Summary
Three of 16 patients with vertebral artery (VA) aneurysms treated by parent artery occlusion suffered ischemic complications.
The cause of the ischemic complications was brain stem or upper cervical spinal cord infarction due to occlusion of the anterior spinal artery (ASA), posterior spinal artery (PSA) and perforating arteries arising from the VA. Angiographic detection of ASA and PSA was studied in 71 consecutive patients (142 VAs) with various diseases who underwent digital subtraction angiography. The ASA and PSA originated from the bilateral VAs in 14% and 9%, unilateral VA in 73% and 35%, and were not detected in 13% and 56%, respectively.
These results indicate that the rate of angiographic detection of the ASA originating from the bilateral VAs is considerably lower than that of previously reported anatomical studies. Special attention must be paid to the ASA, PSA and perforating arteries on preoperative vertebral angiography to prevent ischemic complications associated with therapeutic parent artery occlusion for VA aneurysms.
Key words: anterior spinal artery, posterior spinal artery, therapeutic parent artery occlusion, vertebral artery aneurysm
Introduction
Parent artery occlusion using intravascular techniques is a common treatment of vertebral artery (VA) aneurysms that are difficult to treat by neck clipping or intra-aneurysmal coil embolisation. Care is usually taken to preserve the posterior inferior cerebellar artery (PICA), because occlusion of PICA can lead to infarction of the cerebellum or medulla oblongata. However, ischemic complications sometimes occur due to occlusion of the anterior spinal artery (ASA), posterior spinal artery (PSA) and perforating arteries arising from the VA. Preservation of these small arteries usually receives little attention. Anatomical studies have shown that the ASA originates from the bilateral VAs in 73-84% of all specimens1-3. However, the rate of angiographic detection of ASA is unknown.
We experienced three patients with ischemic complications due to the occlusion of such small arteries among 16 patients treated by parent artery occlusion for VA aneurysm. The present study retrospectively reviews and compares the pre- and postoperative angiographic features of these patients. The rate of angiographic detection of the ASA and PSA in a series of 71 consecutive patients (142 VAs) was also studied. Based on our results, we discuss how to prevent ischemic complications associated with therapeutic parent artery occlusion.
Clinical Materials and Methods
Sixteen patients with VA aneurysms, 11 men and five women aged from 32 to 76 years (mean 54.2 years), 11 with dissecting aneurysm, three with fusiform aneurysm and two with saccular aneurysm, were treated by parent artery occlusion using endovascular procedures. The VA aneurysms were classified according to the relationship between the aneurysm and the origin of the PICA into four types: “proximal to PICA,” “distal to PICA,” “PICA involved” and “no PICA” (table 1).
Table 1.
Case presentations
| Case | Age (year) / Sex | Initial symptoms | Type of aneurysm | Classification of aneurysm |
|---|---|---|---|---|
| 1 | 47 / F | incidental | saccular | no PICA |
| 2 | 62 / F | headache | dissecting | proximal to PICA |
| 3 | 60 / M | incidental | saccular | distal to PICA |
| 4 | 32 / F | SAH | dissecting | PICA involved |
| 5 | 50 / M | infarction | dissecting | no PICA |
| 6 | 65 / M | SAH | dissecting | proximal to PICA |
| 7 | 54 / F | SAH | dissecting | proximal to PICA |
| 8 | 42 / M | infarction | dissecting | proximal to PICA |
| 9 | 76 / M | incidental | fusiform | distal to PICA |
| 10 | 55 / M | SAH | dissecting | proximal to PICA |
| 11 | 61 / M | mass effect | fusiform (partially thrombosed) |
distal to PICA |
| 12 | 42 / M | SAH | dissecting | distal to PICA |
| 13 | 68 / M | incidental | fusiform | distal to PICA |
| 14 | 54/ M | infarction | dissecting | no PICA |
| 15 | 47 / M | SAH | dissecting | no PICA |
| 16 | 53 / M | SAH | dissecting | no PICA |
| SAH = subarachnoid haemorrhage; PICA = posterior inferior cerebellar artery | ||||
The sites of parent artery occlusion were proximal to the aneurysm in the proximal to PICA type (five patients) and no PICA type (five patients), and between the PICA and the aneurysm in the distal to PICA type (five patients). Occlusion was first performed proximal to the aneurysm in the PICA involved type (one patient). However, follow-up angiography showed enlargement of the aneurysm. Therefore, second occlusion was performed distal to the aneurysm via the contralateral VA. The ipsilateral PICA was supplied by an anastomosis with the contralateral PICA.
