Abstract
Background
S-Nitrosoglutathione (GSNO) is the S-nitrosated derivative of glutathione and is thought to be a critical mediator of the down-stream signaling effects of nitric oxide (NO). GSNO has also been implicated as a contributor to various disease states.
Scope of Review
This review focuses on the chemical nature of GSNO, its biological activities, the evidence that it is an endogenous mediator of NO action, and implications for therapeutic use.
Major Conclusions
GSNO clearly exerts its cellular actions through both NO- and S-nitrosation-dependent mechanisms; however, the chemical and biological aspects of this compound should be placed in the context of S-nitrosation as a whole.
General Significance
GSNO is a central intermediate in formation and degradation of cellular S-nitrosothiols with potential therapeutic applications; thus, it remains an important molecule of study.
1. Introduction
S-Nitrosoglutathione (GSNO) is the S-nitrosated derivative of the most abundant cellular thiol, glutathione (GSH). S-Nitrosothiols such as GSNO have been reported to be integral to the chemical biology and physiological functions of nitric oxide (NO). GSNO has variously been thought of as a store of NO, or as an essential component of NO-dependent signal transduction. In addition, there has been significant interest in GSNO as a potential therapeutic agent. In this review, we will describe in detail the chemical nature of GSNO, its biological activities, the evidence that it is an endogenous biological mediator of NO action, and implications for therapeutic use.
2. Synthesis, Structure, and Reactions of GSNO
2.1 Chemical Synthesis
As with other S-nitrosothiols, GSNO can be synthesized from the reaction between GSH and nitrous acid. This reaction is efficient, fast, and occurs with high yield (Equation 1). Mixing GSH with acidified nitrite forms an immediate pink color. Unlike
| [1] |
most low-molecular weight S-nitrosothiols, GSNO can be precipitated with acetone and purified as a solid [1], and this has enhanced its use as an experimental tool. It is also possible to synthesize GSNO through the use of other nitrosating agents such as nitrosonium tetrafluoroborate, nitrosonium chloride, dinitrogen trioxide, and dinitrogen tetraoxide [2,3]. However, for simplicity, compatibility with aqueous solutions, and high yield, acidified nitrite is preferred for biological applications. Although early reports indicated that under anaerobic conditions, S-nitrosothiols could be formed directly from NO and a parent thiol [4], it is likely that in these studies, the NO gas used was contaminated with higher oxides of nitrogen which are potent nitrosating agents.
The molar extinction coefficient of GSNO has been variously reported (e.g., [5–7]), with quite a wide range of values. The original study by Hart [1] gave an extinction coefficient of 922 M−1 cm−1 at 335 nm, and our experience would suggest that this number is correct.
2.2 The formation of GSNO from NO
The mechanism by which GSNO is formed from NO has been the subject of much enquiry. What is abundantly clear is that the reaction is not direct. The addition of NO to GSH would form a thionitroxide which has been implicated as an intermediate in the slow direct oxidation of thiols by NO to form disulfides [8,9]. In fact, the intermediate thionitroxide has been identified as the thiol modification in crystals of hemoglobin exposed to NO [10] and as a putative NO-dependent activating modification of soluble guanylyl cyclase [11]. In these reactions, the NO is reduced to nitrous oxide and other more reduced nitrogen-containing species. Direct redox reactions of NO with GSH are slow [9,12] and unlikely to contribute to the biological consumption of NO. However, the rate constants for the reversible association of NO with GSH to form thionitroxide are unknown.
Under aerobic conditions, GSNO is readily formed from NO and GSH. Kinetic studies have shown that nitrosation in general is limited by the apparent third order reaction between NO and oxygen and depends on the square of the concentration of NO [13–15]. There has been some disagreement over the yield of this reaction, and our studies using an NO-releasing agent and GSH indicated a GSNO yield of approximately 10% with the remainder forming GSSG [16]. The rate of NO production and the oxygen concentration have a major influence on yield [17]. The primary conclusions regarding GSNO formation under aerobic conditions are that oxidation of NO by oxygen is a prerequisite, and nitrosation occurs either through the formation of dinitrogen trioxide or though the addition of NO to a glutathionyl radical formed during the reaction. Gow et al. [18] proposed that at low levels of NO, the thionitroxide discussed above could act as a one electron reductant of oxygen resulting in the formation of GSNO and superoxide. While it is fair to say that this mechanism remains speculative, we have recently shown that the one-electron acceptor ferric cytochrome c can efficiently promote GSNO formation under anaerobic conditions from NO and GSH [19,20]. In this case, we have proposed that GSNO formation requires binding of GSH to cytochrome c followed by the addition of NO. It is likely that mechanisms such as these predominate in the low-oxygen, low-NO environment of cells and tissues. Most studies would agree that nitrosation is not a major fate of NO and that only a very small amount of generated NO is converted into an S-nitrosothiol in a biological system.
2.3 Structure of GSNO and mechanisms of decomposition
While tertiary S-nitrosothiols are often stable, primary compounds are less stable, with GSNO being a notable exception. Why GSNO is significantly more stable than S-nitrosocysteine has been the subject of some investigation. Unfortunately, much of this work is confounded by no clear definition of what element of stability is examined. It has been established (and will be discussed later), that S-nitrosothiols are subject to several mechanisms of decomposition, including S-N bond homolysis, photolytic S-N bond cleavage, metal ion-catalyzed decomposition, and hydrolysis. For this reason, published half-lives of S-nitrosothiols vary dramatically and are clearly condition-dependent. Although no crystal structures of GSNO have been published, inference from other studied molecules can give a reasonable supposition as to its structure. The pink color of GSNO, a manifestation of a weak n →π* absorbance transition at 545 nm, is indicative of a primary S-nitrosothiol with the C-S-N-O bonds in a syn conformation and a dihedral angle close to 0° [21,22]. This is contrasted to the green color of SNAP which is due to a shift in absorbance of the weak band to 590 nm and an anti conformation of the bond (dihedral angle of about 180°) [21]. The anti conformation is preferred in tertiary S-nitrosothiols due to steric hindrance from the bulky carbon substituents [21]. Although spontaneous thermal homolysis of S-nitrosothiols has been discussed in the literature, theoretical and experimental analysis has indicated that activation energies are too high for spontaneous S-N bond homolysis to be a biologically meaningful reaction (reported half-lives are years to hours at 100°C) [22]. Consequently, GSNO does not spontaneously homolyze to form NO, and NO formation from GSNO must require external factors. For these reasons, we suggest that GSNO should never be referred to as an ‘NO donor molecule’ (despite commercial advertising) and that ‘half-lives’ of GSNO measured under one condition bear no relationship to GSNO decomposition rates when it is added to a biological system.
If it is not differences in spontaneous decomposition, what underlies the increased stability of GSNO? The most likely answer to this question is differential susceptibility to metal ion-dependent decomposition. It has been shown that GSNO is significantly less susceptible to catalytic decomposition by copper ions than is Snitrosocysteine [23]. In fact, the decomposition of GSNO by added copper ions is more stoichiometric than catalytic [24]. It has been suggested that this is due to the ability of the γ-glutamyl groups of GSSG to act as a weak copper(II) chelator and prevent catalytic decomposition [24]. This was illustrated by the decomposition of GSNO by γ-glutamyltranspeptidase (GGT) in the presence of trace metal ions [25]. Whereas GSNO was stable under the experimental conditions, peptide bond hydrolysis by GGT liberated S-nitrosocysteinylglycine which rapidly decomposed in a metal ion-dependent process. Moreover, when metal ions are chelated, all biologically relevant S-nitrosothiols exhibit vastly increase stability, and differences between them are less apparent.
An alternative, well-studied chemical mechanism of GSNO decay involves its reaction with other thiols. GSNO can donate the nitroso functional group to another thiol in a mechanism referred to as transnitrosation. The kinetics of this reaction have been studied with various thiols, and second order rate constants are in the range of 1–300 M−1s−1 [26,27]. Transnitrosation can also facilitate metal ion-dependent decomposition if the nascent S-nitrosothiol is more susceptible to this mode of decay [28]. As will be discussed later, transnitrosation from an S-nitrosothiol which cannot not transported into cells to one that can be may facilitate cellular uptake of the nitroso functional group [29]. It is likely that transnitrosation reactions with intracellular and solvent-exposed protein targets modulate many of the biological effects of GSNO. In addition to transnitrosation, a slower reaction between an S-nitrosothiol and a thiol has been reported that results in the formation of glutathione disulfide (GSSG) and lower oxides of nitrogen (nitrous oxide, ammonia, hydroxylamine as well as sulfinamides) [9,30,31]. The reaction between GSNO and GSH has a rate constant of 0.01 M−1s−1 at 37°C and is likely biologically irrelevant, but an interesting feature of this reaction is the proposed formation of HNO as a product [32]. In addition, this reaction leads to a disulfide, which, if occurring on a protein could result in S-glutathiolation of the protein thiol.
This section has discussed chemical mechanisms of decomposition, but in the cellular context, it is likely that biological mechanisms predominate through enzymatic reduction. This will be discussed at length later in this review.
3. Detection methods
3.1 Direct UV/Vis detection
In aqueous solutions, GSNO has the characteristic S-nitrosothiol absorption peak in UV region at 335 nm (e = 922 M−1 cm−1) [1]. Thus, direct detection of GSNO in aqueous solutions is possible using spectrophotometric techniques. Direct assays for GSNO require a separation step, using HPLC [33] or capillary electrophoresis [34], and subsequent UV detection. The main drawback of this methodology is the relative lack of sensitivity (micromolar range) due to low molar absorptivity of GSNO.
