Abstract
Background
Between September 17 and October 3, 2009, hundreds of workers employed in a manufacturing factory in Shenzhen, a city in south China developed a sudden onset of acute gastroenteritis. A retrospective cohort study is designed to identify the risk factors and control this outbreak.
Methods
Information on demographic characteristics, working place, the history of contact with a person having diarrhea and/or vomiting, drink water preference and frequency, eating in the company cafeteria or outside the company, hand-washing habits and eating habits is included. Furthermore, in order to find the contamination source, we investigated the environment around the underground reservoir and collected water samples from the junction between municipal supply water system and underground reservoir to test potential bacteria and virus, examine the seepage tracks on the wall of the underground reservoir from the side of septic tank, and check the integrity and attitude of this lid. Relative risk was presented and Chi-square test was performed. All the analyses were performed with OpenEpi software version 2.3.1 online.
Results
The cohort study demonstrated that the workers who had direct drink water were 3.0 fold more likely to suffer from acute gastroenteritis than those who consumed commercial bottled water. The direct drinking water, water of the tank of buildings, and the underground reservoir were positive only for norovirus. Norovirus was also detected from stool and rectal swab samples from patients with acute gastroenteritis. The underground reservoir was found to be the primary contamination source. Further environmental investigation showed that the norovirus contaminated substance entered into the underground reservoir via access holes in lid covering this underground reservoir.
Conclusion
This acute gastroenteritis outbreak was caused by the secondary supply system contaminated by norovirus in this factory. The outbreak of gastroenteritis cases caused by norovirus frequently occurred in China due to a lack of surveillance and supervision, and due to faults in the construction of such water systems. Therefore, more attentions should pay to the secondary supply water system in China.
Keywords: Norovirus, Acute gastroenteritis, Outbreak, Secondary water supply system
Background
Norovirus is a dominating cause of gastroenteritis in adults and older children. Characteristic symptoms of norovirus consist of nausea, vomiting, and diarrhea generally appear after an incubation period of 24–48 hours and last for about 48–72 hours. Outbreak of gastroenteritis caused by norovirus infection was first discovered in 1968, and had been reported in many places around the world [1-3]. Norovirus also resulted in 50% of all food-borne outbreaks of gastroenteritis in the USA [4]. In addition, norovirus is highly contagious as a few virions are sufficient to cause an illness [5]. Direct contact with vomitus or feces of infected persons, sharing food, water, and/or utensils, and contact with a contaminated environment are all possible routes of norovirus transmission [6-8]. However, outbreaks involving a large number of individuals are usually transmitted through common sources such as food and water [9,10].
Shenzhen is a city in Guangdong province, southern China and is next to Hong Kong. As the manufacturing boom, millions of migrant workers are employed in the manufacturing industry in Shenzhen. From September 17 2009, hundreds of workers in a manufacturing plant in Shenzhen City developed a sudden onset of vomiting and diarrhea. In order to control this outbreak, Shenzhen Bao’an Center for Disease Control and Prevention were invited to identify the causes of this outbreak. On September 26, we conducted a retrospective cohort study to investigate the factors associated with this acute gastroenteritis outbreak in order to identify the source of contamination and the infection route, and finally control and prevent such outbreak in the future.
Methods
Epidemiologic investigation
In this outbreak, study population consisted of suspected cases, probable cases and confirmed cases with the following definitions. Suspected cases were defined as company staff with an onset of vomiting or diarrhea (≥once/day) between September 14 and September 25, 2009. Probable cases were defined as those with an onset of diarrhea (≥three times/day) or vomiting (≥twice/day). Confirmed cases were those probable cases that were tested positive for norovirus by reverse transcription-polymerase chain reaction (RT-PCR). In this investigation, we defined diarrhea as having more than one loose or liquid stools per day, or as having more stools than normal for that person. A retrospective cohort study was conducted and possible risk factors were drinking water or food from the cafeteria in this factory. Information on drinking-water preference and frequency, eating in the factory cafeteria or not, hand-washing habits, eating habits, gender, age, workplace, history of contact with a person with diarrhea and/or vomiting were collected through a questionnaire. Drinking-water preference contained the following two categories: drinking water from directly drinkable-water dispensers (DDWDs) or commercial bottled water. This study was approved by the Ethics Committee of Shenzhen Bao’an Center for Disease Control and Prevention (2009010).
