly higher levels of salivary melatonin both at bedtime and wake time compared with placebo (F1,19 = 44.73, P < 0.001). There was no effect of day; melatonin levels were similar after melatonin administration on Day 1 and Day 2. The levels of melatonin produced by the melatonin pill were not significantly correlated with sleep duration during either of the daytime sleep episodes.
Subjective reports of sleepiness
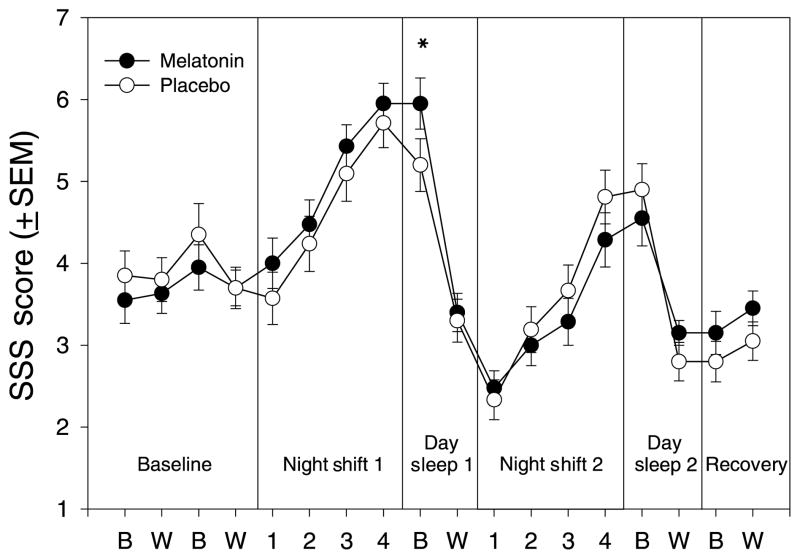
Figure 4 illustrates the subjects’ SSS ratings at bedtime, wake time, and during the night shifts. On Day Sleep 1, subjects were sleepier at bedtime after taking melatonin vs. after taking placebo. The planned contrasts between Baseline and Day Sleep 1 showed a significant Drug by Day interaction for the SSS ratings made at bedtime (F1,20 = 8.155, P = 0.01), and the paired-samples t-test showed that SSS ratings were significantly higher at bedtime after taking melatonin compared with placebo (t1,20 = 3.423, P = 0.003). Melatonin administration did not significantly affect SSS ratings at bedtime on Day Sleep 2, or at wake time on either Day Sleep 1 or Day Sleep 2. Melatonin had no effect on the VAS sleepiness ratings.
Figure 4.
Average Stanford sleepiness scale ratings (mean ± SEM) from bedtime questionnaires, wake time questionnaires, and test bouts during the night shifts. 1 = ‘feeling active and vital; alert; wide awake’ and 7 = ‘almost in reverie; sleep onset soon; must struggle to remain awake.’ (●) The melatonin session, (○) the placebo session. B = Bed-time ratings; W = Wake time ratings; numbers 1–4 indicate the sequential test bouts during the night shifts. *P < 0.01.
Sleepiness increased as the night shifts progressed (Fig. 4), but there were no significant differences between melatonin and placebo on night shift 2, the night shift after the subjects had taken melatonin or placebo before daytime sleep.