3.2 Colorimetric and fluorometric assays
One of the most popular colorimetric methods for GSNO detection is the Saville assay which relies on cleavage of the S-NO bond by mercury(II) [35] followed by reaction of liberated nitrite with N-(1-naphtyl)ethylenediamine dihydrochloride in acidic media to form the colored adduct in the Griess reaction [36]. Sub micromolar levels of S-nitrosothiols can be detected with this method, but discrimination between GSNO and other low molecular weight or protein-based S-nitrosothiols is not possible. In addition, it is difficult to accurately detect S-nitrosothiols in samples with a high background of nitrite. Additional assays that utilize mercury- or copper-dependent decomposition of GSNO have been developed to increase the sensitivity of detection. These methods rely on the reaction of the liberated product with 2,3-diaminonaphthalene to form the fluorescent product, 2,3-napththyltriazole [37]. Nanomolar levels of RSNO can be detected using this approach. This method has been used for detection of GSNO in samples containing thiols, protein, and nitrite, and the quantification is based on the difference between the sample with and without mercuric chloride [38]. Again, the above methods are quantitative, but cannot qualitatively differentiate between GSNO and other S-nitrosothiols. To address this limitation, a method that relies on chromatographic separation of GSNO followed by post-column derivatization with DAF-2 has been developed [39]. GSNO is first resolved by HPLC and then undergoes post-column online enzymatic hydrolysis by GTT to form S-nitrosocysteinylglycine. S-nitrosocysteinylglycine is subjected to decomposition by copper to liberate NO that reacts with DAF-2 forming fluorescent triazole. Another derivatization technique involves reaction with o-phthalaldehyde (OPA) followed by HPLC separation [40]. When GSNO is derivatized with OPA, its extinction coefficient increases to 4800 M−1cm−1; thus increasing the sensitivity by about 5-fold. Moreover, when coupled with fluorescence detection, the sensitivity of this technique increases further. One caveat to this method is that GSNO is reduced to GSH prior to reaction with OPA; hence analysis of GSNO in samples containing GSH requires elimination of cellular thiols prior to analysis.
3.3 NO liberation methods
GSNO can be detected based on stoichiometric release of NO due to homolytic or reductive cleavage of the S-N bond to liberate NO. Liberated NO can be detected with a Clark-type electrode or by chemiluminescent methods.
When using Clark-type electrodes to detect S-nitrosothiols, the sample is exposed to copper to catalyze the release of NO, which is subsequently detected with an NO-electrode [41]. This method allows for quantitative determination of S-nitrosothiols down to 50 nM. One of the advantages of this method is the lack of sample acidification, the importance of which will be discussed later. Homolytic cleavage of the S-N bond can also be accomplished by photolysis. Photolytic cleavage of S-nitrosothiols coupled with chemiluminescent detection has been used to analyze nanomolar levels of S-nitrosothiols [42,43]. In this method, S-nitrosothiols are photolyzed using a lamp with emission maximum at 365 nm, and an inert gas carries NO to the chemiluminescent analyzer in which it reacts with ozone to form the excited state of nitrogen dioxide. Upon decay to its ground state, nitrogen dioxide emits chemiluminescence. Although this method is sensitive, the instrumentation is relatively large, expensive, and not commonly available. The same detection method is used in reductive chemiluminescence methods. Various reagents are placed in a reaction chamber that is purged through to the chemiluminescence reaction cell. The reagents selectively decay the S-nitrosothiol to NO, which is then purged out of solution and detected. The major reagents used are triiodide/acid [44–46], ascorbate/copper ions [47], and cysteine/copper ions [48]. These chemiluminescent techniques are sensitive down to the low nM range, but do not allow for specific determination of GSNO among other S-nitrosothiols.
3.4 HPLC methods with electrochemical and mass spectrometry detection
Recently, the detection of GSNO using HPLC with electrochemical detection has been described [49] and successfully used to detect GSNO in NO-treated neurons and astrocytes. In this method, nitrite is neutralized with ammonium sulfamate [50] to avoid artifactual formation of S-nitrosothiols at low pH, and samples are analyzed by HPLC. This technique also allows for detection of GSH and GSSG, which can be advantageous when studying the metabolism of GSNO and other S-nitrosothiols. Airaki et al. [51] reported simultaneous analysis of GSNO, GSH, and GSSG by LC-ES/MS in plant tissues. This method represents a sensitive and relatively simple technique to study metabolism of S-nitrosothiols and thiol redox status in plants. A gas chromatogaphic-mass spectrometric technique for detection of GSNO has been described and used to study the metabolism of CysNO and GSNO in isolated human erythrocytes [52]. This method involves mercuric chloride decomposition of S-nitrosothiols and subsequent reaction of the product with pentafluorobenzyl bromide prior to analysis by GC-MS. This approach relies on the treatment with 15N isotopes of CysNO and GSNO and thus can be used only for in vitro experiments. An organic mercury compound, p-hydroxymercury benzoate (PHMB) has been used in a single step decomposition and derivatization of GSNO to form the GS-PHMB product that can be detected by reversed chromatography coupled to chemical vapor generation atomic fluorescence spectrometry [53].
3.5 General comments on methodologies
Detection methods discussed above differ in terms of sensitivity, specificity for GSNO vs. other S-nitrosothiols, and suitability to use in biological samples. The presence of GSH and S-nitrosothiols other than GSNO (e.g., CysNO, SNO-albumin) will determine the selection of analytical techniques. In addition, artifactual formation of GSNO from GSH is possible under acidic conditions in the presence of nitrite. Thus, acidification of the sample should be avoided when both nitrite and GSH are present. This limitation can be circumvented by pre-treatment with ammonium sulfamate or sulfanilamide to remove nitrite and N-ethylmaleimide (NEM) to block thiols group on GSH. In addition, the stability of GSNO should be taken into consideration. GSNO, like other S-nitrosothiols, can undergo metal-dependent decomposition, photolysis, and enzymatic degradation. Thus, metal chelators need to be present (e.g., DTPA, neocuproin), samples should be protected from light, and kept on ice to limit enzymatic decomposition. Methods used to collect the samples (e.g., syringe vs. Vacutainers for blood sampling) can result in variable GSNO measurements [53].
4. Biological mechanism of GSNO synthesis
S-Nitrosothiols have been detected in tissues and cells under basal conditions at low, but measurable levels, and have been shown to increase under pathological conditions (e.g., ischemia, iNOS induction). In this section, we will discuss the possible routes for cellular GSNO formation. These involve either the direct reaction of NO/nitrosating species with GSH or take place indirectly via formation of protein-based/low molecular weight S-nitrosothiols followed by subsequent transnitrosation to GSH. As discussed earlier, NO produced by NO synthase does not directly react with GSH to form GSNO, but rather additional reactions are required to engender thiol nitrosation.
4.1 Formation of nitrosating species from NO
NO can be oxidized by molecular oxygen to form dinitrogen trioxide that is a strong nitrosating species [14]. This reaction, described in section 2, is unlikely to occur at any meaningful rate under physiological concentrations of NO and oxygen. It has been suggested that hydrophobic environments such as lipid membranes represent important sites for formation of nitrosating species because both oxygen and NO preferentially partition into membranes [54]. GSNO formation from Proli/NO-derived NO and GSH has been shown to be much more efficient in the presence of low-density lipoprotein [55]. It should be clearly stressed that the formation of nitrosating species in hydrophobic environments does not necessarily lead to increase nitrosation of thiols in that microdomain. In fact, thiol groups in hydrophobic environments are more likely to be protonated and thus would be poorly reactive with nitrosating species. When model transmembrane peptides were used to examine nitrosation, the yield was dramatically decreased when thiol was positioned deeper into the membrane [56]. Whether this mechanism serves to increase S-nitrosothiol formation in vivo is as yet unclear.
4.2 Metal-dependent S-nitrosation
NO can readily react with thiyl radical to generate S-nitrosothiols [17,57]. Hence, formation of thiyl radical from GSH or proteins may enhance the formation of GSNO or protein-based S-nitrosothiols in the presence of NO. Although transition metal ions and protein metal centers are capable of performing a one-electron oxidation of thiol residues, transition metals ions (e.g., Cu, Fe) are tightly sequestered in the cells to prohibit this chemistry. With regards to protein metal centers, there is evidence that the plasma copper-protein ceruloplasmin can increase the generation of GSNO from a mixture of NO and GSH [58], but this has not been demonstrated in whole blood which contains high concentrations of hemoglobin, a potent NO scavenger [59]. Alternatively, dinitrosyl iron complexes (DNICs) are formed in a mixture of iron, thiol, and NO and have been suggested to mediate formation of S-nitrosothiols [60,61]. These complexes have been detected in cells and are considered to represent more stable metabolites of NO and perhaps also mediators of its activity [62,63]. In support of the hypothesis that DNIC are precursors of S-nitrosothiols, it has been shown that nitrosation of bovine serum albumin occurred in the presence of iron and cysteine and involved the intermediacy of DNIC [60]. In addition, the studies by Bosworth et al. showed that cellular DNIC resulted in formation of S-nitrosothiols [64] under anaerobic conditions, but not aerobic, indicating that this mechanism may be more significant under hypoxic conditions. These reports point to the fact that iron homeostasis and more specifically "labile iron" may play a role in formation of GSNO and other S-nitrosothiols in cellular systems.