Laboratory tests
Samples from 29 water specimens were collected, including 13 from the water tanks on the top of five buildings, nine from drinking-water machines, five from bottled water, and two from the underground reservoir. All samples were transported to Bao’an District Center for Disease Control and Prevention. All water samples were tested according to the Standards for Drinking Water Quality (GB5749-2006), including the detection of total coliforms, thermotolerant coliforms, Escherichia coli, Salmonella, Shigella, Campylobacter, and Yersinia enterocolitica[11]. In addition, we also tested for norovirus and rotavirus.
As we suspected noroviruses were the responsible agent, we began to collect the specimens of patients. A total of 12 stool and 9 rectal swab samples were collected to test for norovirus. These samples were also transported into Bao’an District Center for Disease Control and Prevention, and detected for norvirus and rotavirus.
RNA extraction and amplification
Virus concentrations of water samples were based on positively-charged filters from 1-l samples, as described previously [12]. Approximately 50–80-μg stool samples were weighed, diluted 1:10 in nuclease-free H2O, and then vortexed for 30 s. Samples were clarified by centrifugation at 6,800 × g for 10 min at room temperature. Viral RNA was extracted from 140-μl processed samples using a QIAamp Viral RNA kit (Qiagen, Victoria, Australia), according to the manufacturer’s instructions.
Real-time fluorescence RT-PCR
Real-time fluorescence PCR for rotavirus and norovirus was performed using an ABI 7500 real-time PCR system with a commercial kit according to the manufacturer’s instructions (catalog numbers SA-6261 for rotavirus and SA-6251 for norovirus, Beijing Suoao Biotechnology Company Limited, Beijing, China).
Environmental investigation and exclusion of pollution sources
The factory comprised an area of 350,000 m2, consisting of three buildings (A1, A2, A3) used as workshops, three buildings (A21, A22, A23) used as dormitories for workers, one building (A24) as a canteen, one building (A15) as a kitchen, and one building as a repair room. About 13,000 workers ate breakfast, more than 6,000 ate lunch, and over 11,000 ate dinner at the company cafeteria every day. Water was supplied from the municipal water supply system and stored in an underground reservoir with a capacity of 8,000 tons. Water from the underground reservoir was pumped into water tanks on top of eight buildings (A11, A1, A12, A3, A21, A22, A23, and A15), and then supplied to DDWDs provided at multiple sites in these eight buildings. Each floor of the dormitory buildings had two DDWDs.
The environment around the underground reservoir is shown in Figure 1. Three possible contamination sources are indicated by red circles. The sewer pipe was close to the municipal water supply pipe. To exclude contamination of the municipal supply water system from the sewer pipe, we collected water specimens from the junction between the municipal water supply system and the underground reservoir to test for bacteria and viruses. Secondly, to exclude contamination from the septic tank near building A23, we looked for seepage tracks from the side of the septic tank on the wall of the underground reservoir. Finally, we checked the integrity of the reservoir lid in order to determine whether contaminants had entered the underground reservoir through this lid.
Figure 1.
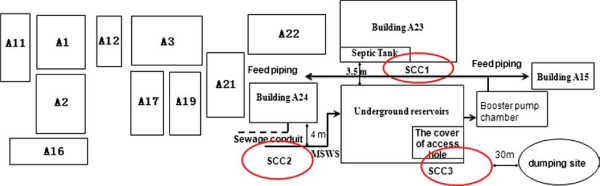
Schematic of environment around underground reservoirs in the factory having gastroenteritis outbreak. SCC1: the first suspected source of contamination. SCC2: the second suspected source of contamination. SCC3: the third suspected source of contamination. MSWS: Municipal supply water system. Building A1, A3, A11, A12, A15, A21, A22, A23 provide DDWD. Building A2, A16, A17, A19 provide bottled water.
Statistical analysis
The distribution of major symptom in workers was summarized by frequency and percent. A retrospective cohort study was conducted to investigate possible risk factors for acute gastroenteritis among a workshop of employees in the company with outbreak of acute gastroenteritis. Chi-square test were performed using OpenEpi software version 2.3.1 online (http://www.openepi.com/OE2.3/Menu/OpenEpiMenu.htm). Relative risks (RR) and 95% confidence intervals (CI) were calculated.