Sleepiness during the night shifts
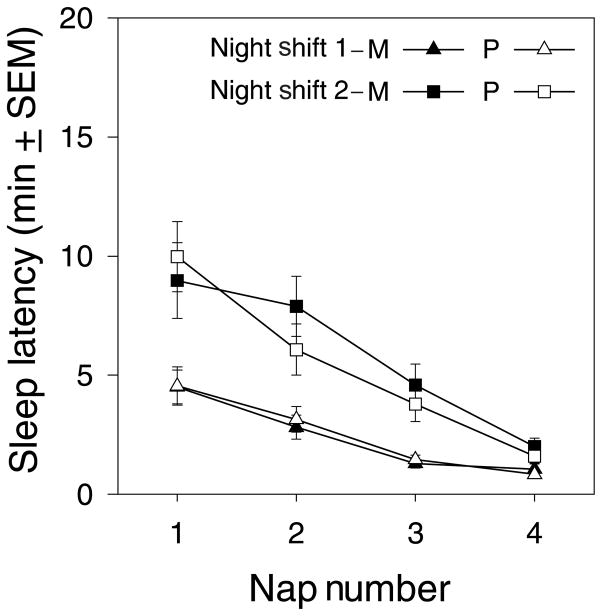
Figure 5 shows the MSLT data from the night shifts. Sleep latencies decreased as the night progressed and were shorter during night shift 1 compared with night shift 2. The same pattern was observed in SSS ratings, i.e. increasing sleepiness as the night progressed and more sleepiness during night shift 1 (Fig. 4). Melatonin administration before Day Sleep 1 had no effect on the MSLT during the subsequent (second) night shift. A repeated measures ANOVA with factors Drug, Night Shift, and Nap showed a significant effect of Night Shift (F1,20 = 48.76, P < 0.001) and Nap number (F1,20 = 22.43, P < 0.001), but no effect of drug.
Figure 5.
Average sleep latencies (mean ± SEM) from the four MSLT naps administered during the night shifts. M = Melatonin session; P = Placebo session. Filled symbols indicate the melatonin session and open symbols indicate the placebo session. Triangles indicate the first night shift and boxes indicate the second night shift.
Performance and mood during the night shift
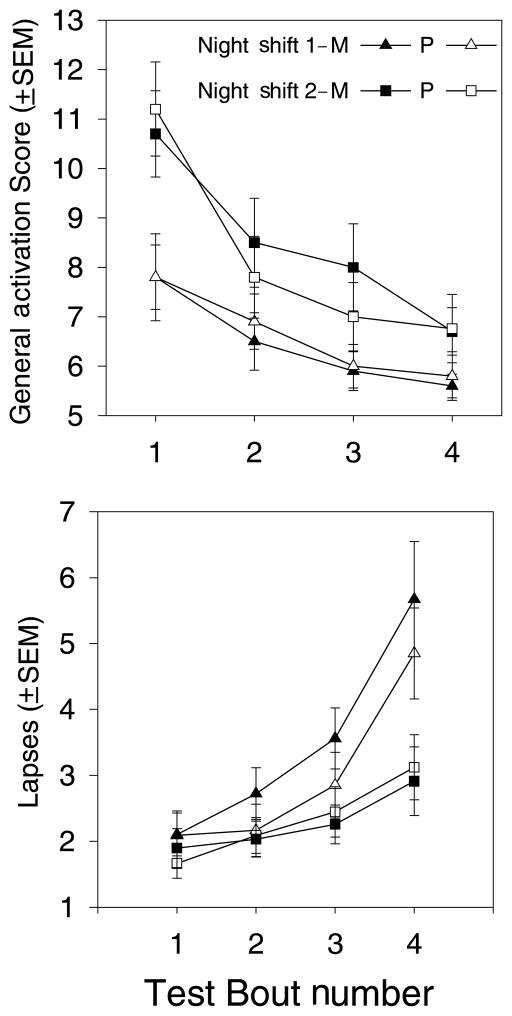
Figure 6 shows two examples of night shift mood and performance measures: general activation on the ADACL and PVT lapses. In general, subjects had better mood and performance during night shift 2 compared with night shift 1, and performance worsened and mood declined across the night shift. Repeated measures ANOVA showed significant main effects of Day and Test Bout for nearly all measures. On the other hand, of more than 20 mood and performance variables measured by the neurobehavioral testing battery during the night shifts, only one showed a main effect of melatonin administration – the subjective rating of effort to achieve performance on the test battery. Further inspection showed that this was an effect of session rather than melatonin administration; subjects reported expending more effort during night shift 1 of the melatonin session (before melatonin was administered for daytime sleep) than during the other night shifts.
Figure 6.
