Abstract
To address the heterogeneous nature of adolescent major depression (MDD), we investigated anhedonia, a core symptom of MDD. We recently reported activation of the kynurenine pathway (KP), a central neuroimmunological pathway which metabolizes tryptophan (TRP) into kynurenine (KYN) en route to several neurotoxins, in a group of highly anhedonic MDD adolescents. In this study, we aimed to extend our prior work and examine the relationship between KP activity and anhedonia, measured quantitatively, in a group of MDD adolescents and in a combined group of MDD and healthy control adolescents. Thirty-six adolescents with MDD (22 medication-free) and 20 controls were included in the analysis. Anhedonia scores were generated based on clinician- and subject-rated assessments and a semi-structured clinician interview. Blood KP metabolites, collected in the AM after an overnight fast, were measured using high-performance liquid chromatography. The rate-limiting enzyme of the KP, indoleamine 2,3-dioxygenase (IDO), was estimated by the ratio of KYN/TRP. Pearson correlation tests were used to assess correlations between anhedonia scores and KP measures while controlling for MDD severity. IDO activity and anhedonia scores were positively correlated in the group psychotropic medication-free adolescents with MDD (r = 0.42, P = 0.05) and in a combined group of MDD subjects and healthy controls (including medicated patients: r = 0.30, P = 0.02; excluding medicated patients: r = 0.44, P = 0.004). In conclusions, our findings provide further support for the role for the KP, particularly IDO, in anhedonia in adolescent MDD. These results emphasize the importance of dimensional approaches in the investigation of psychiatric disorders.
Keywords: Indoleamine 2,3-dioxygenase; Anhedonia; Adolescents; Major depressive disorder; Kynurenine pathway
Introduction
Progress in understanding the neurobiology of major depressive disorder (MDD) in adolescents and adults has been hampered by the disorder’s heterogeneous nature. The major challenge has been that the categorical diagnosis of MDD: (a) relies on a syndromal definition that comprises a cluster of symptoms most likely derived from different etiologies, and (b) does not capture the continuum of symptoms ranging from lesser to greater severity. For this reason, investigating symptoms within the disorder may enhance the likelihood of success in neurobiological investigations of MDD. Anhedonia—the reduced capacity to experience pleasure—is a core symptom of MDD and highly variable among depressed adolescents; can be quantitatively measured and is tied to the reward circuitry; and as such an ideal candidate for a targeted biological investigation in adolescent MDD.
Mounting data suggest a role for the immune system in MDD (Miller 2009; Irwin and Miller 2007). Evidence includes multiple reports of increased levels of pro-inflammatory cytokines in the blood as well as in the cerebrospinal fluid (CSF) of depressed and suicidal adult and adolescent patients (Gabbay et al. 2009a, b; Levine et al. 1999; Lindqvist et al. 2009; Hayley 2011). Other data from animal and human studies have demonstrated that peripheral immunotherapy frequently induces depressive symptoms, particularly anhedonia, as well as leads to concurrent changes in the neural reward circuitry (Eisenberger et al. 2010; Miller 2009; Shuto et al. 1997; Capuron et al. 2007; Majer et al. 2008). These observations suggest that peripheral and brain inflammatory processes are tightly linked and can affect brain function.
The kynurenine pathway (KP) is a central neuroimmunological branch which has been hypothesized to play a key role in linking peripheral inflammation and brain function via production of oxygen radicals and highly potent neurotoxins (Schwarcz and Pellicciari 2002; Amori et al. 2009). The KP is activated by the rate-limiting enzyme indoleamine 2,3-dioxygenase (IDO), which metabolizes tryptophan (TRP) into kynurenine (KYN). KYN is further metabolized to several neurotoxins including 3-hydroxy-kynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), and quinolinic acid (QUIN). Of these, 3-HK and 3-HAA generate reactive radicals which induce oxidative stress and neuronal apoptosis (Goldstein et al. 2000). QUIN is a specific N-methyl-D-aspartate (NMDA) receptor agonist and potent excitotoxin (Schwarcz et al. 1983). Converging evidence indicates that peripheral induction of IDO plays a key role in initiating the KP cascade that leads to neurotoxicity and depressive symptoms (Wichers et al. 2005; Bonaccorso et al. 2002; Capuron et al. 2002, 2003a; Myint et al. 2007). IDO is a cytoplasmatic enzyme which is expressed mainly in antigen-presenting cells such as dendritic cells (Boasso et al. 2005). Two other enzymes, tryptophan 2,3-dioxygenase (TDO) and IDO2, have similar activity but differ in their expression patterns; the former is primarily expressed in the liver while the latter, which was first reported in 2007 (Murray 2007), is predominantly expressed in the kidneys, epididymis, testis, and liver (Ball et al. 2009). IDO is induced by proinflammatory cytokines, particularly by interferon (IFN)-γ, and the IFN-γ pathway is required for the normal upregulation of IDO expression during infection (Kwidzinski and Bechmann 2007).