4.3 Direct addition of NO to GSH
As mentioned previously, Gow et al. [18] reported a mechanism of S-nitrosothiol formation based on a direct addition of NO to a thiol (RSH) to form the radical intermediate RSNOH, and subsequent oxidation of this radical by oxygen or some other one-electron acceptor (e.g., NAD+). This reaction has not been confirmed to take place in the cellular environment, and we have not been able to show that NAD+ is able to oxidize the intermediate formed in this reaction [16]. However, we have reported that ferric cytochrome c can efficiently mediated formation of GSNO from NO and GSH [19]. Here, GSH is able to bind to and slowly reduce ferric cytochrome c. In the presence of NO, the rate of reduction of cytochrome c is greatly enhanced, and it is accompanied by GSNO formation. This reaction reaches almost 100% efficiency under anaerobic conditions and at low NO concentrations. We have proposed a mechanism in which NO reacts with GSH that is bound to cytochrome c, followed by electron transfer in this ternary complex to reduce heme and release GSNO. Higher concentrations of NO decrease the yield of GSNO formation when excess NO reacts with the ternary complex to form GSSG. In support of the role of cytochrome c as a mediator of GSNO synthesis, we have shown that: (1) this reaction takes place under both aerobic and anaerobic conditions; (2) depletion of cytochrome c using specific antibody or cellular knockouts of this protein decreases the formation of S-nitrosothiols; and (3) increasing the pool of ferric cytochrome c correlates with increased S-nitrosothiol generation in NO-producing cells [20]. Although this process is not intrinsically catalytic, reoxidation of ferrous cytochrome c by cellular processes (e.g., cytochrome c oxidase) would provide the recycling of cytochrome c and complete the catalytic cycle. Interestingly, the fact that GSNO formation requires ferric cytochrome c provides a potential link between GSNO formation and the cellular bioenergetic status. Supporting this concept, we demonstrated that the complex I inhibitor antimycin A is able to enhance NO-dependent protein S-nitrosation [20]; thus, it is possible the mitochondrial depolarization and mitochondrial electron transport chain dysfunction could lead to enhanced protein S-nitrosation.
4.4 Protein-catalyzed formation of S-nitrosothiols
Peroxidases have been proposed to represent potential S-nitrosothiol generating enzymes. Possible mechanisms for peroxidase-dependent formation of S-nitrosothiols involve the direct oxidation of NO by peroxidase compounds I and II to form NO+ [65], formation of thiyl radical [66] directly or indirectly (discussed earlier), and formation of nitrosating species (e.g., dinitrogen trioxide). Although myeloperoxidase has been reported to promote N-nitrosation of morpholine and derivatives [65], there is no experimental evidence to support the role of peroxidases in formation of GSNO and other S-nitrosothiols in vivo. Interestingly, tyrosyl radical, which can be formed from the action of peroxidases, has been theoretically implicated in S-nitrosothiol formation through thiyl radical mechanisms [67], or perhaps even through rapid intramolecular electron transfer [68].
5. Cellular mechanisms of GSNO degradation
5.1 GSNO reductase
GSNO reductase, also known as alcohol dehydrogenase 3, class III alcohol dehydrogenase, or GSH-dependent formaldehyde dehydrogenase, is a ubiquitously expressed, NADH-dependent enzyme with the capacity to oxidize medium-chain alcohols and the GSH adducts S-hydroxymethylglutathione (HMGSH) and, most importantly to this review, reduce GSNO [69]. Unlike other members of the alcohol dehydrogenase family, GSNO reductase has little variation in expression across tissues and is the only alcohol dehydrogenase expressed in human and rodent brain, an organ known to be highly regulated by NO- and S-nitrosothiol-dependent signals [70]. GSNO is reduced by GSNO reductase using NADH as a cofactor to produce an intermediate (GSNHOH) which can either react with GSH to produce GSSG and NH2OH or rearrange and then spontaneously hydrolyze to produce GSO2H and ammonia [69]. In either case, nitric oxide is not liberated during GSNO metabolism, and the nitroso moiety is reduced, effectively removing it from the ‘NO pool.’ Strong evidence supports this as an important biological function of this protein and not simply an ex vivo activity [71] making it a novel target for therapy in some pathological conditions. [72,73]. GSNO reductase activity is influenced by both the levels of NADH and GSH; thus, the redox state of a cell may significantly impact the clearance of GSNO and the S-nitroso proteome. In addition, because of GSNO reductase’s role in the oxidation of medium-chain alcohols and HMGSH, levels of these metabolites also impact the rate of GSNO degradation by GSNO reductase. In fact, GSNO reductase-dependent oxidation of HMGSH is increased 8-fold in the presence of GSNO in vitro and more than 20-fold in crude lung and liver lysates. These results highlight the potential impact of alternative GSNO reductase substrates on the biological activity and degradation of GSNO [74].
The role for GSNO reductase in human health and disease has been best characterized in the context of asthma [75]. GSNO reductase is endogenously present in the lung and can inhibit GSNO-mediated smooth muscle relaxation [5]. In addition GSNO knockout mice exhibit an increase in lung S-nitrosothiols and are protected from allogen-induced airway hyperresponsivity.[76]. Allelic variants and single nucleotides polymorphisms in GSNO reductase increase susceptibility to asthma [77], and levels of GSNO in the airways of asthmatics are lower than unaffected individuals [78,79]. It is thought that because GSNO is in transnitrosation equilibrium with protein S-nitrosothiols, enhanced metabolism of GSNO in asthma results in hypo-nitrosation of key proteins (e.g., G protein-coupled receptor 2; Grk2) and pathological consequences, and investigators have developed strategies to replete airway S-nitrosothiols in these patients [80].
While this discussion has focused on the role of GSNO reductase in mammalian cells, it is also expressed in many living organisms and has been implicated in embryonic development in amphioxus, sea squirts, and fruit flies. Moreover, in the plant Arabidopsis, the paraquat resistant 2 gene was recently shown to be a GSNO reductase and is a critical regulator of cell death [81]; thus, GSNO reductase has broad actions in many organisms.
5.2 Carbonyl reductase 1
Carbonyl reductase 1 is the most recent addition to the list of GSNO reducing enzymes. Though classically known to participate in the reduction of a broad spectrum of carbonyl-containing substrates including prostaglandins, steroids, and xenobiotics in phase 1 detoxification, a report in 2008 by Bateman et al. [82] first described its ability to reduce the nitrosyl bond of GSNO instead of carbonyl bonds of its other known substrates. Carbonyl reductase 1 metabolizes GSNO to an intermediate product which can then react with GSH to produce NH2OH and GSSG; thus, similar to GSNO reductase, carbonyl reductase 1 does not liberate NO in its catalytic reaction. Characterization of the kinetics of GSNO reduction by carbonyl reductase 1 revealed GSNO is an good substrate for this enzyme, with kinetic constants comparable to other known substrates [83]; however, a direct comparison between GSNO reductase and carbonyl reductase 1 shows that GSNO reductase is more efficient for specific reduction of GSNO (GSNO reductase: Km = 11 µM, Kcat = 1200 min−1; carbonyl reductase 1: Km = 30 µM, Kcat = 450 min−1). It is important to note that while GSNO reductase is a NADH-dependent enzyme, carbonyl reductase 1 requires NADPH to reduce its substrates; thus, the redox environment, and more specifically relative changes in the NADH/NAD+ versus the NADPH/NADP+ pools, may significantly alter metabolism of GSNO by both of these enzymes.
5.3 Thioredoxin system
The thioredoxin system comprises three main players, thioredoxin (Trx), thioredoxin reductase (TrxR), and NADPH, and it plays a critical role in the control and maintenance redox homeostasis [84]. There are two isoforms of Trx which localize to several subcellular compartments including the cytosol (Trx1), nucleus (Trx1), and mitochondria (Trx2). There, they are responsible for reduction of protein disulfides through their unique active site vicinal dithiols. Reduced Trx (Trx-(SH)2) reacts directly with a protein disulfide through a thiol-switching mechanism to yield a reduced protein dithiol and oxidized Trx (Trx-S2). Trx-S2 is subsequently reduced by TrxR and NADPH to complete the catalytic cycle. Trxs are essential in normal physiology, as knockouts of both isoforms are embryonically lethal. Deregulated Trx function has also been implicated in a host of human pathologies including cancer, lung diseases, and aging.
Although thioredoxin has long been recognized as a target of S-nitrosation [85], an emerging literature has identified Trxs as major players in the reduction of low-molecular weight and protein S-nitrosothiols where it participates in both denitrosation and transnitrosation reactions [86–88]. In its denitrosation capacity, reduced Trx reacts directly with either a low-molecular weight or protein S-nitrosothiol (including GSNO). Through transnitrosation, the low-pKa active-site thiol (Cys32) for Trx becomes S-nitrosated, leaving behind a low-molecular weight or protein thiol. The nitroso group now residing on Trx must then be turned over, and though it is not entirely clear how this occurs, some have suggested that nitroxyl (HNO) is released to form oxidized Trx. This is then reduced by TrxR and NADPH. Because of its more ubiquitous expression than GSNO reductase and carbonyl reductase, several investigators have hypothesized that the Trx system is the primary regulator of the S-nitroso proteome in most tissues, a concept supported by evidence that nearly all small (23–30 kDa) S-nitrosated proteins in HepG2 cells are targets for denitrosation by the thioredoxin system [89].
In addition, it has also been described that Trxs regulate important cellular processes through their transnitrosation activity at cysteine residues not required for its conventional catalytic activity. There are three additional cysteine residues in mammalian Trxs beyond those in the active site (cysteines 62, 69, and 73), and Cys73 has been shown to be S-nitrosated by GSNO when Trx is in its oxidized form [90]. Further, expression of mutant Trx1 lacking both active-site cysteine residues in HeLa cells caused an increase in S-nitroso proteins, 47 of which were reported to be unique Trx1 transnitrosation targets [90]. These studies and others like them demonstrate the thioredoxin system plays a critical role in the maintenance of the S-nitroso proteome by two distinct mechanisms: (1) reduced Trx denitrosates low-molecular weight and protein S-nitrosothiols; and (2) oxidized and S-nitrosated Trx transnitrosates specific protein targets. However, further studies are needed to address the interplay of the thioredoxin system with other GSNO/S-nitrosothiol metabolizing enzymes and its contribution to (patho)physiology of S-nitrosothiols.