Examples of performance test results from the neurobehavioural assessment battery during the night shifts. Filled symbols indicate the melatonin session and open symbols indicate the placebo session. Triangles indicate the first night shift and boxes indicate the second night shift. Top: General activation (GA) scores (mean ± SEM) from the activation–deactivation adjective checklist (ADACL). To complete the ADACL, subjects rated 20 adjectives on whether they described their mood at the time of the test battery. The four possible ratings were ‘definitely feel’, ‘feel slightly’, ‘cannot decide at the moment’ and ‘definitely do not feel.’ The GA subscore is compiled from subjects’ responses to five descriptors (full-of-pep, active, vigorous, energetic, and lively). Bottom: Lapses (mean ± SEM) on the psychomotor vigilance task (PVT). This task measures visual reaction time during a 10-min vigilance task, and any reaction time longer than 0.5 s is considered a lapse. Lapses are plotted as the square root transformation of the number of reaction times >0.5 s.
Individual differences
About half of the subjects had high sleep efficiencies during all 4 daytime sleep episodes, i.e. even during daytime sleep after placebo administration. In these subjects, there was no room for melatonin to confer any substantial benefit over placebo. It was of interest therefore to explore the impact of melatonin administration on those who had difficulty in sleeping during the day. To do these post hoc analyses, we divided our subjects into two groups – those who had a sleep efficiency <85% during either daytime sleep episode of the placebo session (poorer sleepers, n = 11) and those whose sleep efficiencies were >85% during both daytime sleep episodes of the placebo session (better sleepers, n = 9).
In poorer sleepers, melatonin administration resulted in 45 ± 47 more minutes of sleep during Day Sleep 1 compared with placebo, and 13 ± 82 min more sleep during Day Sleep 2 (TST from PSG). Thus, melatonin administration did have a greater impact on poorer sleepers. However, the increase in sleep duration still occurred primarily during the first daytime sleep episode.
It was of interest to see if we could distinguish other differences between better and poorer sleepers aside from their ability to sleep during the day. Poorer sleepers were on average 3.5 years older than better sleepers (28.3 ± 5.1 vs. 24.8 ± 4.1 years), but this difference was not statistically significant (independent samples t-test: t1,18 = −1.654, P = 0.12). The poorer sleepers had baseline sleep times well within normal limits, but interestingly, their baseline sleep durations were significantly lower than those of better sleepers. Poorer sleepers obtained an average of 7.4 ± 0.4 h of sleep during the four baseline sleep episodes compared with better sleepers whose average TST during baseline was 7.7 ± 0.1 h (independent samples t-test: t1,18 = 3.903, P = 0.001). There were no differences in sex distribution or morningness– eveningness between the poorer sleepers and the better sleepers.
DISCUSSION
Sleep
In this simulated night work study with two consecutive daytime sleep episodes, administration of 1.8 mg sustained-release melatonin before daytime sleep prevented the decrease in sleep time that occurs from sleeping at the ‘wrong’ circadian phase. However, this effect was only observed during the first daytime sleep episode. During the second daytime sleep episode, subjects slept less than during baseline and melatonin did not alleviate this decline in sleep.
During the first half of the 8 h daytime sleep episodes, subjects slept for most of the 4 h even after taking placebo. Thus, there was a ceiling effect; there was no room for improvement with melatonin. The effect of melatonin was observed primarily during the second half of the first daytime sleep episode when TST increased by an average of 24 min, bringing subjects’ total sleep time to 7.6 ± 0.4 h after melatonin administration vs. 7.2 ± 0.9 h with placebo. The ability of melatonin to increase daytime sleep near the end of the sleep episode is consistent with the findings of Hughes and Badia (1997). In their study, melatonin increased sleep duration during a 4-h daytime sleep opportunity mainly by increasing TST during the last hour of sleep. In our study, this sleep-maintenance effect was borne out in the last half of the 8-h sleep episode.