Results
Descriptive epidemiology
A total of 396 workers developed gastrointestinal symptoms between September 17 and September 25, 2009. Diarrhea (77%) was the most common symptom (Table 1), and more than 30% of cases had abdominal distention, abdominal pain, or vomiting. But only few patients consulted a physician at the hospital. We collected 12 stool samples and nine rectal swab samples from 21 of these patients, of which 75% (9 of 12) of stool samples and 55.6% of rectal swab samples were positive for norovirus. None of the samples were positive for rotavirus.
Table 1.
The distribution of major symptom in workers from September 14 through 25, 2009
| Symptom | n | % |
|---|---|---|
| Diarrhea |
308 |
77 |
| Abdominal distention |
156 |
39 |
| Abdominal pain |
154 |
39 |
| Vomiting |
140 |
35 |
| Hypodynamia |
104 |
26 |
| Dizziness |
50 |
13 |
| Fever | 22 | 5.5 |
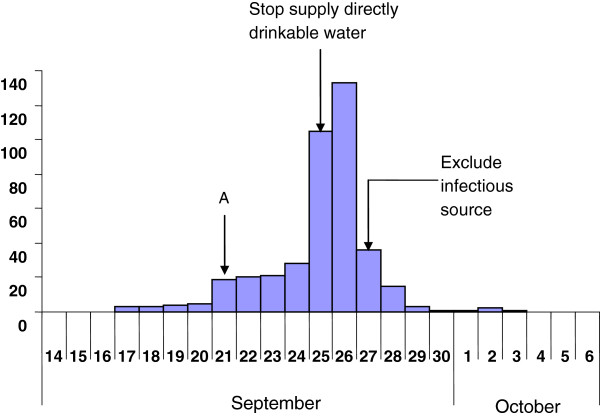
The first case of gastroenteritis occurred on September 17, followed by a rapid increase in the number of cases over the following 4 days (Figure 2). In this factory, five buildings supplied DDWD, while four supplied commercially-bottled water. The incidence of gastroenteritis in the buildings that used DDWD was 2.9% (354/12388), compared to 0.8% (15/1999) in buildings that only provided bottled water (p < 0.0001).
Figure 2.
The time distribution of diarrhea cases amongst workers from September 17 to October 3, 2009.
Risk factors
For the analysis of risk factors in the cohort study, we randomly selected a workshop of 380 employees. Of 380 employees, 38 rotated their days off; 27 declined to join this investigation. Finally 315 employees were interviewed. Of the 162 employees who used a DDWD, 41 (25%) developed gastroenteritis (RR: 3.0, 95% CI:1.7–5.3) (Table 2). In contrast, only one of six employees (17%) who drank bottled water developed gastroenteritis (RR:0.97, 95% CI: 0.16-5.9). The incidence of gastroenteritis among those who had contact with patients having gastroenteritis was the 6.1-fold (95% CI: 3.4–11) higher than those with no contact with infected persons. However, the number of non-responses to this question was high. We further investigated the reason for the lack of response to this question, and found it was difficult for those employees to report whether or not they had contact with vomit or feces from employees with gastroenteritis.
Table 2.
Univariate analysis of risk factors for acute gastroenteritis among employees of a company in Shenzhen City, Sep. 14 to Oct. 3 2009
| |
Non-Response |
Numbers of subjects responding |
Attack rates |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
%(n) |
%(n) |
Exposed |
Not exposed |
Exposed |
Risk ratio |
95% CI# |
|||||
| Cases | Total | Cases | Total | Cases | Total | Yes | No | ||||
| Drink |
|||||||||||
| DDWD* |
0 |
0 |
41 |
162 |
13 |
153 |
25 |
8.5 |
3.0 |
1.7 ~ 5.3 |
|
| Bottled water |
0 |
0 |
1 |
6 |
53 |
309 |
17 |
1.7 |
0.97 |
0.16 ~ 5.9 |
|
| Repast time in company |
|||||||||||
| Breakfast |
0.0(1) |
0.0(10) |
48 |
274 |
6 |
41 |
18 |
15 |
1.2 |
0.55 ~ 2.6 |
|
| Lunch |
0 |
0.0(2) |
53 |
311 |
1 |
4 |
17 |
25 |
0.68 |
0.12 ~ 3.8 |
|
| Supper |
0 |
0.0(5) |
52 |
306 |
2 |
9 |
17 |
22 |
0.77 |
0.22 ~ 2.7 |
|
| Food taken late at night |
0.3(17) |
0.3(88) |
47 |
278 |
7 |
37 |
17 |
19 |
0.89 |
0.44 ~ 1.8 |
|
| Contact with patients |
86(25) |
49(104) |
12 |
22 |
17 |
189 |
55 |
9.0 |
6.1 |
3.4 ~ 11 |
|
| Eating raw and cold food | 0 | 0 | 13 | 47 | 38 | 236 | 28 | 16 | 1.7 | 1.0 ~ 3.0 | |
*: DDWD is drinking direct water disposal.