We have examined the KP and its associated neurotoxicity in adolescent MDD and reported decreased blood TRP and increased IDO activity in highly anhedonic MDD adolescents compared to both controls and non-anhedonic MDD adolescents, along with significant associations between both KYN and neurotoxic load with severity of MDD episode in the anhedonic MDD subgroup (Gabbay et al. 2010a). Additionally, using proton magnetic resonance spectroscopy, we documented associations between KYN and 3-HAA blood levels and striatal total choline (tCho, membrane turnover biomarker) in the subgroup of anhedonic MDD adolescents (Gabbay et al. 2010b). Our findings suggest a specific role for the KP in anhedonia, classified categorically.
Here, we extended our prior work while adopting a dimensional investigative approach and examined the relationship between the KP and anhedonia severity, measured quantitatively. To our knowledge, the specific relationships between KP metabolites and/or IDO activity and anhedonia have not been investigated in adult or in adolescent MDD. Based on the notion that anhedonia is a continuous symptom ranging from normal to pathological, we hypothesized that anhedonia scores would be positively correlated with IDO activity (estimated by KYN/TRP) and KP neurotoxicity (indexed by 3-HAA/KYN and 3-HAA) and negatively correlated with TRP levels in the blood in the MDD group as a whole. We also explored these relationships in the full sample of MDD adolescents and controls.
Methods
Study subjects
Study subjects were the same as previously reported (Gabbay et al. 2010a), excluding individuals missing the necessary assessments to generate anhedonia scores (see below).
The MDD subjects were recruited from the New York University (NYU) Child Study Center, the Bellevue Hospital Department of Psychiatry, and through local advertisements in the New York Metropolitan area. The healthy control (HC) subjects were recruited from the greater New York Metropolitan area by means of advertisements and through families of NYU staff. The study was approved by the NYU School of Medicine Institutional Review Board and the New York City Health and Hospital Corporation. Prior to the baseline clinical evaluation, study procedures were explained to subjects and parents. Participants age 18 and over (N = 10) provided signed informed consent; those under age 18 provided signed assent and a parent provided signed informed consent.
Inclusion and exclusion criteria
Exclusion criteria for all subjects consisted of immune-affecting medications taken in the past 6 months, any immunological or hematological disorder, chronic fatigue syndrome, any infection during the month prior to the blood draw (including the common cold), significant medical or neurological disorders, a positive urine toxicology test, and in females, a positive urine pregnancy test.
Inclusion criteria for adolescents with MDD were DSM-IV-TR diagnosis of MDD with current episode duration ≥6 weeks and a severity score ≥37 on the Children’s Depression Rating Scale-Revised (CDRS-R). Exclusionary diagnoses included a lifetime psychiatric history of bipolar disorder, schizophrenia, pervasive developmental disorder, post-traumatic stress disorder, obsessive-compulsive disorder, Tourette’s disorder, eating disorder, and a substance-related disorder in the past 12 months. HC subjects did not meet criteria for any major current or past DSM-IV-TR diagnoses and had never received psychotropic medication.
