Summary
This report is a clinical and radiologic correlation of anterior spinal arterial distribution ischemia with a thoracic disc herniation affecting the artery of Adamkiewicz. We could only find one other similar reported case.
A 38-year-old woman developed sudden onset of severe back pain and radiculopathy, followed by rapidly evolving paraparesis. The neurological examination was consistent with a deficit caused by anterior spinal artery ischemia. MRI revealed T2 signal change in the thoracolumbar spinal cord and a laterally placed, non-calcified disc herniation. Selective spinal angiography performed 30 hours after onset revealed displacement of the left T9 radicular feeding artery by the disc herniation; at this time the artery was patent. The patient experienced some resolution of symptoms within the first 24 hours and was managed conservatively and made a significant recovery within two weeks.
Appropriately located thoracic disc herniations can disturb the blood supply to the thoracolumbar spinal cord.
Key words: thoracic disc, herniation, ischemia, artery of Adamkiewicz, anterior spinal artery syndrome
Introduction
Anterior spinal artery ischemia results in a characteristic clinical syndrome. This syndrome was more commonly seen when tertiary syphillis was prevalent but may result from vasculitis, dissection or embolisation1. This injury may also be caused by iatrogenic and traumatic insults 2,3,4,5,6. We found only one previous report of a thoracic disc herniation as a cause7. We report a case of rapidly evolving paraplegia associated with back and radicular pain apparently caused by a left-sided T 8/9 thoracic disc herniation.
Case Report
A 38-year-old Caucasian woman in good general health had intermittently experienced episodes of lower thoracic back pain. Such episodes followed strenuous physical activities, lasted 5-10 days, and resolved spontaneously. On this occasion the patient twisted suddenly while getting up from a sofa and had sudden onset of severe mid/low thoracic back pain with a band-like component to the left side. Over the next 3-5 minutes she developed paraplegia and the pain remained intense. When assessed initially at a remotely located hospital the patient appeared to have complete motor paraplegia with some preserved sensation and a presumptive diagnosis of spinal cord “haemorrhage” was made. Her vital signs were within normal limits. There was no history of cardiac, vascular or inflammatory diseases. Because an extrinsic mass lesion could not be excluded steroids were administered and the patient was transferred to a tertiary level hospital. Given the remoteness of the patient's location in Northern Canada the transfer time was lengthy.
When assessed by a neurosurgical resident 12 hours later the vital signs were normal and she was afebrile. The neurologic examination revealed normal mental status, cranial nerves and cerebellar function. Tendon reflexes were normal in the upper extremities. In the lower extremities and over the abdomen reflexes could not be elicited, motor tone was somewhat decreased. Motor examination of the upper extremities was normal. In the legs L2-L5 innervated muscle groups were graded at 2/5 and the plantar flexors were 1/5 bilaterally.
On sensory examination there was a T4 sensory level to pinprick and temperature with intact proprioception. The patient was catheterized, sensation in the sacral segments was intact to touch with marked decrease to pin prick. The bulbocavernosus reflex was weakly intact, rectal tone was present but diminished and the patient had minimal voluntary contraction of the anal sphincter. On examination of the spine there was no palpable deformity and minimal pain to palpation. Voluntary movement of torso, however, provoked significant local pain. The patient complained of pain over the left lower thorax extending to just above the umbilicus. This examination was consistent with neurologic improvement since in the admitting hospital she was described as having no voluntary leg movement.
The provisional diagnosis was of an acutely evolving compressive, destructive or ischemic process affecting the lower thoracic spinal cord causing incomplete paraplegia (sparing posterior column function) and associated with mechanical back pain and a radicular component. The differential diagnosis was: 1) Acute thoracic disc herniation. 2) Pathologic fracture with extradural compression. 3) Haemorrhage - extradural, intradural extramedullary or intraparenchymal. 4) Spinal cord ischemia secondary to vascular compromise from: dissection, embolisation, or focal arterial inflammation or as a rare cause, embolisation of herniated disc material8. Although acute transverse myelitis can present with axial back pain, the presence of radicular pain made this diagnosis unlikely.