#: 95%CI is 95% certificated interval.
Laboratory tests
Of 29 water samples, 14 were positive for total coliforms. However, all water samples were negative for E. coli, Salmonella, Shigella, Campylobacter, Y. enterocolitica, and rotavirus. Twelve of 29 water samples were positive for norovirus, including four of six DDWD samples, two of three water samples from the underground reservoir, and four of five water samples from the tanks on top of the buildings. All five bottled water samples were negative for norovirus, rotavirus, E. coli, Salmonella, Shigella, Campylobacter, Y. enterocolitica, and total coliforms. All positive samples were collected from buildings A12, A13, A14, which only provided water via DDWDs.
After confirming norovirus as the organism responsible for the outbreak, we collected stool and rectal swab samples from patients with acute gastroenteritis and examined them for norovirus. Of 10 stool samples, five were positive for norovirus, and four of nine were positive for norovirus in rectal swab samples. All stool and rectal swab samples were negative for rotavirus.
Total coliforms were observed in 8 of 24 water samples, excluding five bottled water samples, and exceeded 100 colony-forming units/ml, which is the upper limit allowed by the Standard of Water Quality of China. All other indicators met the standard criteria.
Environmental investigation
The distribution of the buildings around the underground reservoir is shown in Figure 1. The three potential contamination routes included the septic tank, seepage conduits, and the lid of the underground reservoir. There was no trace of seepage on the wall of the underground reservoir near to the septic tank, and we therefore excluded the septic tank as a possible source of contamination. Secondly, we found no defects in the conduit pipe so that it was excluded as a potential source. In addition, we checked the integrity of the reservoir lid, and noted eight access holes. Furthermore, the lid was at a similar height to the ground around the underground reservoir (Additional file 1), and there was no shed to protect the water from contaminants falling or flowing into the underground reservoir through these access holes; particles smaller than the access holes in the lid were therefore able to enter the water in the underground reservoir.
Control measures
The supply of DDWD was stopped on September 25 2009, and the incidence of gastroenteritis decreased rapidly 2 days later (Figure 2). On September 27 2009, we suspected that the underground reservoir water was contaminated, and the supply of water from this reservoir was therefore stopped immediately. The occurrence of new cases then dropped rapidly within the next 3 days. Although three new cases still appeared between October 1 and October 2, these cases may have been infected with norovirus before September 29, given that the incubation period of norovirus can be 48–72 hours.
Discussion
The results of this epidemiologic investigation indicated that the outbreak of acute gastroenteritis in the factory under investigation was caused by contamination of a secondary water supply system by norovirus. Noroviruses and total coliforms were detected in the underground reservoir water and the water tanks on top of the buildings, which in turn supplied the DDWDs. Employees in these buildings experienced significantly higher rates of acute gastroenteritis than those living in buildings that supplied bottled water.
To confirm the source of the contaminants in the underground reservoir, we investigated the surrounding environment and found eight access holes in the lid covering the reservoir. Furthermore, the lid was at a similar level to the ground around it, making it possible for contaminated substances to enter the underground reservoir through these access holes. Following the outbreak, a 1.2-m high cover has been erected over the reservoir to prevent contamination via the access holes. No new waterborne acute gastroenteritis cases were reported in this factory within the subsequent 2.5 years.