Clinical assessments
All subjects were assessed by a child and adolescent psychiatrist or psychologist at the NYU Child Study Center. Diagnoses were established using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997), a semi-structured diagnostic interview completed with subjects and parents. Additional assessments included the CDRS-R and the Beck Depression Inventory, 2nd edn (BDI-II) (Beck et al. 1997). Baseline medical assessments included medical history and laboratory tests, constituting complete blood count, metabolic panel, liver and thyroid function tests, urine toxicology tests (assessing amphetamines, barbiturates, benzodiazepines, cocaine, marijuana, methadone, opiates, phencyclidine, and propoxyphene), and a urine pregnancy test for females.
Anhedonia
Anhedonia scores were computed by summing the equally weighted responses associated with anhedonia on: (a) the self-rated BDI-II (item 4: “Loss of Pleasure” and item 12: “Loss of Interest”); (b) the clinician-rated CDRS-R (item 2: “Difficulty Having Fun”); and (c) the K-SADS-PL diagnostic interview (p. 8, “Anhedonia, Lack of Interest, Apathy, Low Motivation, or Boredom” and Affective Disorders Supplement page 1, “Lack of Reactivity of Depressed or Irritable Mood to Positive Stimuli”). Such an approach has been used in several investigations that assessed anhedonia severity (Pizzagalli et al. 2005; Joiner et al. 2003), and scores were shown to correlate with other anhedonia assessments (e.g., Snaith–Hamilton Pleasure Scale) (Leventhal et al. 2006).
Determination of kynurenine pathway metabolite concentrations
All blood samples (10 ml) were drawn between 08:00 and 09:00 a.m. after an overnight fast (≥12 h), processed within 20 min of collection, and stored at −80°C. All analyses were conducted while blind to the clinical status of subjects.
Calibration curves were prepared from stock solutions (100 μg/ml) to yield the respective levels of TRP (1, 2, 4, 8, 12, and 14 μg/ml), KYN (0.05, 0.1, 0.2, 0.4, 1, 2, 4, and 5 μg/ml), and 3-HAA (0.1, 0.2, 0.4, 0.8, 2, and 3 μg/ml) in double-distilled-grade water and human plasma. The internal standard (3-nitro-L-tyrosine, 1 mg/ml) was also added to all calibration standards for recovery purposes.
Plasma (0.20 ml) was combined with 5 μl of 1 μg/μl 3-nitro-L-tyrosine followed by the addition of 0.20 ml of 0.05 M KH2PO4 (pH 6.2). Subsequently, 50 μl of cold 2 M trichloroacetic acid was added, followed by vigorous vortexing. The mixture was centrifuged at 9,500 rpm for 10 min, and the supernatant was transferred into auto-sampler vials for analysis by high-performance liquid chromatography (HPLC). Aliquots of 200 μl of the supernatant were injected into the chromatographic system using a WISP model 717 autosampler (Waters Associates, Milford, MA, USA).
The eluted components of interest were detected as follows: (1) KYN and 3-nitro-L-tyrosine by tandem monitoring with UV absorption at 360 nm; (2) TRP and 3-HAA by fluorescence monitoring at 340 nm (with UV excitation at 285 nm). A gradient elution was used for development of the chromatography in the following manner: the gradient consisted of a combination of two buffers, A (40 mM citrate buffer/0.1 mM succinic acid, disodium salt/0.05% sodium azide) and B (60% methanol/40 mM citrate buffer/0.1 mM succinic acid, disodium salt/0.05% sodium azide). The following sequence of steps was employed: 0–3 min, 0% B; 3–12 min, 0% B–65% B (linear gradient); 12–18 min, 65% B; 18–20 min, 65% B–100% B; 20–24 min 100% B; 24–26 min, 100% B–0% B (linear gradient); 26–30 min 0% B. A flow rate of 0.8 ml/min was used during the 30 min elution sequence of the analytical HPLC C18 column (4.6 × 150 mm, 5 μm, Luna C18, Phenomenex, Torrance, CA, USA).
Data analysis
Pearson correlation coefficients were used to characterize the association of KP metabolite plasma levels with anhedonia scores for the MDD subjects alone as well as for the combined MDD and healthy control groups. To control for the possible confounding effect of MDD severity, analyses were carried out while controlled for CDRS-R scores. Since psychotropic medications can affect the KP, analyses were repeated while excluding medicated subjects. Statistical significance was defined as two-sided P ≤ 0.05, while trends toward significance were defined as P ≤ 0.1.