5.4 γ-Glutamyl transpeptidase
γ-Glutamyltranspeptidase is an extracellular cell surface enzyme involved in the catabolism of glutathione adducts and the processing of thiol-based leukotrienes [5805}. The enzyme either hydrolyzes or, in the presence of a co-substrate such as glycyl-glycine, transfers the γ-glutamate group of glutathione, leaving cysteinyl-glycine [91]. The enzyme is relatively promiscuous and can process many glutathione thiol adducts including glutathione disulfide. Although highly active in the kidney brush border, the enzyme is found on many cell types and tissues [91]. It was shown some time ago that GGT can use GSNO as a substrate, generating S-nitrosocysteinylglycine [25]. This was first reported to have biological consequences, with the associated dipeptide transporter, in the toxicity of GSNO to salmonella tryphimuriam [92] and in the antiproliferative effects of GSNO on T and B lymphocytes [93]. More recently, GGT has been implicated in GSNO processing by mammalian cells and acivicin, and an inhibitor of GGT has been shown to affect some GSNO-mediated cellular responses. In particular, the role of GSNO in the maturation of cystic fibrosis transmembrane regulator has been shown to be GGT-dependent [94].
5.5 Other enzymes-dependent mechanisms
Some studies have suggested a role for other enzymes in GSNO metabolism/degradation. These include cell-surface protein disulfide isomerase (csPDI) [95–97], CuZn superoxide dismutase [98], glutathione peroxidase [99], and xanthine oxidase [100]. In all these cases, the reaction products include NO and glutathione or glutathione disulfide, and thus, do not serve a ‘NO terminase’ function. Moreover, when kinetic measurements have been made, these reactions have been shown to be much less efficient than GSNO reductase, carbonyl reductase 1, or the thioredoxin system [101]. csPDI may have a specific role in platelet-dependent GSNO metabolism [95].
5.6 Non-enzymatic pathways
As mentioned above, GSNO can react directly with other biological molecules including thiols, iron and copper ions [102] and ascorbate [103,104]. In the case of thiols and ascorbate these reactions tend to be slow, and iron and copper ions tend to be sequestered in cells and are present at very low levels. One exception is perhaps the reaction between GSNO and sulfide to generate HSNO. This reaction has recently been studied in detail and may have important cellular consequences [105]
6. Cellular responses to GSNO
Many studies have added GSNO to cells to look for biological effects. Most of these have assumed that GSNO mainly liberates NO and have interpreted the data in such a fashion. This is likely influenced by the commercial labeling of GSNO as an NO donor. Although NO may be released from GSNO in culture, the rate of NO formation is entirely dictated by media conditions and may not be the major mechanism of GSNO decay. This section will discuss other mechanisms by which GSNO can influence cellular responses.
6.1 Mechanisms of Cellular Uptake
GSNO itself is not directly taken up into cells; however, GSNO treatment does cause increases in cellular S-nitrosothiol levels in many conditions. Initially it was hypothesized that GSNO decomposes in the extracellular space to release NO which is then able to diffuse across the cell membrane to S-nitrosate protein targets [97,106]. There is evidence that shows that csPDI can metabolize GSNO to NO or its oxidation products [97] to selectively deliver these compounds to platelets [107]. However, in this case, the effect on S-nitrosothiol levels has not been examined, and this mechanism may not be relevant in all cell types.
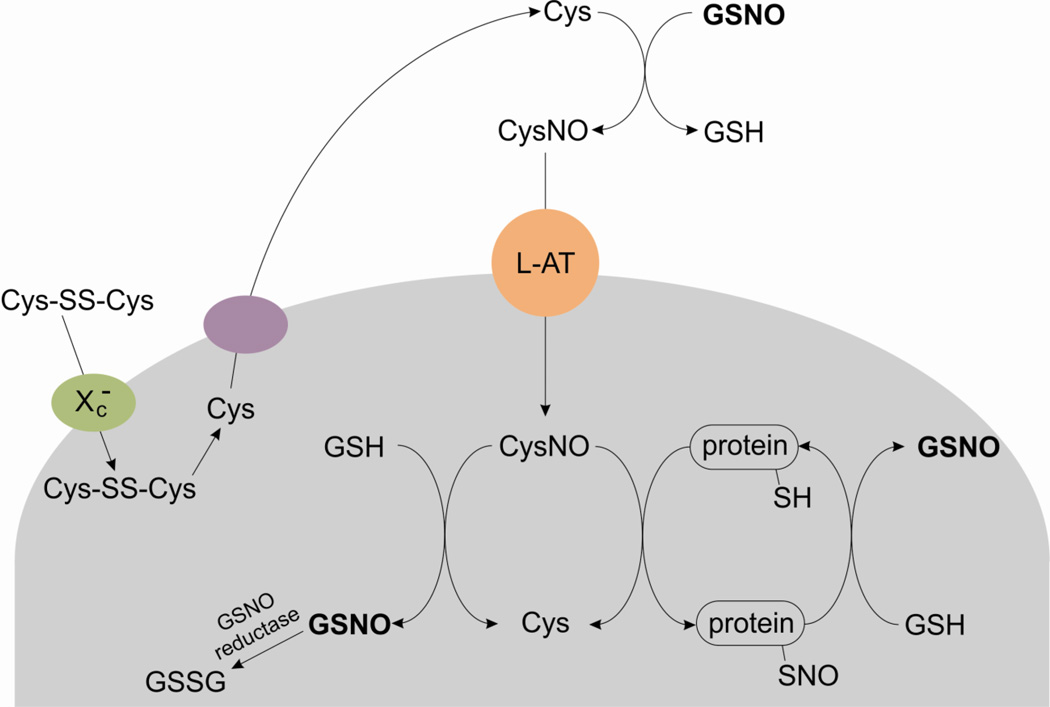
The primary NO-independent mechanism of GSNO uptake into cells requires the transfer of the nitroso group from GSNO to another thiol containing amino acid, L-cysteine, prior to uptake. This transnitrosation reaction produces glutathione and a new low-molecular weight S-nitrosothiol, S-nitroso-L-cysteine (L-CysNO) which is a good substrate for uptake through the amino acid transporter L system (L-AT) [29,108,109]. L-CysNO is readily transported into cells and can either S-nitrosate cellular glutathione to reform GSNO inside the cell or directly S-nitrosate protein thiols to elicit cellular responses. It was observed that the presence of cystine in cell culture media was required for the cellular metabolism of GSNO [110]. The mechanism involves cellular uptake of cystine via the Xc− transporter followed by the intracellular reduction and export of cysteine. GSNO then transnitrosates the newly formed cysteine, and the final step is uptake of L-CysNO by the L-AT system [29,107]. This is summarized in Figure 1.
Figure 1.
A model for cellular S-nitrosothiol uptake. Cystine (Cys-SS-Cys) is transported into cells through Xc− and subsequently reduced to cysteine (Cys) within the cell. Cysteine can then be exported back to the extracellular space where it is transnitrosated by GSNO to form S-nitrosocysteine (CysNO). CysNO is a good substrate for amino acid transporter system L (L-AT) and is avidly taken up by cells; thus, transferring the nitroso function group across the plasma membrane. Additional transnitrosation rations can occur within the cell.
6.2 Protein S-nitrosation as a mechanism for GSNO-dependent cellular responses
There is a large number of proteins known to be S-nitrosated in various (patho)physiological contexts (termed the S-nitroso proteome). In fact, conserved cysteine residues occur in almost all classes of proteins and, in many cases, are important for protein function [111–113]; thus, S-nitrosation of proteins thiols can control diverse cellular processes. With regards to GSNO-dependent effects, S-nitrosation of protein thiols occurs through the direct transfer of a nitroso group from GSNO or another GSNO-derived low molecular weight S-nitrosothiol to a protein thiol through transnitrosation. Despite a plethora of studies examining the effects of GSNO both in vitro and in vivo, few studies have identified the compound which actually S-nitrosates a specific protein thiol, whether it be GSNO or another GSNO-derived low molecular weight S-nitrosothiol (e.g., L-CysNO). Nevertheless, in all these cases, GSNO is the source of the nitroso moiety and a likely intermediate in the protein S-nitrosation process.
S-Nitrosation of proteins increases in biological contexts where NO production is elevated (e.g., inflammation), and some evidence indicates that GSNO is formed under these conditions. In addition, S-nitrosation of specific proteins has been directly implicated in several disease states including parkin in neurodegeneration [114] and ryanodine receptors in heart failure [115]. This can result in both deleterious and beneficial effects. In fact, there is an added layer of complexity in how S-nitrosation controls cellular responses, as some proteins are S-nitrosated basally, and loss of this modification (hypo-nitrosation) results in pathologic effects whereas other are not S-nitrosated to begin with and modification of critical thiols (hyper-nitrosation) has deleterious results. For comprehensive reviews of S-nitrosation and a discussion of the S-nitroso proteome, we refer the reader to the following articles ([116–118]).
Attempts to identify the S-nitroso proteome and the specific role for GSNO in this process in vivo have focused on comparing S-nitrosated proteins from different organs in wild-type and NOS isoform knock-out mice. These studies have revealed tissue- and context- specific protein targets of S-nitrosation. Perhaps more enlightening with respect to GSNO’s role in physiology are similar comparisons in mice with knock out of the primary GSNO metabolizing enzyme, GSNO reductase (also known as GSNOR). These mice are protected from heart failure [119] and asthma [76], but have increased mortality in models of septic shock [120]. Although GSNOR is the most ubiquitous and well-studied enzyme in GSNO metabolism, others have been reported (discussed in detail above). In these cases, the impact of genetic manipulation of alternative GSNO degrading enzymes on the S-nitroso proteome has not been examined to date.