Although total coliforms in the secondary water supply system were above the upper limit required according to drinking water standards [11], no pathogenic bacteria were detected. Norovirus is a robust, chlorine-resistant virus, and 20 ppm chlorine causes no significant reduction in human norovirus infectivity [5,13,14]. Moreover, norovirus in groundwater remains infectious even after storage at room temperature in the dark for 61 days [15]. Meanwhile, norovirus has a high prevalence in groundwater and surface water [16,17]. Overall, these features of norovirus may explain why it is a more common causative agent of waterborne outbreaks of acute gastroenteritis than other viruses. Norovirus is the predominant cause of viral, waterborne outbreaks of acute gastroenteritis, especially in viral drinking-water outbreaks [18]. Secondary water supply systems are common in China. Although many surveillance systems are in place to detect indicator microorganisms in secondary water supply systems every 3 months, surveillance does not cover all such systems, and the detection interval is too long. Sporadic cases and outbreaks of acute gastroenteritis caused by norovirus contamination of drinking water are common in China [19-22], which suggests that norovirus contamination of secondary water supply systems may play an important role in the occurrence of these outbreaks.
There was a notable limitation of the current study. Contact with patients represents one of the major transmission routes for norovirus, but the number of non-responses to this question was high. We were therefore unable to fully investigate the role of transmission by contact in this outbreak.
To our knowledge, this study represents the first report of an outbreak of acute gastroenteritis caused by norovirus contamination of a secondary water supply system in China. Such water systems are common in China, and cases of norovirus-associated acute gastroenteritis are also frequent. However, the contamination sources responsible for most of these norovirus gastroenteritis cases have remained unidentified. The current results highlight the risk of contamination of secondary water supplies by robust norovirus.
Conclusion
Secondary water supply systems in China represent potential sources of acute gastroenteritis outbreaks due to a lack of surveillance and supervision, and due to faults in the construction of such water systems. More attention should be paid to the design and supervision of secondary water supply systems to reduce the incidence of norovirus-related waterborne diseases.
Abbreviations
DDWD: Directly drinkable water dispensers
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
YL design questionnaire, collected the data, conducted the main analyses, and participated in the writing of the manuscript. HG review and interpret the result of analyses, and edit the manuscript. ZX and HZ participated in analyses of data. XZ participated in collecting data. MJ and LZ contributed to design questionnaire and interpretation of analyses. YP re-edited this manuscript in English writing. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
The environment of underground reservoir and access holes of lid covering reservoir.
Contributor Information
Yuan Li, Email: swallowlee2004@tom.com.
Hongxiong Guo, Email: hongxiongguo@gmail.com.
Zhenghui Xu, Email: xuzhenhui79@gmail.com.
Xiaotao Zhou, Email: zhxt000@126.com.
Hailong Zhang, Email: zhanghailongszcdc@163.com.
Lijie Zhang, Email: zhang_li_jie@hotmail.com.
Jing Miao, Email: jinmiao_emily@yahoo.com.
Yi Pan, Email: jun5@cdc.gov.
Acknowledgement
This investigation was partly supported by Jiangsu Provincial Natural Science Fund (Grant No: BK2009432). The authors would like to acknowledge Robert E Fontaine at Centers for Disease Control and Prevention who provided initial guidance at the questionnaire.