In the early cases of the series, the parent artery was occluded using only balloons (Gold valve balloon: Ingenor, Paris, France). In the recent cases of the series, the parent artery with or without the aneurysm was occluded using detachable coils (Interlocking detachable coil: IDC, Guglielmi detachable coil: GDC, and fibered platinum coil: VORTX, Boston Scientific, Natick, MA, USA). The pre- and postoperative digital subtraction angiography (DSA) images of the ASA, PSA and perforating arteries arising from the VA were reviewed in all 16 patients treated by parent artery occlusion. Theangiographic features of the three patients with ischemic complications were compared with those of 13 patients without ischemic complications.
In a separate study, the rates of angiographic detection of the ASA and PSA were assessed in 71 consecutive patients (142 VAs) with various diseases who underwent DSA. DSA images in right and left anterior oblique views were taken in many cases in addition to routine anteroposterior and lateral views. We reviewed the DSA images using both the developed films and the serial images on a television monitor, because the spinal arteries may be detected on only a few images of serial images in some cases.
Results
The outcomes of the treatments and the angiographic findings of the ASA and PSA in patients treated by parent artery occlusion are shown in table2. Complete obliteration of the aneurysms was confirmed by follow-up angiography performed within one week in all patients. The VA stumps were also thrombosed except in one patient in whom a relatively large artery originated from the VA stump (case 9).
Table 2.
Results of treatment and the angiographic findings of the ASA and PSA in the patients treated by parent artery occlusion
| distal to PICA type aneurysms | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Site of | Embolic | Obliteration | Thrombosis | Ischemic | ASA | PSA | |||
| Case | embolisation | materials | of aneurysm | of VA stump | complication | ipsilateral | contralateral | ipsilateral | contralateral |
| 3 | proximal to AN | balloon | ( + ) | ( + ) | ( − ) | ( + ) | ( − ) | ( − ) | ( + ) |
| 9 | intra & proximal to AN | coil | ( + ) | ( − ) | ( − ) | ( − ) | ( + ) | ( − ) | ( − ) |
| 11 | intra & proximal to AN | coil | ( + ) | ( + ) | ( + ) | ( + ) | ( − ) | ( − ) | ( − ) |
| 12 | proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( − ) | ( + ) | ( − ) | ( + ) |
| 13 | intra & proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( − ) | ( + ) | ( + ) | ( − ) |
| proximal to PICA type aneurysms | |||||||||
| 2 | proximal to AN | balloon | ( + ) | ( + ) | ( − ) | ( + ) | ( − ) | ( − ) | ( − ) |
| 6 | proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( + ) | ( − ) | ( − ) | ( − ) |
| 7 | proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( + ) | ( − ) | ( − ) | ( + ) |
| 8 | proximal to AN | coil | ( + ) | ( + ) | ( + ) | ( + ) | ( + ) | ( + ) | ( + ) |
| 10 | proximal to AN | coil | ( + ) | ( + ) | ( + ) | ( − ) | ( + ) | ( − ) | ( + ) |
| no PICA type aneurysms | |||||||||
| 1 | proximal to AN | balloon | ( + ) | ( + ) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) |
| 5 | proximal to AN | balloon | ( + ) | ( + ) | ( − ) | ( − ) | ( + ) | ( − ) | ( − ) |
| 14 | intra & proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( + ) | ( − ) | ( − ) | ( − ) |
| 15 | intra & proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( − ) | ( + ) | ( − ) | ( + ) |
| 16 | intra & proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( − ) | ( + ) | ( − ) | ( − ) |
| PICA involved type aneurysms | |||||||||
| 4 | proximal to AN | balloon | ( − ) | ( + ) | ( − ) | ( − ) | ( + ) | ( − ) | ( + ) |
| intra & proximal to AN | coil | ( + ) | ( + ) | ( − ) | ( − ) | ( + ) | ( − ) | ( + ) | |
| PICA = posterior inferior cerebellar artery; AN = aneurysm; VA = vertebral artery; ASA = anterior spinal artery; PSA = posterior spinal artery | |||||||||
One of five patients with distal to PICA type aneurysms suffered ischemic complications (case 11). This patient with a partially thrombosed VA aneurysm was treated by proximal parent artery occlusion including the aneurysm using GDCs. Seven hours after the procedure, left hemiparesis and right lower limb paresis appeared, although intra- and postoperative systemic heparinization was induced. Emergent left vertebral angiography revealed thrombosis of the VA stump and the ASA which arose only from the ipsilateral VA. Magnetic resonance (MR) imaging showed infarction in the lateral part of the medulla oblongata and anterior part of upper cervical spinal cord (figure 1). Despite medical treatment, the respiratory disorder progressed and he died seven days later. Another patient with the ASA originating only from the affected VA did not experience ischemic complications because the ASA was supplied through an anastomosis with the radiculomedullary artery (case 3). The other three patients without ischemic complication had ASAs arising only from the contralateral VA.