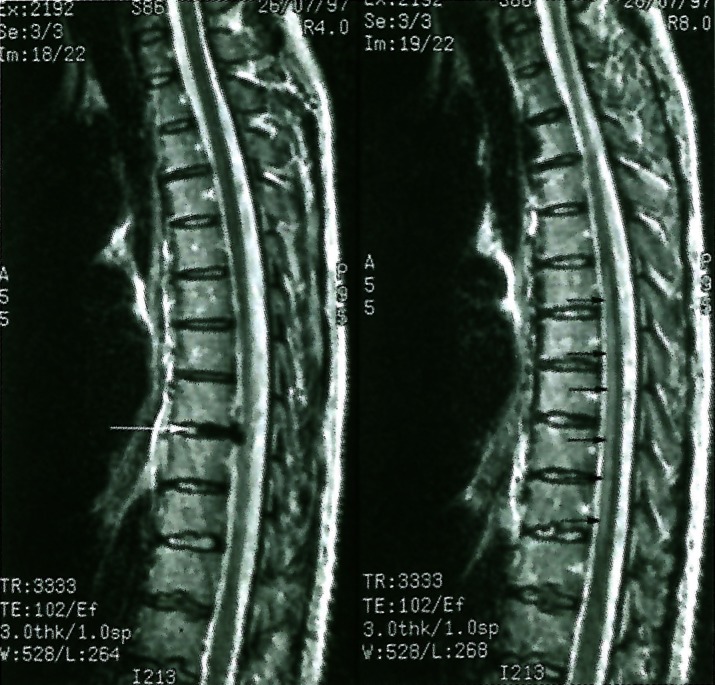
The patient's haemoglobin was normal, the white cell count was slightly elevated, however the patient had received steroids. Accompanying plain films of the thoracolumbar spine did not show an abnormality. An MRI was obtained and T2 sagittal image of the spine revealed a moderate disc herniation at the T8/9 level (figure 1 left) which is left-sided on axial images (figure 2). Surprisingly, given the patient's paraparesis, no cord compression is seen, but the exiting T9 nerve root cannot be defined. Also evident on these images is a region of T2 signal increase within the cord extending from the T5/6 to T10/11 disc level (figure 1 right). The signal change is located within the anterior portion of the spinal cord.
Figure 1.
Left: Off center sagittal T2 image shows left posterior-lateral disc herniation at the T8/T9 level (white arrow). Right: Midline sagittal T2 image shows mildly increased signal in the anterior aspect of the spinal cord from the T5/T6 to T10/T11 disc levels (small black arrows).
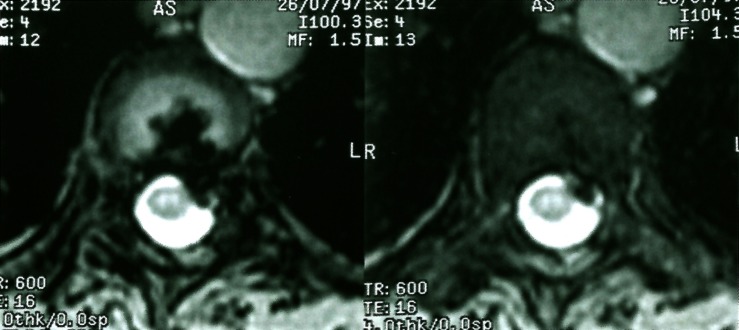
Figure 2.
Transverse images at the T8/T9 disc level show a moderate sized disc herniation extending into the left lateral recess.
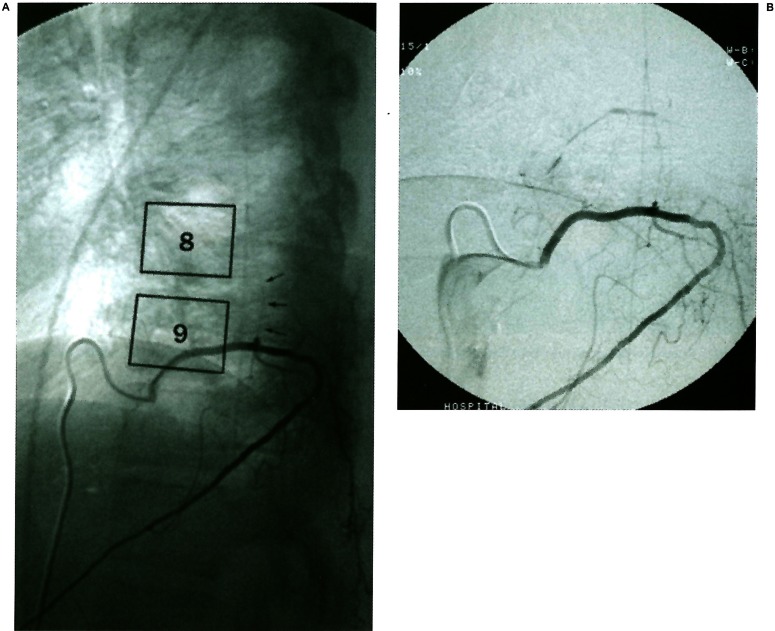
These findings on MRI implicated two diagnoses; acute thoracic disc herniation and a vascular anterior spinal cord syndrome. To further explore for evidence of vascular compromise spinal angiography was obtained. Figure 3 shows lateral views of a contrast injection into the left T9 radicular feeding vessel. The intradural portion of the radicular vessel providing supply to the anterior spinal artery is acutely angulated by the disc protrusion. At this time flow is present throughout the artery.