References
- Lisa L, Christine M, Severine M, Nathalie R, Xi J, Lauren L, Paul S, Jacques L, Ralph B. Human susceptibility and resistance to norovirus virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- Widdowson MA, Sulka A, Bulens SN, Beard RS, Chaves SS, Hammond R, Salehi EDP, Swanson E, Totaro J, Woron R, Mead PS, Bresee JS. Norovirus and foodborne disease, United States, 1991–2000. Emerg Infect Dis. 2005;11:95–102. doi: 10.3201/eid1101.040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, Parashar UD, Ando T, Glass RI, Noel. Epidemiologic and molecular trends of “Norwalk-like Viruses” associated with outbreaks of gastroenteritis in the United States. J infect Dis. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “norovirus-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- Widdowson MA, Sulka A, Bulens SN, Beard RS, Chaves SS, Hammond R, Salehi ED, Swanson E, Totaro J, Woron R, Mead PS, Bresee JS, Monroe SS, Glass RI. Norovirus and foodborne disease, United States, 1991–2000. Emerg Infect Dis. 2005;11:95–102. doi: 10.3201/eid1101.040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KM, Moe CL, Southwick KL, MacCormack JN. Transmission of Norwalk virus during a football game. N Engl J Med. 2000;343:1223–1227. doi: 10.1056/NEJM200010263431704. [DOI] [PubMed] [Google Scholar]
- Parshionikar SU, Willian-True S, Fout GS, Robbins DE, Seys SA, Cassady JD, Harris R. Waterborne outbreak of gastroenteritis associated with a norovirus. Appl Environ Microbiol. 2003;69:5263–5268. doi: 10.1128/AEM.69.9.5263-5268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1775–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer TP, De Jong B, Kühlmann-Berenzon S, Nyrén O, Svenungsson B, Hedlund KO, Ancker C, Wahl T, Andersson Y. A foodborne norovirus outbreak at a manufacturing company. Epidemiol Infect. 2010;138:501–506. doi: 10.1017/S0950268809990756. [DOI] [PubMed] [Google Scholar]
- Lysén M, Thorhagen M, Brytting M, Hjertqvist M, Andersson Y, Hedlund KO. Genetic diversity among food-borne and waterborne norovirus strains causing outbreaks in Sweden. J Clin Microbiol. 2009;47:2411–2418. doi: 10.1128/JCM.02168-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of health of the People’s Republic of China. Standards for drinking water quality (GB-5749-2006) Beijing, China: Adopted by Ministry of Health of the People’s Republic of China; 2007. [Google Scholar]
- American Public Health Association, American Water Works Association, Water Environment Federation. Standard methods for the examination of water and waste water. 18. Washington, DC: American Public Health Association, American Water Works Association, Water Environment Fderation; 1992. [Google Scholar]
- Duizer E, Bijkerk P, Rockx B, De Groot A, Twisk F, Koopmans M. Inactivation of caliciviruses. Appl Environ Microbiol. 2004;70:4538–4543. doi: 10.1128/AEM.70.8.4538-4543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandura JE, Sobsey MD. Viral and bacterial contamination of groundwater from on-site sewer treatment systems. Water Sci Technol. 1997;35:141–146. doi: 10.1016/S0273-1223(97)00249-7. [DOI] [Google Scholar]
- Seitz SR, Leon JS, Schwab KJ, Marshall Lyon G, Melissa D, McDaniels M, Gwen A, Fernandez ML, Lindesmith LC, Baric RS, Moe CL. Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol. 2011;77:6884–6888. doi: 10.1128/AEM.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-G, Jheong W-H, Suh C-I, Kim S-H, Lee J-B, Jeong Y-S, Ko GP, Lib Jang K, Lee G-C, Paik S-Y. National groundwater surveillance of noroviruses in South Korea,2008. Appl Environ Microbiol. 2011;77:1466–1474. doi: 10.1128/AEM.01996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw Tiong G, Gin Karina Y-H, Oon Lynette Lin E, Chen Eileen X, Woo Chee H. Prevalence and genotypes of human noroviruses in tropical urban surface waters and clinical samples in Singapore. Applied Environ Microbiol. 2009;75:4984–4992. doi: 10.1128/AEM.00489-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craun GF, Brunkard JM, Yoder JS, Roberts VA, Joe C, Tim W, Calderon RL, Roberts JM, Beach MJ, Roy SL. Causes of outbreak associated with drinking water in the United States from 1971 to 2006. Clin Microbiol Rev. 2010;23:507–528. doi: 10.1128/CMR.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Weiwu C, Jing D, Zhou S, Xuhui Y, Qinjun K, Renjie H. Analysis of norovirus enteritis outbreaks in Hangzhou City. Chinese J Prevent Med. 2009;10:23–25. [Google Scholar]
- Yueliang Z, Wenlong Z, Maoyu C. Investigation of noroviral gastroenteritis outbreak in a company of Jiangmen City. J Chinese Trop Med. 2009;9:445–457. [Google Scholar]
- Ying Y, Zhaoguo W. A preliminary study on molecular of noroviruses detected in Qingdao. Chinese J Health Lab Technol. 2008;18:2229–2230. [Google Scholar]
- Wu X, Yang X, Jiang L, Shen J, Xie H, Li X, Wu Y, Liu Y, Gao G. Molecular epidemiologic analysis of norovirus from food poisoning affairs in Guangzhou. J Sun Yatsen Univ (Med Sci) 2008;29:486–489. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The environment of underground reservoir and access holes of lid covering reservoir.