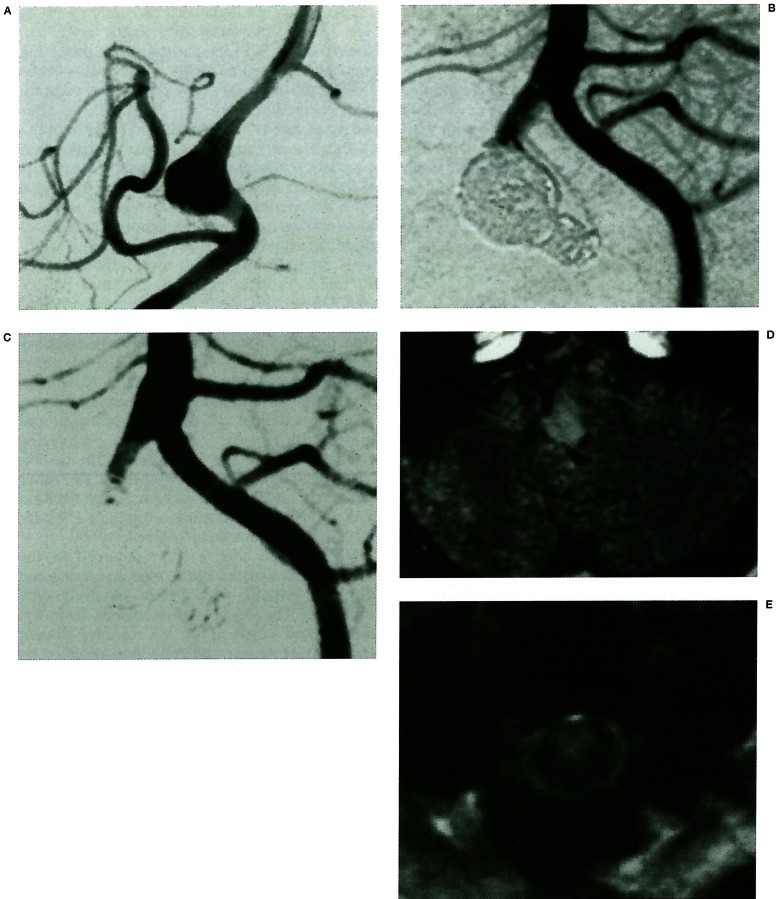
Figure 1.
(Case 11) A 61-year-old man with a “distal to posterior inferior cerebellar artery (PICA)” type fusiform aneurysm presenting with mass effect. A) Right vertebral angiogram showing a fusiform aneurysm located distal to the PICA. B) Left vertebral angiogram after embolisation of both the parent artery and the aneurysm showing the anterior spinal artery (ASA) originating from the vertebral artery (VA) stump. C) Seven hours after embolisation, left hemiparesis and right lower limb paresis appeared. Left vertebral angiogram demonstrating thrombosis of the VA stump and the ASA. D, E) Magnetic resonance (MR) image showing infarction in the lateral part of medulla oblongata and the anterior part of CI level of spinal cord.
Two of five patients with proximal to PICA type aneurysms suffered ischemic complications (cases 8,10). One patient with a dissecting aneurysm presented with slight right heminumbness and facial nerve paresis due to brain stem infarction (case 8). Immediately after proximal parent artery occlusion, right hemihypesthesia and hemiataxia occurred. MR imaging showed infarction in the posterolateral part of the lower medulla and CI level of the spinal cord. Preoperative DSA had shown fine arteries supposed to be the PSA arising from the occluded segment of the VA (figure 2). The symptoms gradually improved after rehabilitation, but mild disability persisted. Another patient with a dissecting aneurysm presented with subarachnoid haemorrhage (case 10). Immediately after proximal parent artery occlusion, right hemi-numbness, hypesthesia, hypoglossal nerve paresis and truncal ataxia emerged. MR imaging showed infarction in the posterolateral part of the junction of the medulla oblongata and spinal cord. However, neither the perforating arteries nor the PSA was visible in the thrombosed segment of the VA (figure 3). The other three patients without ischemic complication had no visible perforating arteries or PSA located in the thrombosed segment of VA.
