Abstract
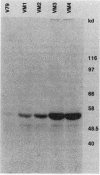
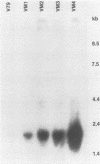
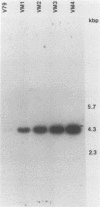
The regulation of IMP dehydrogenase (IMPDH) was analyzed in Chinese hamster V79 cell variants that exhibit different degrees of resistance to the cytotoxic effect of mycophenolic acid, a specific inhibitor of IMPDH. Western blot (immunoblot) analysis with an IMPDH antiserum revealed a 14- to 27-fold increase in the amount of enzyme in the mycophenolic acid-resistant cells. The antiserum was also used to screen for a phage containing the IMPDH cDNA sequence from a lambda gt11 expression library. Northern blot (RNA blot) analyses of total cellular and poly(A)+ RNA showed that an IMPDH cDNA probe hybridized to a 2.2-kilobase transcript, the amount of which was associated with increased resistance. Southern blotting with the probe indicated an amplification of the IMPDH gene in the mycophenolic acid-resistant cells. Our findings suggest that the acquired mycophenolic acid resistance of the V79 cell variants is associated with increases in the amount and activity of IMPDH and the number of IMPDH gene copies.
Full text
PDF
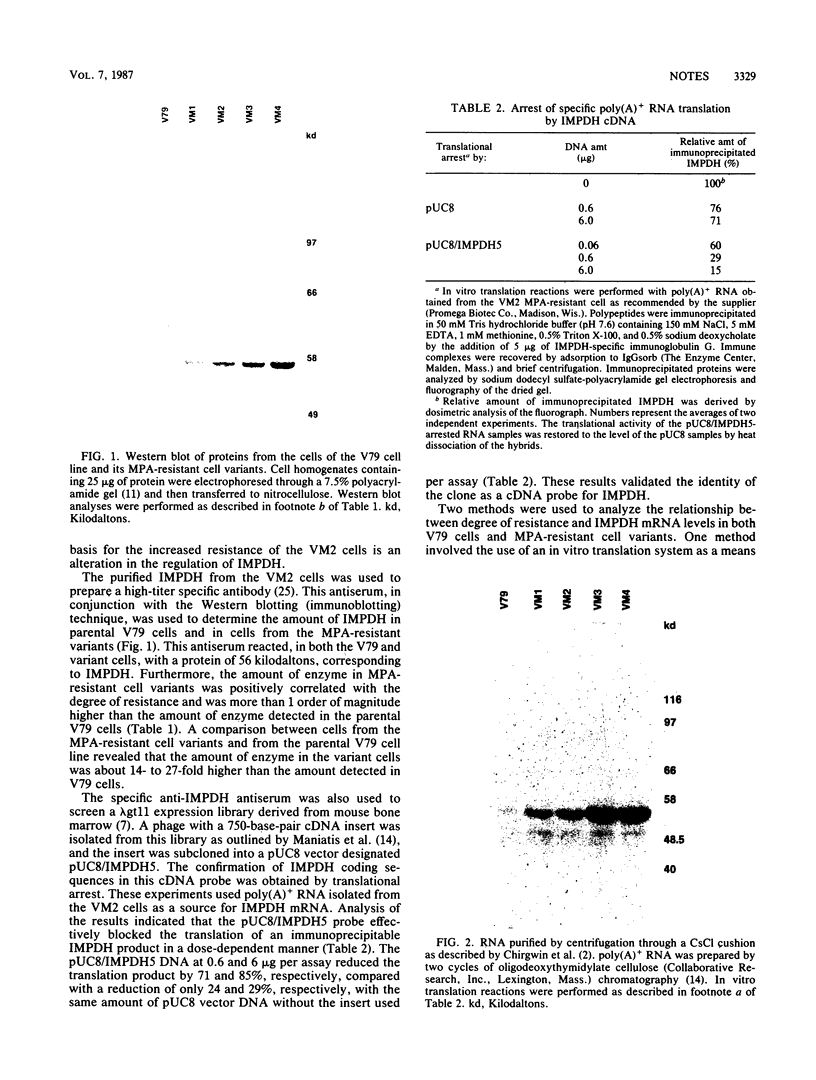


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969 Jul;113(3):515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. W., Pehlke D. M., Kelley W. N. Human IMP dehydrogenase. Kinetics and regulatory properties. Biochim Biophys Acta. 1974 Oct 17;364(2):209–217. doi: 10.1016/0005-2744(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Huberman E., McKeown C. K., Friedman J. Mutagen-induced resistance to mycophenolic acid in hamster cells can be associated with increased inosine 5'-phosphate dehydrogenase activity. Proc Natl Acad Sci U S A. 1981 May;78(5):3151–3154. doi: 10.1073/pnas.78.5.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanalas J. J., Suttle D. P. Amplification of the UMP synthase gene and enzyme overproduction in pyrazofurin-resistant rat hepatoma cells. Molecular cloning of a cDNA for UMP synthase. J Biol Chem. 1984 Feb 10;259(3):1848–1853. [PubMed] [Google Scholar]
- Kittler J. M., Meisler N. T., Viceps-Madore D., Cidlowski J. A., Thanassi J. W. A general immunochemical method for detecting proteins on blots. Anal Biochem. 1984 Feb;137(1):210–216. doi: 10.1016/0003-2697(84)90372-5. [DOI] [PubMed] [Google Scholar]