Results
Subjects
Study subjects consisted of 36 adolescents with MDD (ages 13–19, mean 15.9 ± 1.7, 21 female) and 20 HC (ages 11–20, mean 16.1 ± 2.7, 12 female). Subject groups did not significantly differ with respect to age or gender. Clinical and demographic information for all subjects, including medication use and anhedonia scores, are described in Table 1. Of the 36 adolescents with MDD, 22 (61%) were psychotropic medication-free (20 were psychotropic medication-naïve). All 14 patients on medication had failed to respond to treatment at the time of blood draw.
Table 1.
Clinical and demographic characteristics of adolescents with major depressive disorder (MDD) and healthy controls
| Characteristic | MDD N = 36 | Healthy controls N = 20 |
|---|---|---|
| Age (years) | 15.9 ± 1.7 | 16.1 ± 2.7 |
| Gender (female/male) | 21/15 (58/42%)a | 12/8 (60/40%)a |
| Ethnicity (Caucasian/African American/Hispanic/Asian/other) | 16/5/11/4/0 (44/14/31/11/0%)a | 11/2/1/4/2 (55/10/5/20/10%)a |
| Illness history | ||
| Duration of Illness (months) | 18.8 ± 17.6 (3–84)b | 0 |
| History of suicide attempts | 0.6 ± 0.8 (0–3)b | 0 |
| Medication-naïve/medication-free/medicated | 20/2/14 (56/6/39%)a | 0 |
| CDRS-Rc | 58.4 ± 14.0 (37–97)b | 18.3 ± 2.6 (17–27)b |
| BDI-IId | 23.0 ± 10.6 (8–53)b | 1.9 ± 2.8 (0–11)b |
| Anhedonia scores | 0.578 ± 0.218 (0.083–1.00)b | 0.015 ± 0.036 (0.00–0.014)b |
| Current comorbidity | ||
| ADHDe | 1 (3%)a | 0 |
| Any anxiety disorder | 9 (25%)a | 0 |
| Any comorbidity | 10 (28%)a | 0 |
Respective percentages
Range
Children’s Depression Rating Scale-Revised
Beck Depression Inventory, 2nd edn
Attention Deficit Hyperactivity Disorder
Association between KP metabolite plasma levels and anhedonia scores
MDD group
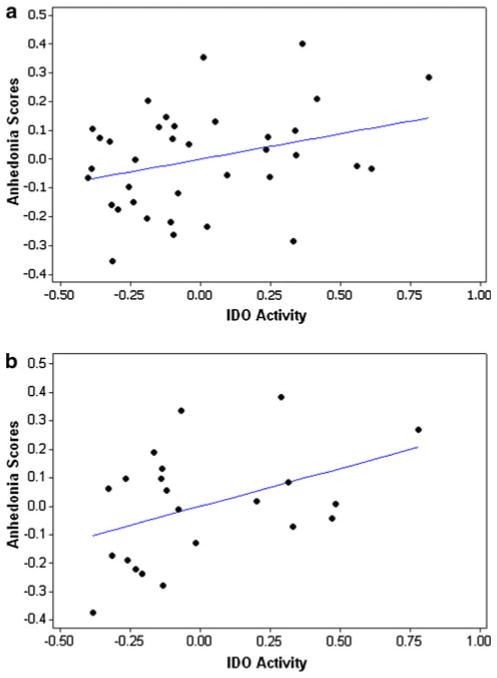
We observed a trend toward a significant correlation between anhedonia scores and IDO activity for the MDD group while controlling for severity (r = 0.31, P = 0.06, Fig. 1a). When medicated subjects were excluded, a significant correlation between IDO activity and anhedonia scores was observed in the medication-free MDD group (r = 0.42, P = 0.05, Fig. 1b).
Fig. 1.