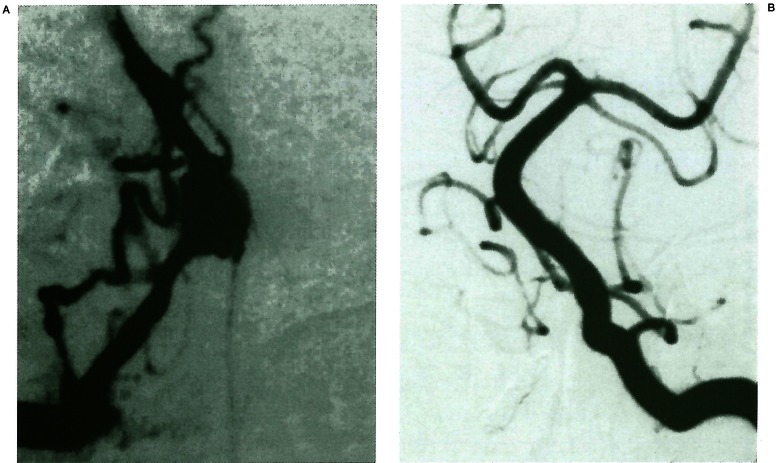
Figure 2.
(Case 8) A 42-year-old man with a “proximal to PICA” type dissecting aneurysm presenting with brain stem infarction. A) Right vertebral angiogram showing a “proximal to PICA” type dissecting aneurysm. Note the small arteries (arrows), supposed to be the posterior spinal artery (PSA) or perforating arteries, arising from the proximal VA. Arrowhead indicates the ipsilateral PICA. B) Left vertebral angiogram after parent artery occlusion showing preservation of the PICA and disappearance of the small arteries. Immediately after the procedure, right hemihypesthesia and hemiataxia occurred. C, D) MR images on admission showing a small infarction in the right posterolateral part of the medulla oblongata, e, f) MR images performed one day after the embolisation showing enlargement of the lesion of the medulla and a new infarction at the CI level of the spinal cord (white arrows).
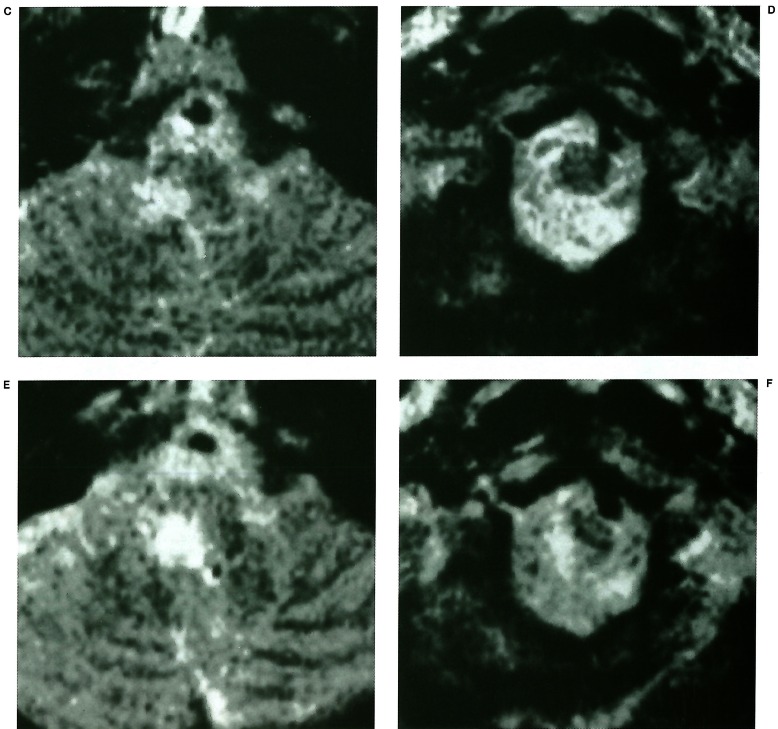
Figure 3.