It was of interest that our subjects did not display the profound insomnia typically associated with sleeping at the wrong circadian phase. Overall, subjects slept remarkably well during the day with average sleep efficiencies >85%. This is possibly because of the fact that our subjects had a long time to become accustomed to sleeping in the laboratory before daytime sleep was attempted, and they were young and healthy. Perhaps of even greater consequence were the dark, quiet laboratory bedrooms, which protected subjects from the usual disturbances that interfere with the sleep of night shift workers. Furthermore, we restricted caffeine intake and prohibited alcohol consumption in our sample. Nevertheless, when we looked only at the ‘poorer sleepers’, melatonin administration extended sleep duration by about 45 min during the first daytime sleep episode. This increase in daytime sleep duration is similar to that observed with short-acting benzodiazepines (Schweitzer et al. 1991; Walsh et al. 1988). It should be noted that our ‘poorer sleepers’ actually slept fairly well during the day, and it is conceivable that melatonin could confer even greater benefits on individuals who have more difficulty in sleeping at the wrong phase of the circadian cycle, e.g. middle-aged or older workers (e.g. Moline et al. 1992; Dijk et al. 1999).
In our protocol, subjects were required to remain in bed for the full 8 h during each sleep episode. It is possible that this protocol requirement diminished the effect of melatonin by forcing subjects to try to sleep when they wanted to get up, particularly during the placebo session. Most real night shift workers curtail their time in bed to less than 8 h (e.g. Pilcher et al. 2000). If our subjects had been allowed to choose when to get out of bed, we might have observed greater differences in TST between the melatonin and placebo sessions. On the other hand, because the sleep-promoting effect of melatonin during Day Sleep 1 was manifested mostly towards the end of the sleep episode, it is possible that the required 8 h in bed magnified the effect of melatonin, i.e. that the sleep-promoting effect of melatonin would not have been observed if subjects had been allowed to get out of bed earlier.
It is unclear why melatonin administration did not increase sleep duration during the second daytime sleep episode. The salivary melatonin levels achieved by administration of 1.8 mg sustained-release melatonin were equivalent before and after the daytime sleep episodes on Day Sleep 1 and Day Sleep 2. Nevertheless, subjects reported feeling sleepier after taking melatonin than after taking placebo on Day 1 but not on Day 2, and they slept more after melatonin compared with placebo on Day 1 but not on Day 2, suggesting a possible tolerance to melatonin’s sleep-promoting effects. Behavioural tolerance to melatonin administration has not been previously described in the literature, and no studies have specifically examined this question. Nevertheless, melatonin administration has been shown to have an inhibitory effect on the expression of melatonin receptors in animals (Gauer et al. 1993), and thus it is possible that elevated melatonin levels on Day 1 could have affected the expression of melatonin receptors in these subjects, such that melatonin receptors were down-regulated on Day 2. Although the receptor-mediated mechanism of melatonin’s sleep-promoting effect has not yet been elucidated, it is not unreasonable to hypothesize that prolonged elevation of daytime melatonin levels could have altered the expression of melatonin receptors in these subjects as it does in rats. Similar receptor regulation is observed in other G-protein coupled receptor systems (Ross 1996).
The lack of a sleep-promoting effect on Day Sleep 2 could also be related to the pharmacokinetics of the sustained-release preparation of melatonin that was used in this study. Melatonin has a short-half life of about ½ hour and 1.8 mg of regular, i.e. immediate release, melatonin would be expected to elevate melatonin levels for approximately 4–5 h. The subjects in this study had elevated melatonin levels for more than 8.5 h after taking the melatonin pill, as shown by the salivary melatonin levels measured at wake time. The median wake time was 16.30 h and the subjects’ normal nocturnal rise in endogenous melatonin secretion would have been expected to begin a few hours later (at around 21:00–23:00 h). Thus, it is possible that the elevation of melatonin levels by exogenous melatonin and the onset of endogenous melatonin secretion may have overlapped in some subjects. If this occurred, subjects’ melatonin levels would have never decreased to the usual barely detectable daytime levels before the Day 2 sleep episode. It is possible that a sharp onset of increased melatonin levels, as occurs with endogenous melatonin secretion, is the important neural signal for sleep promotion, rather than a sustained elevation in melatonin levels as may have been produced in this study. Future investigations could systematically address the relationship between pharmacokinetics of melatonin and its sleep-promoting properties.
