Abstract
Background
Invasive aspergillosis (IA) is an important cause of morbidity and mortality in immunocompromised individuals. This study was conducted to identify a desirable target DNA sequence for the diagnosis of aspergillosis using real-time quantitative polymerase chain reaction (qPCR).
Methods
Genomic DNA was extracted from Aspergillus, Candida, and bacteria species, and qPCR was applied to validate a partial ribosomal DNA 28S-ITS2 sequence. Ethylenediaminetetraacetic acid-anticoagulated blood samples were collected from 72 febrile hematological patients, while total DNA was isolated from plasma and whole blood for the Aspergillus qPCR. The results were analyzed using a receiver operating characteristic curve. All cases were evaluated using the revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) diagnostic criteria.
Results
Use of qPCR yielded positive results for 15 Aspergillus species but negative results for Candida species, bacterial strains, and human DNA. The limit of detection was one copy per microliter of DNA. Analytical sensitivity and specificity were six copies of DNA and 100%, respectively. The standard curve showed that qPCR was reliable for Aspergillus detection and that significantly more DNA copies were obtained from whole blood than from plasma (P < 0.001). At a cut-off value ≥ 25 copies/μL, the diagnostic sensitivity and specificity for IA using 28S-ITS2 qPCR were 90.9% and 73.4%, respectively.
Conclusions
The use of qPCR with whole blood to detect and verify the 28S-ITS2 sequence is a specific and useful way to diagnose IA.
Keywords: Hematological malignancies, Invasive aspergillosis, Real-time quantitative polymerase chain reaction, 28S-ITS2
Background
Invasive aspergillosis (IA) is an important cause of morbidity and mortality in immunocompromised individuals. Patients at the highest risk are those with prolonged periods of neutropenia due to chemotherapy for acute leukemia or hematopoietic stem cell transplantation (HSCT) for hematological malignancies or solid tumors [1,2]. Conventional diagnostic tests such as blood cultures are not useful or practical for the diagnosis of Aspergillus spp. fungemia [3]. Further, non-culture–based techniques used in the past lack the sensitivity and specificity needed for the testing of immunocompromised patients [4-7]. Additionally, established IA is difficult to treat and has a death rate of 80–90%, which has driven researchers to develop more reliable and effective methods for its timely diagnosis [1,2,8,9]. Polymerase chain reaction (PCR) assays (conventional, nested, and real-time) have recently been developed for diagnosing fungal infections, especially quantitative real-time PCR (qPCR), which shows better potential for clinical application [1,10-14]. A range of gene region targets (cytochrome p450; heat shock proteins; the 18S, 5.8S, and 28S domains; and the internal transcribed space [ITS]) have been used in studies employing qPCR methods. Over the past few years, the ribosomal DNA gene region, composed of the 18S (1800 bp), 5.8S (159 bp), and 28S (3396 bp) genes, has been shown to be a promising target in which the three above-mentioned genes are separated by the ITS1 (361 bp) and ITS2 (231 bp) genes. Until now, numerous studies have used target sequences from this region to detect Aspergillus DNA using PCR methods [10,15-18]. However, a consensus is not available with regard to the specific target sequence, specimen type, extraction method, or PCR format and platform. Hence, the objectives of our study were to search for a new potential target sequence for Aspergillus, assess the qPCR value, and determine the qPCR cut-off index for the presumptive clinical diagnosis of IA.
Methods
Preparation of fungal and bacterial cultures
All the strains were isolated from clinical patients. The tested strains consisted of: Aspergillus (15 strains), including A. fumigatus (four strains), A. niger (five strains), A. flavus (four strains), A. nidulans (one strain), and A. terreus (one strain); Candida (24 strains), including C. albicans (12 strains), C. tropicalis (four strains), C. parapsilosis (three strains), C. pseudotropicalis (one strain), C. stellatoidea (one strain), C. krusei (one strain), and C. lipolytica (one strain); Monilia guilliermondii (one strain); and assorted bacteria (10 strains) including Staphylococcus aureus, Streptococcus pneumoniae, Neisseria meningitidis, Shigella, Haemophilus parainfluenzae, Escherichia coli, Klebsiella pneumoniae (two strains), and Bacterium aeruginosum (two strains).