(Case 10) A 55-year-old man with a “proximal to PICA” type dissecting aneurysm presenting with subarachnoid haemorrhage. A) Right vertebral angiogram showing a “proximal to PICA” type dissecting aneurysm. Note that neither the perforating arteries nor PSA are shown in the proximal VA. B) Left vertebral angiogram after embolisation showing the right PICA supplied via the contralateral VA. After the procedure, right hemi-numbness, hypesthesia, truncal ataxia and right hypoglossal nerve paresis emerged. C) MR image on the next day showing an infarction in the posterolateral part of the junction of the medulla oblongata and spinal cord.
No ischemic complication occurred in the five patients with no PICA type aneurysm and the one patient with PICA involved type aneurysm. The ASA originated only from the thrombosed segment of VA in one patient (case 14). However, the ASA was supplied by an anastomosis with the radiculomedullary arteries after embolisation. Neither the ASA nor the PSA was visible in the thrombosed segment of the VA in the other five patients.
Angiography detected the ASA and PSA originating from the bilateral VAs in 14% and 9% of the 71 cases, the unilateral VA in 73% and 35% of cases, and did not detect these vessels in 13% and 56% of cases, respectively.
Discussion
Angiographic and anatomic characteristics of the AS A, PSA and perforating arteries
The ASA is formed by the union of paired anterior ventral spinal arteries originating from the VA near the origin of basilar artery and forms anastomoses with the anterior branches of the radicular arteries 2,4,5. According to anatomical studies, the ASA originates bilaterally in 73-84% of all specimens1-3. However, our angiographic study found bilateral origins of the ASA in only 14% of cases. The main reason for this discrepancy is that the caliber of the anterior ventral spinal artery varies greatly. If the anterior ventral spinal artery is hypoplastic on one side, the blood flow is much less than on the other side. Such a hypoplastic artery would not be detected by DSA.
Angiographic visualization of the PSA was difficult in the past5, but this is now possible through advances in the resolution of DSA. There are no data concerning the rate of angiographic detection of PSA. We visualized the PSA in over 40% of cases. This rate is higher than expected. Based on anatomical studies, the PSA arises from the intracranial or extracranial portion of the VA or from the PICA and often anastomoses with branches of the PICA or posterior branches of the radicular arteries 2,4.
Detection of the perforating arteries arising from the VA was difficult even by high resolution DSA. Anatomical studies indicate that the perforating arteries arising from the VA supply the anterior and lateral parts of the medulla oblongata 6. These arteries have rich anastomoses with the perforating arteries from the VA, basilar artery, PICA, and anterior inferior cerebellar artery3.
Prevention of ischemic complications associated with therapeutic VA occlusion
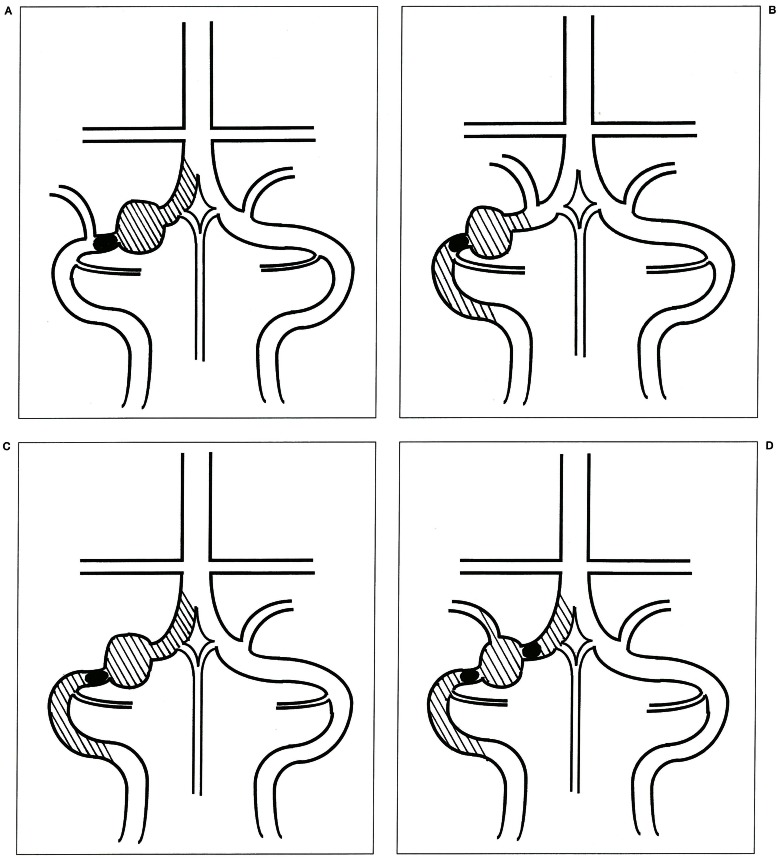
Parent artery occlusion for VA aneurysms will result in thrombosis of the stagnant VA stump unless large arteries are present in this segment. Therefore, we must pay careful attention to the small arteries originating from the VA stump as well as from the occluded segment of the VA. These small arteries are the ASA in cases of the “distal to PICA” type (figure 4A), the PSA in cases of the “proximal to PICA” type (figure 4B), and both the ASA and PSA in cases of the “no PICA” (figure 4C) or “PICA involved” type VA aneurysms (figure 4D).