Scatter plots indicating partial correlations between IDO activity and anhedonia scores, controlling for CDRS-R scores. a (top) all MDD subjects, r = 0.31, P = 0.06. b (bottom) medication-free MDD subjects, r = 0.42, P = 0.05
Whole group (MDD and HC)
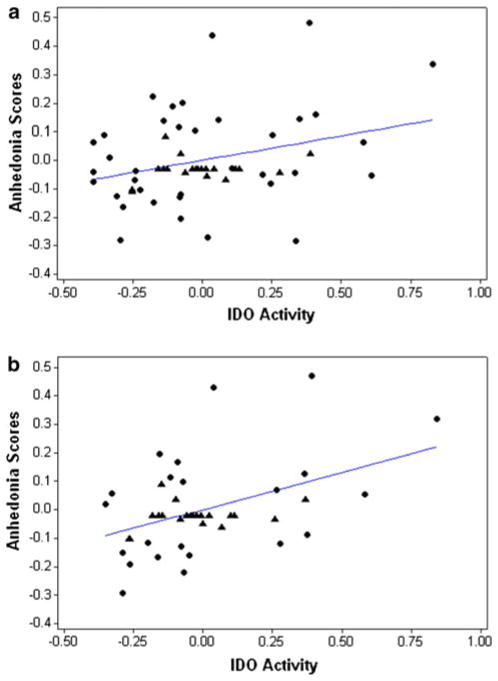
A significant positive correlation between IDO activity and anhedonia scores was observed in all subjects (r = 0.30, P = 0.02, Fig. 2a). This correlation remained significant when medicated patients were excluded (r = 0.44, P = 0.004, Fig. 2b).
Fig. 2.
Scatter plots indicating partial correlations between IDO activity and anhedonia scores, controlling for CDRS-R scores. a (top) all subjects, r = 0.30, P = 0.02. b (bottom) all subjects excluding medicated MDD patients, r = 0.44, P = 0.004. Control subjects are indicated by triangles while MDD subjects are indicated by circles. Note that the range of anhedonia scores in HC group was relatively small (0.0–0.14), so the HC subjects appear clustered together in the plots. The appearance of lower IDO activity and anhedonia scores in the MDD group than in the HC group is a consequence of the statistical control for depression severity and does not reflect the uncontrolled range of either measure
No significant correlations or trends toward significance were found between TRP, KYN, 3-HAA, or 3-HAA/KYN blood levels and anhedonia scores in either the whole sample or the MDD group alone.
Discussion
This is the first study to examine the relationship between KP activity and anhedonia as assessed dimensionally. We found a significant positive correlation between IDO activity (reflected by the ratio KYN/TRP) and anhedonia scores in psychotropic-free depressed adolescents as well as in a combined group of depressed adolescents and healthy controls.
The present findings are consistent with our previous studies implicating the KP in adolescent MDD, where increased IDO activity and neurotoxicity were present only in the subgroup categorized as highly anhedonic, as well as studies by other research groups reporting increased urinary excretion of KYN and 3-HK in anhedonic depressed women (Curzon and Bridges 1970) and decreased plasma TRP/competing amino acids ratios in several studies of highly anhedonic MDD subjects (Cowen et al. 1989; Anderson et al. 1990; Maes et al. 1996). The correlation we established between IDO activity and anhedonia, assessed quantitatively, supports the view that psychiatric symptoms should be studied as dimensions ranging from normal to pathological in addition to their traditional binary/categorical classification. Such an approach provides greater power as well as insight into the underlying biological mechanisms of psychiatric symptoms. Notably, our correlation between IDO activity and anhedonia scores was more significant when medicated patients were excluded and may be explained by the anti-inflammatory properties of antidepressants (Maes et al. 1999; Kubera et al. 2001).
Another important observation is the relatively narrow range of IDO activity in the healthy control group versus the wide range in MDD patients. This phenomena is not uncommon in biological research of psychiatric illness, which by itself suggests the heterogeneous nature of MDD and supports our dimensional investigative approach.