Another explanation for the beneficial effect on Day Sleep 1 and not Day Sleep 2 is that melatonin’s sleep-promoting effects may vary as a function of sleep deprivation and prior wakefulness. Subjects had been awake for 25 h before Day Sleep 1 began, whereas they were only awake for 16 h prior to Day Sleep 2. It is possible that melatonin’s sleep-promoting properties are modulated by the organism’s homoeostatic sleep drive. If this is the case, it would appear that melatonin has greater sleep-promoting effects when there is more ‘sleep need’.
In studies where real night workers’ subjective ratings of daytime sleep were assessed across several consecutive days after melatonin administration, the data for a soporific effect have been equivocal and day-by-day sleep estimates have not been reported. In two of these studies (Folkard et al. 1993; Jorgensen and Witting 1998), 5 mg or 10 mg melatonin (or placebo) was administered to workers at bedtime after the night shift for 2–6 consecutive days, and daytime sleep durations increased by about 25 min. In another study (James, M et al. 1998), however, workers took 6 mg melatonin or placebo before daytime sleep for 4 consecutive days, and although melatonin decreased the number of self-reported awakenings from sleep compared with placebo, it had no effect on total sleep time. The sleep-promoting effects of melatonin on several consecutive days of daytime sleep needs further study before we will know if melatonin administration can be helpful to shift workers who must sleep during the day on two or more consecutive days.
Melatonin administration did not appear to result in hang-over effects in our study. Subjects did not report any more sleepiness at wake time after melatonin administration compared with placebo, nor did they perform worse on any of the cognitive performance tests during the night shift following melatonin administration compared with placebo. Similarly, melatonin administration did not appear to result in rebound insomnia; there were no differences in any sleep parameters on the recovery night between placebo and melatonin.
Sleep architecture
Our study showed no effect of melatonin on sleep stages during daytime sleep. These results are consistent with those of Dijk et al. (1995), who were unable to discern any effect of melatonin administration on daytime sleep stages. Their subjects had a night of partial sleep deprivation, and our subjects had full nights of wakefulness preceding their daytime sleep episode. On the other hand, these findings contrast with results from two studies where melatonin was administered before daytime sleep after a full night sleep (Hughes and Badia 1997; Matsumoto 1999). In both of these studies, melatonin increased Stage 2 sleep compared with placebo. In addition, melatonin decreased SWS in one study (Hughes and Badia 1997) and increased REM sleep in the other (Matsumoto 1999). It is possible that melatonin affects sleep architecture differently depending on the strength of the homeostatic pressure.
Sleepiness and performance during the night shifts
The first night shift was an acute sleep deprivation night, and thus the observed sleepiness on the MSLT and impairment on the neurobehavioral test battery were predictable and expected. During the second night shift, however, subjects showed marked sleepiness and performance decrements despite the fact that they had slept well during the preceding daytime sleep episode. The increase in daytime sleep to baseline levels, which was produced by melatonin, did not result in any benefit during the subsequent night shift. For instance, average sleep latencies on the MSLT were <6 min during the second night shift, and subjects were most impaired during the last 20 min of the night shift when they had average sleep latencies of about 2 min. This time of day is of particular concern vís a vís public safety and sleep related accidents because it is close to the time that real night shift workers travel home from work in the morning. The very short MSLT sleep latencies in our study suggest that despite a fairly long daytime sleep episode, night workers can be pathologically sleepy during, and especially towards the end of the night shift, and are therefore vulnerable to on-the-job accidents and bouts of unintended sleep at work and while they are driving home from the night shift. This impairment is because of circadian rhythm misalignment, and is not likely to be helped with countermeasures that focus solely on increasing daytime sleep duration. Countermeasures such as shifting circadian rhythms to align with the shift in the sleep-wake schedule (Eastman and Martin 1999; Sharkey and Eastman 2000), scheduling appropriately timed naps (Bonnet and Arand 1994; Schweitzer et al. 1992), and using alerting drugs such as caffeine (Bonnet and Arand 1994; Schweitzer et al. 1992; Wright et al. 1997) or modafinil (Pigeau et al. 1995) could be employed in conjunction with strategies to increase daytime sleep duration in order to obtain maximal benefit.