Czapek’s agar and Sabouraud’s agar (Beijing AoBoXing Bio-Tech Co., Ltd., China) were inoculated with conidiospores from Aspergillus mycelia and Candida, respectively. Both were incubated at 30°C for 18–72 h until a mycelial mat formed. Blood agar, chocolate agar, and Mueller-Hinton agar plates (Beijing Laboratory Biology Technology of China, Beijing, China) were inoculated with different bacterial species and incubated at 37°C for 12–16 h. A proportion of the mycelial mat weighing approximately 10–20 mg was harvested with sterile forceps and placed into a sterile 1.5-mL Eppendorf tube with 600 μL of phosphate-buffered saline (PBS).
Patient group
Seventy-two patients with hematological malignancies suffering from fever, four patients at normal temperature, and 10 healthy volunteers who visited our Hematology Department and Bone Transplantation Center between September 2010 and April 2011 were enrolled in the study. The study protocol was approved by the author’s institutional ethics committee, namely the Ethics Committee of General Hospital of Chinese Peoples’ Liberation Army, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to specimen collection. Five-milliliter ethylenediaminetetraacetic acid (EDTA)-anticoagulated blood samples were collected when the patients were febrile [19], which was defined as an axillary temperature > 38.3°C on a single occasion or temperature > 38°C for >1 h (according to the National Comprehensive Cancer Network). An aliquot of 200 μL of plasma was centrifuged from the whole blood and total DNA was extracted from the plasma and the remnant whole blood. We then prospectively viewed the charts, electronic medical records, and laboratory records of these patients for the prior 60 days. IA was diagnosed on the basis of the revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria [20]. Using these definitions, we classified the study population into four probability levels: proven IA, probable IA, possible IA, and no IA.
DNA extraction
(i) From fungal specimens
The mycelial mats harvested in PBS were centrifuged for 1 min at 9,000 × g, and DNA was then extracted using a Biospin Fungus Genomic DNA Extraction Kit in accordance with the manufacturer’s instructions (BioFlux, China). In this process, a sample is first lysed in LE buffer, and then DNA in the sample is liberated. After DA buffer is added and the mixture is centrifuged at 14,000 × g for three minutes, the impurity is discarded. Released DNA is bound exclusively and specifically to the Biospin membrane in the presence of E Bingding Buffer under appropriate salt iron and pH conditions. Denatured proteins and other contaminants are removed by several washing procedures. The DNA is then eluted from the membrane using 30 μL of elution buffer.
(ii) From bacterial specimens
The bacterial harvest was centrifuged for one min at 12,000 × g, while genomic DNA was extracted using a Bacterial DNAout Kit according to the manufacturer’s instructions (Andy Bio, USA) for gram-positive and -negative bacteria.
(iii) From clinical specimens
Total DNA was simultaneously extracted from whole blood and 200 μL of supernatant plasma that had been centrifuged from the whole blood. Red blood cell lysis solution (Solarbio, China) was used to remove the red blood cells from the whole blood and obtain a white blood cell sediment. LE buffer was added to the white blood cells or 200 μL of plasma, and the following DNA extraction steps were undertaken in accordance with the manufacturer’s instructions of the Biospin Kit used for the fungal specimens. A 30-μL elution volume of DNA was ultimately obtained [19]. Human DNA was extracted using a DNA Purification Kit (Promega, USA) according to the relevant protocols.
As controls, an array of known positive samples from A. fumigatus and known negative samples from C. albicans was included in every set of DNA extractions. A maximum of 12 samples, including one positive and one negative control, was processed at any given time. The DNA concentration was determined from the absorbance at 260 nm measured using a UV spectrometer (Amersham Biosciences Co., USA).