6.3 Protein S-glutathiolation from GSNO
As mentioned above, in addition to transnitrosation, GSNO can react slowly with thiols to form a disulfide resulting in S-glutathiolation. In pure systems, some protein thiols appear to have a preference for S-thiolation over S-nitrosation [121,122]. For example, bovine serum albumin showed extensive S-nitrosation after incubation with both SNAP and GSNO, whereas creatine kinase favored S-thiolation when exposed to GSNO and S-nitrosation when exposed to SNAP [122]. From a thermodynamic perspective, it is likely that disulfide formation is the eventual end-point, as the reaction is effectively reversible. The preference for S-nitrosation or S-thiolation is likely to be due to tuning of reaction kinetics by the thiol environment, and as in the above example, that nature of the initial S-nitrosothiol. There is evidence that it is not GSNO per se but a degradation product of GSNO (namely glutathione disulfide-S-oxide) that results in protein S-glutathiolation [123,124], which may limit the biological importance of this reaction as it is not clear if these compounds can be formed from GSNO in vivo. The question of whether S-thiolation is an important cellular response to S-nitrosothiol formation or exposure has not been fully addressed and is not often considered. Protein S-thiolation was recognized quite early as cellular response to NO [125], however whether GSNO is an intermediate in this process remains unclear.
6.4 Canonical NO-dependent responses to GSNO
It is worth mentioning that as a low-molecular weight S-nitrosothiol, GSNO is a good S-nitrosating agent; however, in some contexts, it may also releases NO. Because of this property, GSNO has been used an NO donor, and many reports have identified canonical NO-dependent responses, completely independent of protein S-nitrosation. As mentioned above, NO release from GSNO depends strongly on environmental factors (e.g., transition metal ions, light, etc.); however, there is evidence for an intracellular flavoprotein-dependent mechanism for NO liberation from S-nitrosothiols [126], although it’s identity remains unclear.
7. Use of GSNO in human clinical trials
To date, there have been nearly 20 clinical trials investigating the therapeutic efficacy of GSNO in multiple pathological contexts though most have focused on its effects in cardiovascular diseases. GSNO has been administered through intravenous infusion [127], as an aerosolized inhalant [128], and more recently, as a topical gel [129] and poly vinyl alcohol film [130]. The best-characterized effects of GSNO in humans are on its direct and selective action in platelets. In this case, GSNO is thought to act primarily as an NO donor [131–134]. Though the mechanism of its selective action in platelets is not clear, some have suggested that it is driven by the high expression csPDI on the surface of platelets. As discussed above, csPDI can metabolize GSNO to produce NO, a well-known anti-platelet compound, and decrease coagulation and thrombosis [95]. In addition, release of NO in the vasculature has significant vasodilatory effects which may also contribute to the effects observed with GSNO administration. GSNO has been shown to decrease embolism from symptomatic carotid plaques and after carotid endarterectomy [135], carotid angioplasty [131], and vein graft [136] by limiting platelet activation. Its beneficial effects in the vasculature extend to cardiac left ventricular function [127], systemic vasodilation [137], and preeclampsia [138]. Topical administration of a GSNO gel has also been shown to increase clitoral blood flow and has been suggested as a treatment for female sexual dysfunction [129].
Beyond its vascular effects in humans, GSNO has been examined in several additional conditions. These include as an anti-fungal agent in nails [139] and as a therapeutic agent in cystic fibrosis [128]. Topical administration of GSNO was shown to improve onychomycosis through an S-nitrosothiol-dependent mechanism [139]. This is particularly noteworthy, as the nail bed represents a physical barrier impenetrable to many anti-fungal agents. Moreover, in patients with cystic fibrosis, a condition correlated with depleted airway S-nitrosothiol levels, aerosolized GSNO was well-tolerated and increased oxygen saturation acutely [128]. These effects were also suggested to be through NO-independent mechanisms.
There are many other conditions in which S-nitrosothiols are changed in humans. In fact, as a NO-dependent post-translational modification of proteins, S-nitrosothiols levels have been used as an indicator of NO production in biological samples and are known to increase upon infusion with sodium nitrite [140], nitrate ingestion [141], treatment with NO-releasing aspirin [142], and dietary flavonoid consumption [143]. Perhaps more interesting, however, is documentation of a correlation of S-nitrosothiol levels and disease pathology in humans. As examples, cerebrospinal fluid of patients with multiple sclerosis [144] and airway lining fluid in asthmatics treated with budesonide [145] have elevated S-nitrosothiols compared to their respective controls. It is these types of studies and the wealth of literature supporting a role for GSNO and other S-nitrosothiols in many (patho)physiological contexts that will likely motivate additional clinical trials in this area.
8. Final Remarks
This review has focused on the chemical and biological aspects of GSNO and its potential as a therapeutic agent, but it should be placed in the context of S-nitrosation as a whole. GSNO may not be the best way to increase cellular S-nitrosothiol levels, and it is clearly only one part of the story with respect to NO-dependent thiol modification. However, GSNO is well-tolerated in humans and is likely a central intermediate in cellular nitrosation events. Consequently, it remains an important molecule of study.
Highlights.
GSNO exerts its actions through nitric oxide- and S-nitrosation-dependent mechanisms.
GSNO effects should be placed in the context of S-nitrosation as a whole.
A role for GSNO in pathology will likely drive its development as a therapeutic agent.
Acknowledgements
This work was supported by the Redox Biology Program at the Medical College of Wisconsin (to N.H.), an Interdisciplinary Cancer Research Post-Doctoral Fellowship from the Cancer Center of the Medical College of Wisconsin (to A.R.D.), and the National Institutes of Health [grant number R01-GM-55792 (to N.H.)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hart TW. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of L-cysteine and glutathione. Tetrahedron Lett. 1985;26:2013. [Google Scholar]
- 2.Williams DLH. S-Nitrosation and the reactions of S-Nitroso Compounds. Chemical Society Reviews. 1985:191. [Google Scholar]
- 3.Oae S, Kim YH, Fukushima D, Shinhama K. New syntheses of thionitrites and their chemical reactivities. J. Chem. Soc. Perkin Trans. 1978;1:913. [Google Scholar]
- 4.Ignarro LJ, Barry BK, Gruetter DY, Edwards JC, Ohlstein EH, Gruetter CA, Baricos WH. Guanylate cyclase activation of nitroprusside and nitrosoguanidine is related to formation of S-nitrosothiol intermediates. Biochem. Biophys. Res. Commun. 1980;94:93. doi: 10.1016/s0006-291x(80)80192-6. [DOI] [PubMed] [Google Scholar]
- 5.Fang K, Johns R, Macdonald T, Kinter M, Gaston B. S-nitrosoglutathione breakdown prevents airway smooth muscle relaxation in the guinea pig. Am. J. Physiol Lung Cell Mol. Physiol. 2000;279:L716–L721. doi: 10.1152/ajplung.2000.279.4.L716. [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Akerboom TP, Sies H, Thomas JA. S-Nitrosylation and S-glutathiolation of protein sulfhydryls by S-Nitrosoglutathione. Arch. Biochem. Biophys. 1999;362:67. doi: 10.1006/abbi.1998.1013. [DOI] [PubMed] [Google Scholar]
- 7.Mathews WR, Kerr SW. Biological activity of S-nitrosothiols: the role of nitric oxide. J. Pharmacol. Exp. Ther. 1993;267:1529. [PubMed] [Google Scholar]
- 8.Pryor WA, Church DF, Govindan CK, Crank G. Oxidation of thiols by nitric oxide and nitrogen dioxide: Synthetic utility and toxicological implications. J. Org. Chem. 1982;47:159. [Google Scholar]
- 9.Hogg N, Singh RJ, Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett. 1996;382:223. doi: 10.1016/0014-5793(96)00086-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YL, Houk KN. Thionitroxides, RSNHO: The Structure of the SNO Moiety in "S-Nitrosohemoglobin" A Possible NO Reservoir and Transporter. J. Am. Chem. Soc. 2006;128:1422. doi: 10.1021/ja057097f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernhoff NB, Derbyshire ER, Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21602. doi: 10.1073/pnas.0911083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkes LK, Wardman P. Kinetics of the reaction between nitric oxide and glutathione: implications for thiol depletion in cells. Free Radic. Biol. Med. 2004;37:549. doi: 10.1016/j.freeradbiomed.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Wink DA, Darbyshire JF, Nims RW, Saavedra JE, Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation reaction by intermediates generated in the NO/O2 reaction. Chem. Res. Toxicol. 1993;6:23. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein S, Czapski G. Mechanism of the nitrosation of thiols and amines by oxygenated NO solutions: the nature of the nitrosating intermediates. J. Am. Chem. Soc. 1996;118:3419. [Google Scholar]