CONCLUSION
Melatonin administration prevented the decrease in sleep length which can occur from sleeping during the day. However, this beneficial effect occurred during the first daytime sleep episode only. Melatonin conferred no benefit over placebo during the second episode of daytime sleep, suggesting a possible tolerance to melatonin. Regardless of treatment (melatonin or placebo) daytime sleep duration were longer than those reported in several previous studies, emphasizing the importance of a quiet, protected sleep environment for improving daytime sleep. Although sleep duration were fairly long for daytime sleep (>7 h), subjects remained very sleepy during the subsequent night shift. Other strategies such as phase shifting circadian rhythms to align with the night-work, day-sleep schedule, are needed to help night shift workers overcome the negative effects of having to work at an inappropriate circadian phase.
Acknowledgments
We thank Sheri Allen, Vanitha P. Asokan, Shannon Kalmer, Stacia K. Martin, and Craig P. Stewart for assistance with data collection. We thank Stacia K. Martin for helpful comments on the manuscript and Phil Gehrman for statistical advice. We are indebted to Frank Diaz, Dr Ed Stepanski, and Dr James Wyatt for assisting with the polysomnographic sleep scoring validation. We are grateful to the volunteers who participated in this study. This research was supported by MH11239 to KMS and NS35695 to CIE. Melatonin and matching placebo were donated by Ecological Formulas, Concord CA.
References
- Akerstedt T. Psychological and psychophysiological effects of shift work. Scand J Work Environ Health. 1990;16 (Suppl 1):67–73. doi: 10.5271/sjweh.1819. [DOI] [PubMed] [Google Scholar]
- Akerstedt T. Sleepiness as a consequence of shift work. Sleep. 1988;11:17–34. doi: 10.1093/sleep/11.1.17. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Torsvall L, Gillberg M. Sleepiness and shift work: field studies. Sleep. 1982;5:S95–S106. doi: 10.1093/sleep/5.s2.s95. [DOI] [PubMed] [Google Scholar]
- Akerstedt T. Work hours, sleepiness and the underlying mechanisms. J Sleep Res. 1995;4 (Suppl 2):15–22. doi: 10.1111/j.1365-2869.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Akerstedt T. Work schedules and sleep. Experientia. 1984;40:417–422. doi: 10.1007/BF01952374. [DOI] [PubMed] [Google Scholar]
- Arendt J, Borbely AA, Franey C, Wright J. The effects of chronic, small doses of melatonin given in the late afternoon on fatigue in man: a preliminary study. Neurosci Lett. 1984;45:317– 321. doi: 10.1016/0304-3940(84)90245-3. [DOI] [PubMed] [Google Scholar]
- Arendt J. Safety of melatonin in long-term use? J Biol Rhythms. 1997;12:673–681. doi: 10.1177/074873049701200624. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. The use of prophylactic naps and caffeine to maintain performance during a continous operation. Ergonomics. 1994;37:1009–1020. doi: 10.1080/00140139408963714. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Daytime sleepiness. Quantification of a behavioral state. Neurosci Biobehav Rev. 1987;11:307–317. doi: 10.1016/s0149-7634(87)80016-7. [DOI] [PubMed] [Google Scholar]
- Dahlgren K. Adjustment of circadian rhythms and EEG sleep functions to day and night sleep among permanent nightworkers and rotating shiftworkers. Psychophysiology. 1981;18:381–391. doi: 10.1111/j.1469-8986.1981.tb02469.x. [DOI] [PubMed] [Google Scholar]
- Dawson D, Encel N. Melatonin and sleep in humans. J Pineal Res. 1993;15:1–12. doi: 10.1111/j.1600-079x.1993.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516.2:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Roth C, Landolt HP, et al. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci Lett. 1995;201:13–16. doi: 10.1016/0304-3940(95)12118-n. [DOI] [PubMed] [Google Scholar]
- Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4 (Suppl 2):4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light treatment for sleep disorders: consensus report. VI. Shift work. J Biol Rhythms. 1995;10:157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Stewart KT, Mahoney MP, Liu L, Fogg LF. Dark goggles and bright light improve circadian rhythm adaptation to night-shift work. Sleep. 1994;17:535–543. doi: 10.1093/sleep/17.6.535. [DOI] [PubMed] [Google Scholar]
- Folkard S, Arendt J, Clark M. Can melatonin improve shift workers’ tolerance of the night shift? Some preliminary findings. Chronobiol Int. 1993;10:315–320. doi: 10.3109/07420529309064485. [DOI] [PubMed] [Google Scholar]
- Folkard S, Monk TH. Hours of Work: Temporal Factors in Work-Scheduling. John Wiley & Sons Ltd; New York: 1985. [Google Scholar]
- French J, Hughes R, Whitmore J, Neville K, Strollo P, Reiter RJ. Diurnal melatonin induced effects on oral temperature and subjective fatigue. Sleep Res. 1993;22:83. [Google Scholar]
- Gauer F, Masson-Pevet M, Pevet P. Melatonin receptor density is regulated by rat pars tuberalis and suprachiasmatic nuclei by melatonin itself. Brain Res. 1993;602:153–156. doi: 10.1016/0006-8993(93)90256-m. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- James SP, Mendelson WB, Sack DA, Rosenthal NE, Wehr TA. The effect of melatonin on normal sleep. Neuropsychopharmacology. 1987;1:41–44. doi: 10.1016/0893-133x(87)90008-x. [DOI] [PubMed] [Google Scholar]
- James M, Tremea MO, Jones JS, Krohmer JR. Can melatonin improve adaptation to night shift? Am J Emergency Med. 1998;16:367–370. doi: 10.1016/s0735-6757(98)90129-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen KM, Witting MD. Does exogenous melatonin improve day sleep or night alertness in emergency physicians working night shifts? Ann Emerg Med. 1998;31:699–704. doi: 10.1016/s0196-0644(98)70227-6. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD. Intersociety symposium on new and neglected hormones: melatonin. Federation Proceedings. 1960;19:590–592. [Google Scholar]
- Lerner AB, Nordlund JJ. Melatonin clinical pharmacology. J Neural Transm. 1978;13 (Suppl):339–347. [PubMed] [Google Scholar]
- Lieberman HR, Waldhauser F, Garfield G, Lynch HJ, Wurtman RJ. Effects of melatonin on human mood and performance. Brain Res. 1984;323:201–207. doi: 10.1016/0006-8993(84)90290-7. [DOI] [PubMed] [Google Scholar]
- Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. JAM A. 1998;279:1908–1913. doi: 10.1001/jama.279.23.1908. [DOI] [PubMed] [Google Scholar]
- Matsumoto M. The hypnotic effects of melatonin treatment on diurnal sleep in humans. Psychiatry Clin Neurosciences. 1999;53:243–245. doi: 10.1046/j.1440-1819.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public policy: consensus report. Sleep. 1988;11:100–109. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moline ML, Pollak CP, Monk TH, et al. Age-related differences in recovery from simulated jet lag. Sleep. 1992;15:28–40. doi: 10.1093/sleep/15.1.28. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Richardson GS. Medical implications of shift-work. Annu Rev Med. 1985;36:607–617. doi: 10.1146/annurev.me.36.020185.003135. [DOI] [PubMed] [Google Scholar]
- Nave R, Peled R, Lavie P. Melatonin improves evening napping. Eur J Pharmacol. 1995;275:213–216. doi: 10.1016/0014-2999(94)00769-4. [DOI] [PubMed] [Google Scholar]
- Nickelsen T, Demisch L, Demisch K, Radermacher B, Schoffling K. Influence of subchronic intake of melatonin at various times of the day on fatigue and hormonal levels: a placebo-controlled, double-blind trial. J Pineal Res. 1989;6:325–334. doi: 10.1111/j.1600-079x.1989.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Pigeau R, Naitoh P, Buguet A, et al. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. I. Effects on mood, fatigue, cognitive performance and body temperature. J Sleep Res. 1995;4:212–228. doi: 10.1111/j.1365-2869.1995.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Lambert BJ, Huffcutt AI. Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep. 2000;23:155–163. [PubMed] [Google Scholar]