- Kuttan R., Robins R. K., Saunders P. P. Inhibition of inosinate dehydrogenase by metabolites of 2-beta-D-ribofuranosyl thiazole-4-carboxamide. Biochem Biophys Res Commun. 1982 Aug;107(3):862–868. doi: 10.1016/0006-291x(82)90602-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucas D. L., Webster H. K., Wright D. G. Purine metabolism in myeloid precursor cells during maturation. Studies with the HL-60 cell line. J Clin Invest. 1983 Dec;72(6):1889–1900. doi: 10.1172/JCI111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui M. S., Faderan M. A., Liepnieks J. J., Natsumeda Y., Olah E., Jayaram H. N., Weber G. Modulation of IMP dehydrogenase activity and guanylate metabolism by tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide). J Biol Chem. 1984 Apr 25;259(8):5078–5082. [PubMed] [Google Scholar]
- McConlogue L., Dana S. L., Coffino P. Multiple mechanisms are responsible for altered expression of ornithine decarboxylase in overproducing variant cells. Mol Cell Biol. 1986 Aug;6(8):2865–2871. doi: 10.1128/mcb.6.8.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Okada M., Shimura K., Shiraki H., Nakagawa H. IMP dehydrogenase. II. Purification and properties of the enzyme from Yoshida sarcoma ascites tumor cells. J Biochem. 1983 Nov;94(5):1605–1613. [PubMed] [Google Scholar]
- Proffitt R. T., Pathak V. K., Villacorte D. G., Presant C. A. Sensitive radiochemical assay for inosine 5'-monophosphate dehydrogenase and determination of activity in murine tumor and tissue extracts. Cancer Res. 1983 Apr;43(4):1620–1623. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloski J. A., Blair O. C., Sartorelli A. C. Alterations in glycoprotein synthesis and guanosine triphosphate levels associated with the differentiation of HL-60 leukemia cells produced by inhibitors of inosine 5'-phosphate dehydrogenase. Cancer Res. 1986 May;46(5):2314–2319. [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Sweeney M. J., Gerzon K., Harris P. N., Holmes R. E., Poore G. A., Williams R. H. Experimental antitumor activity and preclinical toxicology of mycophenolic acid. Cancer Res. 1972 Sep;32(9):1795–1802. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman B. Characterization of mutant murine lymphoma cells with altered inosinate dehydrogenase activities. J Biol Chem. 1983 Jan 10;258(1):523–528. [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Weber G., Olah E., Lui M. S., Kizaki H., Tzeng D. Y., Takeda E. Biochemical commitment to replication in cancer cells. Adv Enzyme Regul. 1980;18:3–26. doi: 10.1016/0065-2571(80)90005-9. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Butow R. A., Hannon Y. P. Rapid electroelution of nucleic acids from agarose and acrylamide gels. Anal Biochem. 1982 Sep 1;125(1):125–130. doi: 10.1016/0003-2697(82)90392-x. [DOI] [PubMed] [Google Scholar]