Our finding of a relationship between anhedonia and IDO may be related to IDO being the rate-limiting enzyme of the KP and its role in initiating the KP cascade and subsequent neurometabolic alterations. Clinical trials in patients with hepatitis C who underwent immunotherapy support this perspective. Findings include increased plasma IDO activity (reflected by KYN/TRP ratios) coupled with parallel increases of KYN, QUIN, and kynurenic acid (KA) in the CSF in response to treatment with IFN-α (Raison et al. 2009). Since metabolites other than KYN and 3-HK do not cross the blood–brain barrier, these findings suggest that peripheral activation of IDO leads to parallel activation of the KP in the brain. Preclinical data confirm this notion. For example, O’Connor et al. reported that a peripheral immunological challenge with lipopolysaccharides (LPS) in mice resulted in elevated brain expression of IDO and cytokine mRNAs along with depressive symptoms (i.e., increased duration of immobility in forced-swim and tail suspension tests). Authors also documented that direct and indirect peripheral inhibition of IDO blocked the central transcription of IDO in the brain and the development of depressive-like behaviors in mice following LPS (O’Connor et al. 2008). Importantly, the direct inhibition of IDO (with the antagonist 1-methyltryptophan) did not affect cytokines but still prevented depressive symptoms (O’Connor et al. 2008). Moreover, several studies in mice have specifically linked peripheral and central IDO activation to anhedonic behaviors assessed by decreased preference for sucrose (Henry et al. 2008; Moreau et al. 2008).
At the same time, Raison et al. (2009) also reported significant correlations between KYN, QUIN and KA with specific immunological variables (e.g., IFN-α, TNF receptor) in the CSF and with depressive symptoms. Similarly, immune system stimulation with LPS has also been shown to induce cytokine production in the mouse brain, particularly the pro-inflammatory cytokine IL-1β, with concurrent production of IDO mRNA in microglia (Henry et al. 2008). This suggests that the KP is only one player in the generalized inflammatory response associated with depression (McNally et al. 2008).
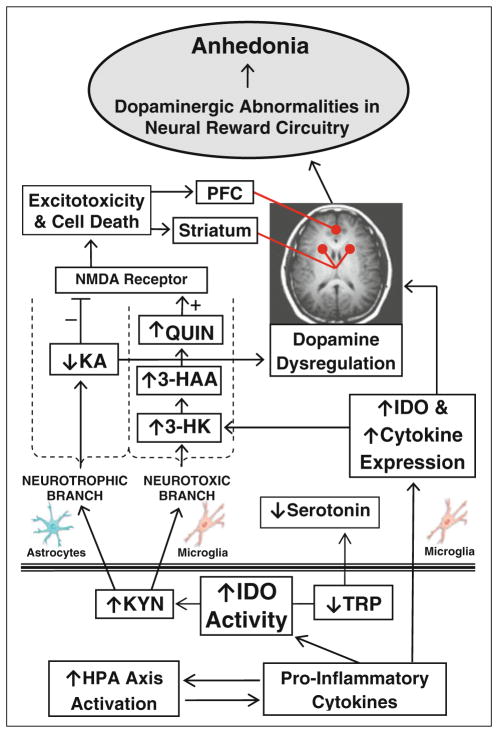
As illustrated in Fig. 3, activation of the KP neurotoxic branch can contribute to dopaminergic alterations within the neural reward circuitry via several linked processes. The KP neurotoxin, QUIN, directly alters the mesolimbic dopaminergic system and induces dopaminergic and GABAergic neuronal death (Kurachi et al. 2000; Sumiyoshi et al. 2004; Beskid and Finkiewicz-Murawiejska 1992; Araujo et al. 2000) while KA, another KP metabolite, tightly controls the firing of midbrain dopaminergic neurons, with decreased endogenous KA resulting in decreased dopamine release (Erhardt et al. 2009; Wu et al. 1994). Additionally, animal studies suggest that 3-HK and 3-HAA toxicity is dependent upon transporter-mediated cellular intake with brain region selectivity; striatal and cortical neurons (key regions within the neural reward circuitry) are particularly vulnerable to these toxins (Okuda et al. 1998; Heyes et al. 2001).
Fig. 3.