- Quera-Salva MA, Defrance R, Claustrat B, DeLattre J, Guilleminault C. Rapid shift in sleep time and acrophase of melatonin secretion in short shift work schedule. Sleep. 1996;19:539–543. [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects Public Health Service. U.S. Government Printing Office; Washington, D.C: 1968. [Google Scholar]
- Roden M, Koller M, Pirich K, Vierhapper H, Waldhauser F. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol. 1993;265:R261–R267. doi: 10.1152/ajpregu.1993.265.1.R261. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Niswender GD. Radioimmunoassay of serum concentrations of melatonin in sheep exposed to different lighting regimens. Endocrinology. 1975;98:482–489. doi: 10.1210/endo-98-2-482. [DOI] [PubMed] [Google Scholar]
- Ross EM. Pharmacodynamics: mechanisms of drug action and the relationship between drug concentration and effect. In: Hardman JG, Limbird LE, Molino PB, Ruddon RW, Gilman AG, editors. The Pharmacological Basis of Theraputics. McGraw-Hill; New York: 1996. pp. 29–41. [Google Scholar]
- Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- Schweitzer PK, Koshorek G, Muehlbach MJ, et al. Effects of estazolam and triazolam on transient insomnia associated with phase-shifted sleep. Human Psychopharmacol-Clin Exp. 1991;6:99–107. [Google Scholar]
- Schweitzer PK, Muehlbach MJ, Walsh JK. Countermeasures for night work performance deficits. the effect of napping or caffeine on continuous performance at night. Work Stress. 1992;6:355–365. [Google Scholar]
- Sharkey KM, Eastman CE. Melatonin phase shifts human circadian rhythms in a simulated night work study. J Sleep Res. 2000;9 (Suppl 1):173. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BM, Turner C, Mills SL, Nicholson AN. Hypnotic activity of melatonin. Sleep. 2000;23:663–669. [PubMed] [Google Scholar]
- Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983;227:587–591. [PubMed] [Google Scholar]
- Thayer RE. Factor analytic and reliability studies on the activation-deactivation adjective check list. Psychol Report. 1978;42:747–756. doi: 10.2466/pr0.1978.42.3.747. [DOI] [PubMed] [Google Scholar]
- Tilley AJ, Wilkinson RT, Warren PSG, Watson B, Drud M. The sleep and performance of shift workers. Hum Factors. 1982;24:629–641. doi: 10.1177/001872088202400601. [DOI] [PubMed] [Google Scholar]
- U.S. Congress and Office of Technology. Assessment Biological Rhythms: Implications for the Worker. U.S. Government Printing Office; Washington DC: 1991. [Google Scholar]
- Walsh JK, Sugerman JL, Muehlbach MJ, Schweitzer PK. Physiological sleep tendency on a simulated night shift: adaptation and effects of triazolam. Sleep. 1988;11:251–264. doi: 10.1093/sleep/11.3.251. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Tepas DI, Moses PD. The EEG sleep of night and rotating shift workers. In: Johnson LC, Tepas DI, Colquhoun WP, editors. Biological Rhythms, Sleep and Shift Work. Spectrum Publications; New York: 1981. pp. 371–381. [Google Scholar]
- Weitzman ED, Kripke DF, Goldmacher D, McGregor P, Nogeire C. Acute reversal on the sleep-waking cycle in man. Arch Neurol. 1970;22:483–489. doi: 10.1001/archneur.1970.00480240003001. [DOI] [PubMed] [Google Scholar]
- Wright KP, Badia P, Myers BL, Plenzler SC. Combination of bright light and caffeine as a countermeasure for impaired alertness and performance during extended sleep deprivation. J Sleep Res. 1997;6:26–35. doi: 10.1046/j.1365-2869.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Dijk DJ, Ritz-DeCecco A, Ronda JM, Czeisler CA. Effects of physiologic and pharmacologic doses of exogenous melatonin on sleep propensity and consolidation in healthy young men and women are circadian phase-dependent. Sleep Res Online. 1999;2 (Suppl 1):636. [Google Scholar]
- Zhdanova IV, Wurtman RJ, Lynch HJ, et al. Sleep-inducing effects of low doses of melatonin ingested in the evening. Clin Pharmacol Ther. 1995;57:552–558. doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]