Figure 3.
A) Unsubtracted lateral view from spinal angiogram during injection of left T9 vascular pedicle showing outlined T8 and T9 vertebra and the posteriorly displaced radiculo-medullary input at the level of the disc (arrows). B) Slightly magnified subtracted view of the same image.
Our interpretation of this clinical and radiographic presentation was an acute herniation of the T8/9 thoracic disc causing severe back pain and also radiculopathy. We hypothesized that the disc rupture had acutely compressed and/or stretched the T9 radicular vessel leading to temporary spasm or occlusion. The decreased flow had lead to ischemia of the anterior spinal cord.
The herniated disc was thus presumed responsible for both the pain and neurologic deficit which was improving at the time she was admitted to us. We were concerned that any additional manipulation of the radicular vessel could lead to recurrent spasm and possibly occlusion resulting in increased neurologic deficit. Given the left lateral foraminal position of the disc we favored a transpedicular or trans-facet pedicle-sparing approach. These approaches could lead to manipulation of the radicular vessel within the root sleeve. Therefore we elected to delay surgical intervention, kept the patient on bed rest, and treated her with analgesics and aspirin. The patient's neurologic status was monitored frequently over the first three in-hospital days. Her deficits continued to resolve and she became ambulatory at ten days post-injury.
Discussion
Although the potential for herniated thoracic discs to compromise spinal cord blood flow has been suggested in several publications, we could find only one other case report of this occurrence in the literature. Mansour et Al 19877 reported the case of a women who presented with acute onset of a Brown-Sequard syndrome associated with severe thoracic back and radicular pain. CT myelography demonstrated a calcified migrated disc fragment within the left T9/10 foramen. This patient underwent transpedicular removal of the fragment; the authors described microscopic visualization of compression of the radicular vessel within the foramen.
The clinical presentation of our patient was consistent with ischemia in the anterior spinal artery distribution1. Importantly, the sensory level was clearly higher than the level of the herniated disc indicating that the deficit was not caused solely by direct cord compression due to the herniated thoracic disc. Furthermore, in cases of paraplegia secondary to acutely herniated thoracic discs, the herniation is usually central instead of lateral, as in our case9. The radicular artery involved was confirmed to be the arteria radicularis magna (artery of Adamkiewicz) on spinal angiography. The T2 signal change observed in the anterior spinal cord extended within the vascular territory of the anterior spinal artery normally fed by the artery of Adamkiewicz; and was consistent with reports of the MRI appearance of anterior spinal vascular compromise 10,2. The patient's deficit was maximal soon after onset and improved rapidly, suggesting that the interruption of flow within the vessel was either transient or incomplete. We propose three possible mechanisms for this sequence of events: 1) abrupt arterial stretch led to focal spasm with partial or complete occlusion, 2) the insult to the vessel lead to thrombosis which resolved, or 3) decreased flow in the vessel caused frank ischemia which was ameliorated by collateral flow.
In some respects it is surprising that this clinical syndrome is not observed more commonly. Thoracic disc herniation most commonly occurs between T9 and T12 11,12. The artery of Adamkiewicz enters the dural root sleeve just distal to the costovertebral joint3,13 within the foramen and then ascends 1-3 levels before joining the anterior spinal artery. It most commonly arises between T9-Ll from the left side. This artery is frequently the lowest radicular feeder to the spinal cord4,14. If the artery is occluded proximal to the bifurcation of the intercostal artery retrograde filling can occur. This is the basis of the low incidence of neurologic deficit following intercostal artery ligation at the aorta. The intercostal artery gives origin to the medial trunk, which is the artery of Adamkiewicz. There are a few branches proximal to this vessel's dural entry but none thereafter until it joins the anterior spinal artery. Thus if the Adamkiewicz artery is occluded after dural entry the only available collateral supply is from the small descending anterior spinal artery or from the small posterior radicular arteries which form a highly variable posterolateral arterial plexus. Whether Adamkiewicz artery occlusion can cause spinal cord infarction has been controversial since the primate studies of Fried et Al15 in which only direct occlusion of the descending anterior spinal artery (and not of the radicular artery alone) leads to obvious deficits. However, sufficient clinical reports exist to justify the belief that occlusion of this artery can lead to spinal infarction in some patients5,6,16.