- 15.Mirza UA, Chait BT, Lander HM. Monitoring reactions of nitric oxide with peptides and proteins by electrospray ionization-mass spectrometry. J. Biol. Chem. 1995;270:17185. doi: 10.1074/jbc.270.29.17185. [DOI] [PubMed] [Google Scholar]
- 16.Keszler A, Zhang Y, Hogg N. The Reaction between Nitric Oxide, Glutathione and Oxygen in the Presence and Absence of Protein: How are S-Nitrosothiols Formed? Free Radic. Biol. Med. 2009;48:55. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J. Biol. Chem. 2003;278:15720. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 18.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J. Biol. Chem. 1997;272:2841. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Keszler A, Azarova NA, Nwanze N, Perlegas A, Shiva S, Broniowska KA, Hogg N, Kim-Shapiro DB. A novel role for cytochrome c: Efficient catalysis of S-nitrosothiol formation. Free Radic. Biol. Med. 2010;48:255. doi: 10.1016/j.freeradbiomed.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broniowska KA, Keszler A, Basu S, Kim-Shapiro DB, Hogg N. Cytochrome c-mediated formation of S-nitrosothiol in cells. Biochem. J. 2011 doi: 10.1042/BJ20111294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartberger MD, Houk KN, Powell SC, Mannion JD, Lo KY, Stamler J, Toone EJ. Theory Spectroscopy and crystolographic analysis of S-nitrosothiols: Conformational distribution dictates spectroscopic behavior. J. Am. Chem. Soc. 2000;122:5889. [Google Scholar]
- 22.Bartberger MD, Mannion JD, Powell SC, Stamler JS, Houk KN, Toone EJ. S-N dissociation energies of S-nitrosothiols: on the origins of nitrosothiol decomposition rates. J Am Chem Soc. 2001;123:8868. doi: 10.1021/ja0109390. [DOI] [PubMed] [Google Scholar]
- 23.Dicks AP, Swift HR, Williams DL, Butler AR, Al-Sa'doni HH, Cox BG. Identification of Cu+ as the effective reagent in nitric oxide formation from S-nitrosothiols (RSNO) J. Chem. Soc. Perkin Trans. 1996;2:481. [Google Scholar]
- 24.Noble DR, Swift HR, Williams DLH. Nitric oxide release from S-nitrosoglutathione (GSNO) Chem. Commun. 1999:2317. [Google Scholar]
- 25.Hogg N, Singh RJ, Konorev E, Joseph J, Kalyanaraman B. S-Nitrosoglutathione as a substrate for gamma-glutamyl transpeptidase. Biochem. J. 1997;323:477. doi: 10.1042/bj3230477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogg N. The kinetics of S-Transnitrosation - a reversible second-order reaction. Anal. Biochem. 1999;272:257. doi: 10.1006/abio.1999.4199. [DOI] [PubMed] [Google Scholar]
- 27.Meyer DJ, Kramer H, Ozer N, Coles B, Ketterer B. Kinetics equilibria of S-nitrosothiol-thiol exchange between glutathione, cysteine, penicillamines and serum albumin. FEBS Lett. 1994;345:177. doi: 10.1016/0014-5793(94)00429-3. [DOI] [PubMed] [Google Scholar]
- 28.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 1996;271:18596. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. USA. 2004;101:7891. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnelle DR, Stamler JS. NO+, NO and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 31.Singh SP, Wishnok JS, Keshive M, Deen WM, Tannenbaum SR. The chemistry of the S-nitrosoglutathione/glutathione system. Proc. Natl. Acad. Sci. USA. 1996;93:14428. doi: 10.1073/pnas.93.25.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton SA, Choi YB, Sucher NJ, Pan ZH, Stamler JS. Redox state, NMDA receptors and NO-related species. Trends Pharmacol. Sci. 1996;17:186. doi: 10.1016/0165-6147(96)20028-8. [DOI] [PubMed] [Google Scholar]
- 33.Welch GN, Upchurch GRJ, Loscalzo J. S-nitrosothiol detection. Methods Enzymol. 1996;268:293. doi: 10.1016/s0076-6879(96)68031-8. [DOI] [PubMed] [Google Scholar]
- 34.Stamler JS, Loscalzo J. Capillary zone electrophoretic detection of biological thiols and their S-nitrosated derivatives. Anal. Chem. 1992;64:779. doi: 10.1021/ac00031a014. [DOI] [PubMed] [Google Scholar]
- 35.Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst. 1958;83:670. [Google Scholar]
- 36.Griess P. Bemerkungen zu der Abhandlung der HH. Weselky und Benedikt Ueber einige Azoverbindungen. Chemische Berichte. 1879;12:426. [Google Scholar]
- 37.Cook JA, Kim SY, Teague D, Krishna MC, Pacelli R, Mitchell JB, Vodovotz, Nims RW, Christodoulou D, Miles AM, Grisham MB, Wink DA. Convenient colorimetric and fluorometric assays for S-nitrosothiols. Anal. Biochem. 1996;238:150. doi: 10.1006/abio.1996.0268. [DOI] [PubMed] [Google Scholar]
- 38.Park JK, Kostka P. Fluorometric detection of biological S-nitrosothiols. Anal. Biochem. 1997;249:61. doi: 10.1006/abio.1997.2159. [DOI] [PubMed] [Google Scholar]
- 39.Bramanti E, Angeli V, Mester Z, Pompella A, Paolicchi A, D'Ulivo A. Determination of S-nitrosoglutathione in plasma: comparison of two methods. Talanta. 2010;81:1295. doi: 10.1016/j.talanta.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Tsikas D, Sandmann J, Holzberg D, Pantazis P, Raida M, Frolich JC. Determination of S-nitrosoglutathione in human and rat plasma by high-performance liquid chromatography with fluorescence and ultraviolet absorbance detection after precolumn derivatization with o-phthalaldehyde. Anal. Biochem. 1999;273:32. doi: 10.1006/abio.1999.4209. [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer S, Schrammel A, Schmidt K, Mayer B. Electrochemical determination of s-nitrosothiols with a clark-type nitric oxide electrode. Anal. Biochem. 1998;258:68. doi: 10.1006/abio.1998.2562. [DOI] [PubMed] [Google Scholar]
- 42.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. USA. 1992;89:7674. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alpert C, Ramdev N, George D, Loscalzo J. Detection of S-nitrosothiols and other nitric oxide derivatives by photolysis-chemiluminescence spectrometry. Anal. Biochem. 1997;245:1. doi: 10.1006/abio.1996.9947. [DOI] [PubMed] [Google Scholar]
- 44.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic. Res. 2003;37:1. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 45.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem. 1998;258:322. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 46.Lu WP, Ragsdale SW. Reductive activation of the coenzyme A/acetyl-CoA isotopic exchange reaction catalyzed by carbon monoxide dehydrogenase from Clostridium thermoaceticum and its inhibition by nitrous oxide and carbon monoxide. J. Biol. Chem. 1991;266:3554. [PubMed] [Google Scholar]
- 47.Nagababu E, Rifkind JM. Determination of s-nitrosothiols in biological fluids by chemiluminescence. Methods Mol. Biol. 2011;704:27. doi: 10.1007/978-1-61737-964-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang K, Ragsdale NV, Carey RM, Macdonald T, Gaston B. Reductive Assays for S-Nitrosothiols: Implications for Measurements in Biological Systems. Biochem. Biophys. Res. Commun. 1998;252:535. doi: 10.1006/bbrc.1998.9688. [DOI] [PubMed] [Google Scholar]
- 49.Yap LP, Sancheti H, Ybanez MD, Garcia J, Cadenas E, Han D. Determination of GSH, GSSG, and GSNO using HPLC with electrochemical detection. Methods Enzymol. 2010;473:137. doi: 10.1016/S0076-6879(10)73006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsikas D. Measurement of physiological S-nitrosothiols: a problem child and a challenge. Nitric Oxide. 2003;9:53. doi: 10.1016/s1089-8603(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 51.Airaki M, Sanchez-Moreno L, Leterrier M, Barroso JB, Palma JM, Corpas FJ. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant Cell Physiol. 2011;52:2006. doi: 10.1093/pcp/pcr133. [DOI] [PubMed] [Google Scholar]
- 52.Tsikas D, Sandmann J, Rossa S, Gutzki FM, Frolich JC. Gas Chromatographic-Mass Spectrometric Detection of S-Nitroso-cysteine and S-Nitroso-glutathione. Anal. Biochem. 1999;272:117. doi: 10.1006/abio.1999.4177. [DOI] [PubMed] [Google Scholar]
- 53.Bramanti E, Angeli V, Mester Z, Pompella A, Paolicchi A, D'Ulivo A. Determination of S-nitrosoglutathione in plasma: comparison of two methods. Talanta. 2010;81:1295. doi: 10.1016/j.talanta.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Miller MJS, Joshi MS, Thomas DD, Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA. 1998;95:2175. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR, Denicola A. Membrane "Lens" Effect: Focusing the Formation of Reactive Nitrogen Oxides from the NO/O2 Reaction. Chem. Res. Toxicol. 2007;20:709. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Andrekopoulos C, Xu Y, Joseph J, Hogg N, Feix J, Kalyanaraman B. Decreased S-Nitrosation of peptide thiols in the membrane interior. Free Radic. Biol. Med. 2009 doi: 10.1016/j.freeradbiomed.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madej E, Folkes LK, Wardman P, Czapski G, Goldstein S. Thiyl radicals react with nitric oxide to form S-nitrosothiols with rate constants near the diffusion-controlled limit. Free Radic. Biol. Med. 2008;44:2013. doi: 10.1016/j.freeradbiomed.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol Formation Catalyzed by Ceruloplasmin. Implication for cytoprotective mechanism in vivo. J. Biol. Chem. 1999;274:27069. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc. Natl. Acad. Sci. USA. 2004;101:11477. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boese M, Mordvintcev P, Vanin AF, Busse R, Mülsch A. S-Nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem. 1995;270:29244. doi: 10.1074/jbc.270.49.29244. [DOI] [PubMed] [Google Scholar]
