Abstract
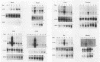
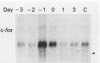
Expression of the c-fos gene during murine perinatal development was studied. Before birth, all eight of the prenatal organs tested expressed undetectable or low levels of c-fos mRNA. On the day of birth, there occurred a 10- to 100-fold increase in the level of c-fos message in all of these organs. The expression was transient, in that 1 day after birth, the level of c-fos mRNA precipitously dropped. The c-fos gene expression at birth is unrelated to the expression of the c-myc gene and major histocompatibility complex class I genes, which display distinct kinetics during the perinatal development. The c-fos gene was also expressed locally and transiently in the gravid uterus 1 to 2 days prior to delivery. These results indicate that an event associated with birth induced c-fos gene expression in the mother and newborn.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Meek J., Edwards S. A. Product of the cellular oncogene, c-fos, observed in mouse and human tissues using an antibody to a synthetic peptide. EMBO J. 1985 Apr;4(4):941–947. doi: 10.1002/j.1460-2075.1985.tb03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka T., Gubits R. M., van der Noen H. M. Beta-adrenergic stimulation of c-fos gene expression in the mouse submandibular gland. Mol Cell Biol. 1986 Aug;6(8):2984–2989. doi: 10.1128/mcb.6.8.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Casey M. L., Winkel C. A., Porter J. C., MacDonald P. C. Endocrine regulation of the initiation and maintenance of parturition. Clin Perinatol. 1983 Oct;10(3):709–721. [PubMed] [Google Scholar]
- Cawson M. J., Anderson A. B., Turnbull A. C., Lampe L. Cortisol, cortisone, and 11-deoxycortisol levels in human umbilical and maternal plasma in relation to the onset of labour. J Obstet Gynaecol Br Commonw. 1974 Oct;81(10):737–745. doi: 10.1111/j.1471-0528.1974.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Challis J. R., Mitchell B. F. Hormonal control of preterm and term parturition. Semin Perinatol. 1981 Jul;5(3):192–202. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H., Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatr Res. 1977 Aug;11(8):889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H., Slotkin T. A. The "stress" of being born. Sci Am. 1986 Apr;254(4):100–107. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- Liggins G. C. Initiation of spontaneous labor. Clin Obstet Gynecol. 1983 Mar;26(1):47–55. doi: 10.1097/00003081-198303000-00009. [DOI] [PubMed] [Google Scholar]
- MacDonald P. C., Schultz F. M., Duenhoelter J. H., Gant N. F., Jimenez J. M., Pritchard J. A., Porter J. C., Johnston J. M. Initiation of human parturition. I. Mechanism of action of arachidonic acid. Obstet Gynecol. 1974 Nov;44(5):629–636. [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Does the human fetal adrenal play a role in parturition? Am J Obstet Gynecol. 1973 Feb 15;115(4):521–525. doi: 10.1016/0002-9378(73)90400-6. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Tremblay J. M., Adamson E. D., Verma I. M. Tissue and cell type-specific expression of two human c-onc genes. Nature. 1983 Aug 4;304(5925):454–456. doi: 10.1038/304454a0. [DOI] [PubMed] [Google Scholar]
- Olver R. E. Of labour and the lungs. Arch Dis Child. 1981 Sep;56(9):659–662. doi: 10.1136/adc.56.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer-Ohlsson S., Rydnert J., Goustin A. S., Larsson E., Betsholtz C., Ohlsson R. Cell-type-specific pattern of myc protooncogene expression in developing human embryos. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5050–5054. doi: 10.1073/pnas.82.15.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders A. W., van der Korput J. A., van Steenbrugge G. J., Romijn J. C., Trapman J. Expression of cellular oncogenes in human prostatic carcinoma cell lines. Biochem Biophys Res Commun. 1985 Oct 30;132(2):548–554. doi: 10.1016/0006-291x(85)91168-4. [DOI] [PubMed] [Google Scholar]
- Rüther U., Garber C., Komitowski D., Müller R., Wagner E. F. Deregulated c-fos expression interferes with normal bone development in transgenic mice. 1987 Jan 29-Feb 4Nature. 325(6103):412–416. doi: 10.1038/325412a0. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Hamamori Y., Yamashita T., Fukumoto Y., Takai Y. Involvement of three intracellular messenger systems, protein kinase C, calcium ion and cyclic AMP, in the regulation of c-fos gene expression in Swiss 3T3 cells. FEBS Lett. 1986 Nov 10;208(1):39–42. doi: 10.1016/0014-5793(86)81527-7. [DOI] [PubMed] [Google Scholar]
- ULSTROM R. A., COLLE E., REYNOLDS J. W., BURLEY J. Adrenocortical steroid metabolism in newborn infants, period. J Clin Endocrinol Metab. 1961 Apr;21:414–425. doi: 10.1210/jcem-21-4-414. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Wan Y. J., Orrison B. M., Lieberman R., Lazarovici P., Ozato K. Induction of major histocompatibility class I antigens by interferons in undifferentiated F9 cells. J Cell Physiol. 1987 Feb;130(2):276–283. doi: 10.1002/jcp.1041300214. [DOI] [PubMed] [Google Scholar]