Our reluctance to operate on this patient in the acute setting derived from the absence of spinal cord compression and concern that manipulation of the involved vessel might lead to a severe anterior spinal cord syndrome.
We feel justified in our conservative approach given the fact that the patient was improving and that the natural history of pain related to thoracic disc herniation is often improvement without surgery17.
Conclusions
We believe that the possibility of compromise of the Adamkiewicz artery should be considered in patients presenting with a laterally positioned lower thoracic disc herniation and anterior spinal cord syndrome.
Acknowledgements
The authors thank Dr Roberto Heros for his careful reading of the manuscript and his insightful comments.
References
- 1.Satran R. Spinal cord infarction. Stroke. 1988;19:529–532. doi: 10.1161/01.str.19.4.529. [DOI] [PubMed] [Google Scholar]
- 2.Rogers FB, Osier TM, et al. Isolated stab wound to the artery of Adamkiewicz: case report and review of the literature [see comments] J Trauma. 1997;43(3):549–551. doi: 10.1097/00005373-199709000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, Ebraheim NA, et al. Vulnerability of great medullary artery. Spine. 1996;21:1852–1855. doi: 10.1097/00007632-199608150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Dommisse GF. The blood supply of the spinal cord. A critical vascular zone in spinal surgery. J Bone Joint Surg [Br] 1974;56:225–235. [PubMed] [Google Scholar]
- 5.Boglino C, Martins AG, et al. Spinal cord vascular injuries following surgery of advanced thoracic neuroblastoma: an unusual catastrophic complication. Med Pediatr Oncol. 1999;32:349–352. doi: 10.1002/(sici)1096-911x(199905)32:5<349::aid-mpo7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Mawad ME, Rivera V, et al. Spinal cord ischemia after resection of thoracoabdominal aortic aneurysms: MR findings in 24 patients. Am J Roentgenol. 1990;155:1303–1307. doi: 10.2214/ajr.155.6.2122684. [DOI] [PubMed] [Google Scholar]
- 7.Mansour H, Hammoud F, Vlahovitch B. [Brown-Sequard syndrome caused by foramen and calcified disk herniation, responsible for direct compression of Adamkiewicz's artery] Neurochirurgie. 1987;33:478–481. [PubMed] [Google Scholar]
- 8.Moorhouse DF, Burke M, et al. Spinal cord infarction caused by cartilage embolus to the anterior spinal artery. Surg Neurol. 1992;37:448–452. doi: 10.1016/0090-3019(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Terry AF, McSweeney T, Jones HW. Paraplegia as a sequela to dorsal disc prolapse. Paraplegia. 1981;19:111–117. doi: 10.1038/sc.1981.25. [DOI] [PubMed] [Google Scholar]
- 10.Elksnis SM, Hogg JP, Cunningham ME. MR imaging of spontaneous spinal cord infarction. J Comput Assist Tomogr. 1991;15:228–232. doi: 10.1097/00004728-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Sekhar LN, Jannetta PJ. Thoracic disc herniation: operative approaches and results. Neurosurgery. 1983;12:303–305. doi: 10.1227/00006123-198303000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Stillerman CB, Chen TC, et al. Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg. 1998;88:623–633. doi: 10.3171/jns.1998.88.4.0623. [DOI] [PubMed] [Google Scholar]
- 13.Alleyne CH, Jr, Cawley CM, et al. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurg. 1998;89:791–795. doi: 10.3171/jns.1998.89.5.0791. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Baeza A, Muset-Lara A, et al. The arterial supply of the human spinal cord: a new approach to the arteria radicularis magna of Adamkiewicz [see comments] Acta Neurochir. 1991;109:57–62. doi: 10.1007/BF01405699. [DOI] [PubMed] [Google Scholar]
- 15.Fried LC, G Di Chiro, Doppman JL. Ligation of major thoraco-lumbar spinal cord arteries in monkeys. J Neurosurg. 1969;31:608–614. doi: 10.3171/jns.1969.31.6.0608. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro L, Leite I, et al. Spontaneous thoracolumbar spinal cord infarction: report of six cases. Acta Neurol Scand. 1992;86:563–566. doi: 10.1111/j.1600-0404.1992.tb05487.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown CW, et al. The natural history of thoracic disc herniation. Spine. 1992;17:S97–102. doi: 10.1097/00007632-199206001-00006. [DOI] [PubMed] [Google Scholar]