- 61.Bosworth CA, Toledo J, Zmijewski JW, Li Q, Lancaster JR., Jr The mechanism of cellular nitrosothiol formation from nitric oxide. Free Radic. Biol. Med. 2008;45:S110. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lancaster JR, Jr, Hibbs JB., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1223. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanin AF. Dinitrosyl iron complexes and S-nitrosothiols are two possible forms for stabilization and transport of nitric oxide in biological systems. Biochemistry (Mosc.) 1998;63:782. [PubMed] [Google Scholar]
- 64.Bosworth CA, Toledo JC, Zmijewski JW, Li Q, Lancaster JR. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. USA. 2009;106:4671. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lakshmi VM, Nauseef WM, Zenser TV. Myeloperoxidase Potentiates Nitric Oxide-mediated Nitrosation. J. Biol. Chem. 2005;280:1746. doi: 10.1074/jbc.M411263200. [DOI] [PubMed] [Google Scholar]
- 66.Burner U, Obinger C. Transient-state and steady-state kinetics of the oxidation of aliphatic and aromatic thiols by horseradish peroxidase. FEBS Lett. 1997;411:269. doi: 10.1016/s0014-5793(97)00713-8. [DOI] [PubMed] [Google Scholar]
- 67.Lancaster JR. Nitroxidative, Nitrosative, and Nitrative Stress: Kinetic Predictions of Reactive Nitrogen Species Chemistry Under Biological Conditions. Chem. Res. Toxicol. 2006;19:1160. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H, Xu Y, Joseph J, Kalyanaraman B. Intramolecular Electron Transfer between Tyrosyl Radical Cysteine Residue Inhibits Tyrosine Nitration Induces Thiyl Radical Formation in Model Peptides Treated with Myeloperoxidase, H2O2, and NO2-: EPR SPIN TRAPPING STUDIES. J. Biol. Chem. 2005;280:40684. doi: 10.1074/jbc.M504503200. [DOI] [PubMed] [Google Scholar]
- 69.Jensen DE, Belka GK, Du Bois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 1998;331:659. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoog JO, Ostberg LJ. Mammalian alcohol dehydrogenases--a comparative investigation at gene and protein levels. Chem. Biol. Interact. 2011;191:2. doi: 10.1016/j.cbi.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 71.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 72.Green LS, Chun LE, Patton AK, Sun X, Rosenthal GJ, Richards JP. Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase. Biochemistry. 2012;51:2157. doi: 10.1021/bi201785u. [DOI] [PubMed] [Google Scholar]
- 73.Sun X, Qiu J, Strong SA, Green LS, Wasley JW, Blonder JP, Colagiovanni DB, Mutka SC, Stout AM, Richards JP, Rosenthal GJ. Discovery of potent and novel S-nitrosoglutathione reductase inhibitors devoid of cytochrome P450 activities. Bioorg. Med. Chem. Lett. 2011;21:5849. doi: 10.1016/j.bmcl.2011.07.103. [DOI] [PubMed] [Google Scholar]
- 74.Staab CA, Alander J, Brandt M, Lengqvist J, Morgenstern R, Grafstrom RC, Hoog JO. Reduction of S-nitrosoglutathione by alcohol dehydrogenase 3 is facilitated by substrate alcohols via direct cofactor recycling and leads to GSH-controlled formation of glutathione transferase inhibitors. Biochem. J. 2008;413:493. doi: 10.1042/BJ20071666. [DOI] [PubMed] [Google Scholar]
- 75.Henderson EM, Gaston B. SNOR and wheeze: the asthma enzyme?, Trends in Molecular Medicine In Press. Corrected Proof. 2005 doi: 10.1016/j.molmed.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from Experimental Asthma by an Endogenous Bronchodilator. Science. 2005;308:1618. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu H, Romieu I, Sienra-Monge JJ, Estela del Rio-Navarro B, Anderson DM, Jenchura CA, Li H, Ramirez-Aguilar M, del Carmen Lara-Sanchez I, London SJ. Genetic variation in S-nitrosoglutathione reductase (GSNOR) and childhood asthma. Journal of Allergy and Clinical Immunology. 2007;120:322. doi: 10.1016/j.jaci.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ, Loscalzo J, Stamler JS. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc. Natl. Acad. Sci. USA. 1993;90:10957. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaston B, Sears S, Woods J, Hunt J, Ponaman M, McMahon TJ, Stamler JS. Bronchodilator s-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351:1317. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 80.Moya MP, Gow AJ, McMahon TJ, Toone EJ, Cheifetz IM, Goldberg RN, Stamler JS. S-nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc. Natl. Acad. Sci. USA. 2001;98:5792. doi: 10.1073/pnas.091109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen R, Sun S, Wang C, Li Y, Liang Y, An F, Li C, Dong H, Yang X, Zhang J, Zuo J. The Arabidopsis PARAQUAT RESISTANT2 gene encodes an S-nitrosoglutathione reductase that is a key regulator of cell death. Cell Res. 2009;19:1377. doi: 10.1038/cr.2009.117. [DOI] [PubMed] [Google Scholar]
- 82.Bateman RL, Rauh D, Tavshanjian B, Shokat KM. Human carbonyl reductase 1 is an S-nitrosoglutathione reductase. J. Biol. Chem. 2008:M807125200. doi: 10.1074/jbc.M807125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Staab CA, Hartmanova T, El-Hawari Y, Ebert B, Kisiela M, Wsol V, Martin HJ, Maser E. Studies on reduction of S-nitrosoglutathione by human carbonyl reductases 1 and 3. Chem. Biol. Interact. 2011;191:95. doi: 10.1016/j.cbi.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Sengupta R, Holmgren A. The role of thioredoxin in the regulation of cellular processes by S-nitrosylation. Biochim. Biophys. Acta. 2012;1820:689. doi: 10.1016/j.bbagen.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 85.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat. Cell Biol. 2002;4:743. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell DA, Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1:154. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 87.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sengupta R, Holmgren A. Thioredoxin and Thioredoxin Reductase in Relation to Reversible S-Nitrosylation. Antioxid. Redox Signal. 2012 doi: 10.1089/ars.2012.4716. [DOI] [PubMed] [Google Scholar]
- 89.Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, Stoyanovsky DA. Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry. 2007;46:8472. doi: 10.1021/bi700449x. [DOI] [PubMed] [Google Scholar]
- 90.Wu C, Liu T, Chen W, Oka S, Fu C, Jain MR, Parrott AM, Baykal AT, Sadoshima J, Li H. Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol. Cell Proteomics. 2010;9:2262. doi: 10.1074/mcp.M110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meister A, Tate SS, Griffith OW. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- 92.De Groote MA, Granger D, Xu Y, Campbell G, Prince R, Fang FC. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA. 1995;92:6399. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henson SE, Nichols TC, Holers VM, Karp DR. The ectoenzyme gamma-glutamyl transpeptidase regulates antiproliferative effects of S-nitrosoglutathione on human T and B lymphocytes. J. Immunol. 1999;163:1845. [PubMed] [Google Scholar]
- 94.Zaman K, McPherson M, Vaughan J, Hunt J, Mendes F, Gaston B, Palmer LA. S-Nitrosoglutathione increases cystic fibrosis transmembrane regulator maturation. Biochem. Biophys. Res. Commun. 2001;284:65. doi: 10.1006/bbrc.2001.4935. [DOI] [PubMed] [Google Scholar]
- 95.Root P, Sliskovic I, Mutus B. Platelet cell surface disulfide isomerase mediated S-Nitrosoglutathione consumption. Biochem. J. 2004;382:575. doi: 10.1042/BJ20040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bell SE, Shah CM, Gordge MP. Protein disulfide-isomerase mediates delivery of nitric oxide redox derivatives into platelets. Biochem J. 2007;403:283. doi: 10.1042/BJ20061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramachandran N, Root P, Jiang X-M, Hogg PJ, Mutus B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc. Natl. Acad. Sci. USA. 2001;98:9539. doi: 10.1073/pnas.171180998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jourd'heuil D, Laroux FS, Miles AM, Wink DA, Grisham MB. Effect of superoxide dismutase on the stability of S-nitrosothiols. Arch. Biochem. Biophys. 1999;361:323. doi: 10.1006/abbi.1998.1010. [DOI] [PubMed] [Google Scholar]
- 99.Hou Y, Guo Z, Li J, Wang PG. Seleno compounds and glutathione peroxidase catalyzed decomposition of S-nitrosothiols. Biochem. Biophys. Res. Commun. 1996;228:88. doi: 10.1006/bbrc.1996.1620. [DOI] [PubMed] [Google Scholar]
- 100.Trujillo M, Alvarez MN, Peluffo G, Freeman BA, Radi R. Xanthine Oxidase-mediated Decomposition of S-Nitrosothiols. J. Biol. Chem. 1998;273:7828. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- 101.Staab CA, Hellgren M, Hoog JO. Medium- and short-chain dehydrogenase/reductase gene and protein families : Dual functions of alcohol dehydrogenase 3: implications with focus on formaldehyde dehydrogenase and S-nitrosoglutathione reductase activities. Cell Mol. Life Sci. 2008;65:3950. doi: 10.1007/s00018-008-8592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams DL. S-nitrosothiols and role of metal ions in decomposition to nitric oxide. Methods Enzymol. 1996;268:299. doi: 10.1016/s0076-6879(96)68032-x. [DOI] [PubMed] [Google Scholar]