Overview of the proposed model of the kynurenine pathway in anhedonia. Plasma levels of tryptophan (TRP), kynurenine (KYN), 3-hydroxyanthranilic acid (3-HAA), and KYN/TRP ratio (IDO) were assayed in blood samples from depressed and control adolescents. TRP, KYN, and 3-hydroxykynurenine (3-HK) can cross the blood–brain barrier. PFC prefrontal cortex
Clearly, the KP is one of the players in the cascade in which peripheral activation of the immune system results in alterations in the mesolimbic dopamine system, with cytokines and the hypothalamic–pituitary–adrenal (HPA) axis being tightly linked as well (illustrated in Fig. 3). Cytokines initiate the KP via induction of IDO. At the same time, considerable evidence indicates that cytokine therapy induces alterations in the mesolimbic dopaminergic system and in reward processing (Eisenberger et al. 2010; Miller 2009). Similarly, immune system activation is strongly connected to HPA axis activity (Capuron et al. 2003b), with alterations in the latter being one of the most consistent findings in anhedonic depressed patients (Tremblay et al. 2005).
Several limitations should be noted. Our estimates of IDO activity are based on KYN/TRP ratio rather than directly assessing the enzyme activity. The ratio of KYN/ TRP is a commonly used and widely accepted method of estimating IDO activity (Schrocksnadel et al. 2006). There are, however, several issues to this method. TRP in blood is highly labile and easily changed, and as such can affect our estimates of IDO activity (Badawy 2009). TRP is partly bound to albumin, with competitive binding by thyroxine and fatty acids (Badawy 2009), while brain uptake of TRP is dependent on free blood TRP levels (Kema et al. 2000). Further, since TRP is also metabolized to KYN by TDO, the ratio KYN/TRP indirectly indicates the sum of IDO + TDO activities. Diet and processes that influence the equilibrium between free TRP and protein-bound TRP modify is availability for uptake in the brain (Kema et al. 2000). However, addressing this concern, all subjects had to fast for at least 12 h before the blood draw. Additionally, we did not measure the major neurotoxins of the KP such as QUIN and 3-HK.
In summary, the results of the present study support a role for the KP in anhedonia. Future studies should involve different patient populations as well as measure KP enzyme activity directly.
Acknowledgments
This study was supported by grants from NIH (AT002395, MH077072, AT004576), the American Foundation for Suicide Prevention, the NYU School of Medicine General Clinical Research Center grant (M01-RR00096), the Leon Levy Foundation, and generous gifts from the Anita Saltz Foundation and from Bruce and Claude Wasserstein.
Statement regarding human subjects: All procedures described in this manuscript have been approved by the NYU Institutional Review Board and New York City Health and Hospital Corporation; procedures comply with the ethical standards established in the 1964 Declaration of Helsinki. All subjects involved in the study provided informed consent prior to inclusion.
Contributor Information
Vilma Gabbay, Email: Vilma.Gabbay@nyumc.org, Department of Child and Adolescent Psychiatry, NYU Child Study Center, NYU School of Medicine, New York University Langone Medical Center, 577 First Avenue, New York, NY 10016, USA. Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd #35, Orangeburg, NY 10962, USA.
Benjamin A. Ely, Department of Child and Adolescent Psychiatry, NYU Child Study Center, NYU School of Medicine, New York University Langone Medical Center, 577 First Avenue, New York, NY 10016, USA
James Babb, Radiology, Research, NYU School of Medicine, Bellevue C&D Building 122, 462 First Avenue, New York, NY 10016, USA.
Leonard Liebes, Cancer Institute, Tisch Hospital, NYU School of Medicine, 550 First Avenue, New York, NY 10016, USA.