Figure 4.
Schematic drawings of the occluded segment (area colored black) and the VA stump supposed to be thrombosed (area of oblique lines) in four types of VA aneurysms. A) “distal to PICA” type. B) “proximal to PICA” type. C) “no PICA” type. D) “PICA involved” type.
The ASA, PSA and perforating arteries form various anastomoses, and these are the anatomical basis for the safety of therapeutic parent artery occlusion. In our study, two (cases 3,14) of the three patients (cases 3,11,14) in whom the ASA originated only from the affected side of the VA did not experience ischemic complications because of the collateral flow from the radiculomedullary arteries. However, parent artery occlusion can cause brain stem or cervical spinal cord infarction if the collateral flow is insufficient. The ascending branch of the PSA supplies the restiform body and the gracile and cuneate tubercles, and the descending branch supplies the posterior part of the cervical spinal cord2. The symptoms of occlusion of the PSA are mainly deep sensory disturbance and truncal ataxia. The ASA supplies the pyramids, the medial lemniscus, the interolivery bundle, the posterior longitudinal fasciculus and the anterolateral part of the cervical spinal cord2. ASA occlusion can cause catastrophic symptoms such as tetraparesis or respiratory disturbance as in our case ll. Therefore, if the ASA originates only from the side of the affected VA, we must be cautious in determining the indications for parent artery occlusion, even though prevention of re-rupture has the first priority in the treatment of ruptured aneurysms.
Two mechanisms of occlusion of the arteries arising from the VA are proposed: direct obliteration of the orifice of the arteries by embolic materials, and gradual occlusion caused by thrombosis of the stagnant VA stump. Direct obliteration can be prevented by embolisationof a shorter segment of the VA. We embolize the parent artery including the aneurysm, and use fibered coils, which have a high thrombogenicity, together with GDCs or IDCs. Gradual occlusion may be prevented by intra- and postoperative systemic heparinization.
The occurrence of ischemic complication associated with parent artery occlusion is difficult to predict. The balloon occlusion test can predict haemodynamic ischemia, but cannot predict ischemia due to occlusion of the ASA, PSA and perforating arteries due to late thrombosis of the stump of the parent artery. Therefore, we believe that careful observation of preoperative angiography is most important.
Conclusions
We emphasize the need for great care in detecting the ASA and the PSA on preoperative vertebral angiography to prevent ischemic complications associated with therapeutic VA occlusion.
References
- 1.Govsa F, Aktan ZA, et al. Origin of the anterior spinal artery. Surg Radiol Anat. 1996;18:189–193. doi: 10.1007/BF02346126. [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira E, Rhoton AL, Jr, et al. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985;24:293–352. doi: 10.1016/0090-3019(85)90042-4. [DOI] [PubMed] [Google Scholar]
- 3.Akar ZC, Dujovny M, et al. Microsurgical anatomy of the intracranial part of the vertebral artery. Neurol Res. 1994;16:171–180. doi: 10.1080/01616412.1994.11740221. [DOI] [PubMed] [Google Scholar]
- 4.Krayenbühl HA, Yasargil MG. Cerebral angiography. 2nd edition. PhiladelphiaLippincott: Lippincott; 1968. pp. 141–155. [Google Scholar]
- 5.Newton TH, Potts DG. Radiology of the skull and brain. book 2. Vol 2. St Louis: CV Mosby; 1974. pp. 1659–1709. [Google Scholar]
- 6.Lister JR, Rhoton AL, Jr, et al. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170–199. [PubMed] [Google Scholar]