- 103.Holmes AJ, Williams DLH. Reaction of ascorbic acid with S-nitrosothiols: clear evidence for two distinct reaction pathways. J. Chem. Soc. Perkin Trans. 2000;2:1639. [Google Scholar]
- 104.Scorza G, Pietraforte D, Minetti M. Role of ascorbate and protein thiols in the release of nitric oxide from S-nitroso-albumin and S-nitroso-glutathione in human plasma. Free Radic. Biol. Med. 1997;22:633. doi: 10.1016/s0891-5849(96)00378-4. [DOI] [PubMed] [Google Scholar]
- 105.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012;134:12016. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramachandran N, Jacob S, Zielinski B, Curatola G, Mazzanti L, Mutus B. N-Dansyl-S-nitrosohomocysteine a fluorescent probe for intracellular thiols and S-nitrosothiols. Biochim. Biophys. Acta. 1999;1430:149. doi: 10.1016/s0167-4838(98)00286-6. [DOI] [PubMed] [Google Scholar]
- 107.Zhu J, Li S, Marshall ZM, Whorton AR. A cystine-cysteine shuttle mediated by xCT facilitates cellular responses to S-nitrosoalbumin. Am. J. Physiol. Cell Physiol. 2008;294:C1012–C1020. doi: 10.1152/ajpcell.00411.2007. [DOI] [PubMed] [Google Scholar]
- 108.Rassaf T, Bryan NS, Maloney RE, Specian V, Kelm M, Kalyanaraman B, Rodriguez J, Feelisch M. NO adducts in mamlian blood cells: too much or too little? Nat. Med. 2003;9:481. doi: 10.1038/nm0503-481. [DOI] [PubMed] [Google Scholar]
- 109.Takehara Y, Kanno T, Yoshioka T, Inoue M, Utsumi K. Oxygen-dependent regulation of mitochondrial energy metabolism by nitric oxide. Arch. Biochem. Biophys. 1995;323:27. doi: 10.1006/abbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- 110.Zeng H, Spencer NY, Hogg N. Metabolism of S-nitrosoglutathione by endothelial cells. Am. J. Physiol. 2001;281:H432–H439. doi: 10.1152/ajpheart.2001.281.1.H432. [DOI] [PubMed] [Google Scholar]
- 111.Claiborne A, Yeh JI, Conn Mallett T, Luba J, Crane EJ, III, Charrier V, Parsonage D. Protein-sulfenic acids: Diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 112.Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 113.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung KKK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-Nitrosylation of Parkin Regulates Ubiquitination and Compromises Parkin’s Protective Function. Science. 2004;304:1328. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 115.Gonzalez DR, Treuer A, Sun QA, Stamler JS, Hare JM. S-Nitrosylation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009;54:188. doi: 10.1097/FJC.0b013e3181b72c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 117.Hogg N, Broniowska KA. The Chemical Biology of S-Nitrosothiols. Antioxid. Redox Signal. 2012 doi: 10.1089/ars.2012.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doulias PT, Greene JL, Greco TM, Tenopoulou M, Seeholzer SH, Dunbrack RL, Ischiropoulos H. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16958. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6297. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential Roles of S-Nitrosothiols in Vascular Homeostasis and Endotoxic Shock. Cell. 2004;116:617. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 121.Coles SJ, Easton P, Sharrod H, Hutson SM, Hancock J, Patel VB, Conway ME. S-Nitrosoglutathione inactivation of the mitochondrial and cytosolic BCAT proteins: S-nitrosation and S-thiolation. Biochemistry. 2009;48:645. doi: 10.1021/bi801805h. [DOI] [PubMed] [Google Scholar]
- 122.Konorev EA, Kalyanaraman B, Hogg N. Modification of creatine kinase by S-nitrosothiols : S-nitrosation vs. S-thiolation. Free Radic. Biol. Med. 2000;28:1671. doi: 10.1016/s0891-5849(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 123.Tao L, English AM. Protein S-Glutathiolation Triggered by Decomposed S-Nitrosoglutathione. Biochemistry. 2004;43:4028. doi: 10.1021/bi035924o. [DOI] [PubMed] [Google Scholar]
- 124.Li J, Huang FL, Huang KP. Glutathiolation of proteins by glutathione disulfide S-oxide derived from S-nitrosoglutathione. Modifications of rat brain neurogranin/RC3 and neuromodulin/GAP-43. J. Biol. Chem. 2001;276:3098. doi: 10.1074/jbc.M008260200. [DOI] [PubMed] [Google Scholar]
- 125.Padgett CM, Whorton AR. Cellular responses to nitric oxide - role of protein S-thiolation/dethiolation. Arch. Biochem. Biophys. 1998;358:232. doi: 10.1006/abbi.1998.0859. [DOI] [PubMed] [Google Scholar]
- 126.Riego JA, Broniowska KA, Kettenhofen NJ, Hogg N. Activation and Inhibition of Soluble Gunylyl Cyclase by S-Nitrosocysteine:Involvement of Amino Acid Transport System L. 2009:269–274. doi: 10.1016/j.freeradbiomed.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rassaf T, Poll LW, Brouzos P, Lauer T, Totzeck M, Kleinbongard P, Gharini P, Andersen K, Schulz R, Heusch G, Modder U, Kelm M. Positive effects of nitric oxide on left ventricular function in humans. Eur. Heart J. 2006;27:1699. doi: 10.1093/eurheartj/ehl096. [DOI] [PubMed] [Google Scholar]
- 128.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am. J. Respir. Crit Care Med. 2002;165:922. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 129.Souto S, Palma P, Fregonesi A, Palma T, Reis LO. Vascular modifications of the clitoris induced by topic nitric oxide donor gel--preliminary study. J. Sex Med. 2011;8:484. doi: 10.1111/j.1743-6109.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- 130.Simoes MM, de Oliveira MG. Poly(vinyl alcohol) films for topical delivery of S-nitrosoglutathione: effect of freezing-thawing on the diffusion properties. J. Biomed. Mater. Res. B Appl. Biomater. 2010;93:416. doi: 10.1002/jbm.b.31598. [DOI] [PubMed] [Google Scholar]
- 131.Kaposzta Z, Clifton A, Molloy J, Martin JF, Markus HS. S-nitrosoglutathione reduces asymptomatic embolization after carotid angioplasty. Circulation. 2002;106:3057. doi: 10.1161/01.cir.0000041251.07332.28. [DOI] [PubMed] [Google Scholar]
- 132.Salas E, Langford EJ, Marrinan MT, Martin JF, Moncada S, de Belder AJ. S-nitrosoglutathione inhibits platelet activation and deposition in coronary artery saphenous vein grafts in vitro and in vivo. Heart. 1998;80:146. doi: 10.1136/hrt.80.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Molloy J, Martin JF, Baskerville PA, Fraser SC, Markus HS. S-nitrosoglutathione reduces the rate of embolization in humans. Circulation. 1998;98:1372. doi: 10.1161/01.cir.98.14.1372. [DOI] [PubMed] [Google Scholar]
- 134.Langford EJ, Brown AS, Wainwright RJ, de Belder AJ, Thomas MR, Smith RE, Radomski MW, Martin JF, Moncada S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344:1458. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 135.Kaposzta Z, Baskerville PA, Madge D, Fraser S, Martin JF, Markus HS. L-arginine and S-nitrosoglutathione reduce embolization in humans. Circulation. 2001;103:2371. doi: 10.1161/01.cir.103.19.2371. [DOI] [PubMed] [Google Scholar]
- 136.Salas E, Langford EJ, Marrinan MT, Martin JF, Moncada S, Debelder AJ. S-nitrosoglutathione inhibits platelet activation and deposition in coronary artery saphenous vein grafts in vitro and in vivo. Heart. 1998;80:146. doi: 10.1136/hrt.80.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rassaf T, Kleinbongard P, Preik M, Dejam A, Gharini P, Lauer T, Erckenbrecht J, Duschin A, Schulz R, Heusch G, Feelisch M, Kelm M. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: experimental and clinical Study on the fate of NO in human blood. Circ. Res. 2002;91:470. doi: 10.1161/01.res.0000035038.41739.cb. [DOI] [PubMed] [Google Scholar]
- 138.Lees C, Langford E, Brown AS, de Belder A, Pickles A, Martin JF, Campbell S. The effects of S-nitrosoglutathione on platelet activation, hypertension, and uterine and fetal Doppler in severe preeclampsia. Obstetrics & Gynecology. 1996;88:14. doi: 10.1016/0029-7844(96)00070-1. [DOI] [PubMed] [Google Scholar]
- 139.Finnen MJ, Hennessy A, McLean S, Bisset Y, Mitchell R, Megson IL, Weller R. Topical application of acidified nitrite to the nail renders it antifungal and causes nitrosation of cysteine groups in the nail plate. Br. J. Dermatol. 2007;157:494. doi: 10.1111/j.1365-2133.2007.08063.x. [DOI] [PubMed] [Google Scholar]
- 140.Pluta RM, Oldfield EH, Bakhtian KD, Fathi AR, Smith RK, Devroom HL, Nahavandi M, Woo S, Figg WD, Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS. One. 2011;6:e14504. doi: 10.1371/journal.pone.0014504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37:395. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 142.Carini M, Aldini G, Orioli M, Piccoli A, Tocchetti P, Facino RM. Chemiluminescence and LC-MS/MS analyses for the study of nitric oxide release and distribution following oral administration of nitroaspirin (NCX 4016) in healthy volunteers. J. Pharm. Biomed. Anal. 2004;35:277. doi: 10.1016/S0731-7085(03)00531-4. [DOI] [PubMed] [Google Scholar]
- 143.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008;88:1018. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 144.Calabrese V, Scapagnini G, Ravagna A, Bella R, Foresti R, Bates TE, Giuffrida Stella AM, Pennisi G. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J. Neurosci. Res. 2002;70:580. doi: 10.1002/jnr.10408. [DOI] [PubMed] [Google Scholar]
- 145.Kharitonov SA, Donnelly LE, Montuschi P, Corradi M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax. 2002;57:889. doi: 10.1136/thorax.57.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]