References
- Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009;109(2):316–325. doi: 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Parry-Billings M, Newsholme EA, Poortmans JR, Cowen PJ. Decreased plasma tryptophan concentration in major depression: relationship to melancholia and weight loss. J Affect Disord. 1990;20(3):185–191. doi: 10.1016/0165-0327(90)90143-v. [DOI] [PubMed] [Google Scholar]
- Araujo DM, Cherry SR, Tatsukawa KJ, Toyokuni T, Kornblum HI. Deficits in striatal dopamine D(2) receptors and energy metabolism detected by in vivo microPET imaging in a rat model of Huntington’s disease. Exp Neurol. 2000;166(2):287–297. doi: 10.1006/exnr.2000.7514. [DOI] [PubMed] [Google Scholar]
- Badawy AB. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2009;24:809–815. doi: 10.1177/0269881108098965. [DOI] [PubMed] [Google Scholar]
- Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2, 3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41(3):467–471. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35(8):785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Beskid M, Finkiewicz-Murawiejska L. Quinolinic acid: effects on brain catecholamine and c-AMP content during L-dopa and reserpine administration. Exp Toxicol Pathol. 1992;44(2):66–69. doi: 10.1016/S0940-2993(11)80189-2. [DOI] [PubMed] [Google Scholar]
- Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2, 3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105(4):1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003a;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32(11):2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003b;160(7):1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Parry-Billings M, Newsholme EA. Decreased plasma tryptophan levels in major depression. J Affect Disord. 1989;16(1):27–31. doi: 10.1016/0165-0327(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Curzon G, Bridges PK. Tryptophan metabolism in depression. J Neurol Neurosurg Psychiatry. 1970;33(5):698–704. doi: 10.1136/jnnp.33.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23(2):91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. 2009a;115(1–2):177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol. 2009b;19(4):423–430. doi: 10.1089/cap.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry. 2010a;51(8):935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2010b;34(1):37–44. doi: 10.1016/j.pnpbp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Leopold MC, Huang X, Atwood CS, Saunders AJ, Hartshorn M, Lim JT, Faget KY, Muffat JA, Scarpa RC, Chylack LT, Jr, Bowden EF, Tanzi RE, Bush AI. 3-Hydroxyky-nurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39(24):7266–7275. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- Hayley S. Toward an anti-inflammatory strategy for depression. Front Behav Neurosci. 2011;5:19. doi: 10.3389/fnbeh.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipo-polysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Ellis RJ, Ryan L, Childers ME, Grant I, Wolfson T, Archibald S, Jernigan TL. Elevated cerebrospinal fluid quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain. 2001;124(Pt 5):1033–1042. doi: 10.1093/brain/124.5.1033. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21(4):374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res. 2003;119(3):243–250. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kema IP, de Vries EG, Muskiet FA. Clinical chemistry of serotonin and metabolites. J Chromatogr B Biomed Sci Appl. 2000;747(1–2):33–48. doi: 10.1016/s0378-4347(00)00341-8. [DOI] [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21(2):199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- Kurachi M, Sumiyoshi T, Shibata R, Sun YJ, Uehara T, Tanii Y, Suzuki M. Changes in limbic dopamine metabolism following quinolinic acid lesions of the left entorhinal cortex in rats. Psychiatry Clin Neurosci. 2000;54(1):83–89. doi: 10.1046/j.1440-1819.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. J Mol Med. 2007;85(12):1351–1359. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62(12):1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, Scharpe S. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20(4):370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Maes M, Wauters A, Verkerk R, Demedts P, Neels H, Van Gastel A, Cosyns P, Scharpe S, Desnyder R. Lower serum L-tryptophan availability in depression as a marker of a more generalized disorder in protein metabolism. Neuropsychopharmacology. 1996;15(3):243–251. doi: 10.1016/0893-133X(95)00181-C. [DOI] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22(6):870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13(6):501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23(2):149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Andre C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22(7):1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MF. The human indoleamine 2, 3-dioxygenase gene and related human genes. Curr Drug Metab. 2007;8(3):197–200. doi: 10.2174/138920007780362509. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98(1–2):143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008;14(5):511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2009;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364(1–2):82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303(1):1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747(2):348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Tsunoda M, Uehara T, Tanaka K, Itoh H, Sumiyoshi C, Kurachi M. Enhanced locomotor activity in rats with excitotoxic lesions of the entorhinal cortex, a neurodevelopmental animal model of schizophrenia: behavioral and in vivo microdialysis studies. Neurosci Lett. 2004;364(2):124–129. doi: 10.1016/j.neulet.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62(11):1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10(6):538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Schwarcz R, Shepard PD. Excitatory amino acid-induced excitation of dopamine-containing neurons in the rat substantia nigra: modulation by kynurenic acid. Synapse. 1994;16(3):219–230. doi: 10.1002/syn.890160307. [DOI] [PubMed] [Google Scholar]