Primer and probe design
Ribosomal subunit gene sequences of Aspergillus were accessed in NCBI GenBank (accession no. EF634384.1) and aligned using BLAST. Potential primer-binding sites were selected from the sequences conserved among Aspergillus (EF634384.1, 353–953 bp) but not among the human genome and other clinical relevant fungal genera except for Penicillium and Paecilomyces species. Primers were designed with the aid of Primer Express 3.0 software (Applied Biosystems,USA). The following primers targeting the 28S-ITS2 region of the ribosomal subunit gene, which was not examined in earlier studies, were used: forward primer, 5′-GTCCGGTCCTCGAGCGTAT-3′ (435–453); and reverse primer, 5′-GTTCAGCGGGTATCCCTACCT-3′ (534–554). They are approximately positioned on the A. fumigatus ribosomal 28S-ITS2 gene: GTCCGGTCCTCGAGCGTATGGGGCTTTGTCACCTGCTCTGTAGGCCCGGCCGGCGCCAGCCGACACCCAACTTTATTTTTCTAAGGTTGACCTCGGATCAGGTAGGGATACCCGCTGAAC (EF634384.1, 435–554 bp; ITS2, 435–521 bp; 28S, 522–554 bp). The hydrolysis probe sequence was fluorescence labeled with 6-carboxyfluorescein at the 5' end as the reporter dye and None Fluorescence Quencher modified by Minor Groove Binder at the 3' end as the quencher 5′-FAM-CTTTGTCACCTGCTCTG-NFQ-MGB-3′ (458–474) (Figure 1).
Figure 1.
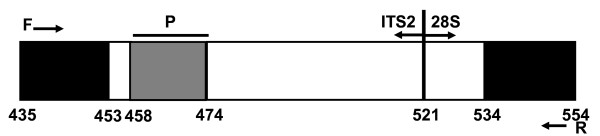
The target sequence, primer, and probe. The 1,154-bp sequence of ribosomal DNA includes an 18S ribosomal RNA gene, a partial sequence (<0–12 bp), internal transcribed spacer 1 (ITS-1, 13–195 bp), the 5.8S ribosomal RNA gene (196–352 bp), internal transcribed spacer 2 (ITS-2, 353–521 bp), 28S ribosomal RNA gene, and a partial sequence (522–>1,154 bp). The target sequence (435–554) includes ITS2 and 28S as well as the partial sequence 28S-ITS2.
Real-time qPCR
To minimize contamination, the PCR assays were set up in a class 2 laminar flow cabinet in a separate room from the DNA extractions. The qPCR procedure was performed using 40-μL reaction mixes on Mx3000P (Stratagene, USA). Each reaction contained 20 μL of 2× QuantiTect Probe PCR Master Mix Buffer (Qiagen, Germany), 1 μL of each primer, 0.5 μL of the probe (American Biosystems, USA), 6 μL of template DNA, and 11.5 μL of RNase-Free Water (Qiagen). The conditions for the qPCR were as follows: 50°C for 2 min to digest the PCR products including dUMP using uracil-N-glycosylase; 95°C for 10 min to activate a hot start DNA polymerase; and 40 cycles of denaturing at 94°C for 15 sec and annealing and extension at 59°C for 1 min. Each sample was processed in a set of two counterparts. When the difference in the quantification cycle (Cq) value of the two counterparts was >1, the sample processing was repeated in another qPCR set until the Cq difference was <1 cycle to ensure concordance and accuracy. Two positive controls containing copies of A. fumigatus, two negative controls containing copies of C. albicans, and two blank controls containing water were also included with each run to monitor inter-assay consistency. All DNA extractions and qPCR procedures were performed with the operator blinded to the strains’ species and the patients’ clinical features. The results were shown by initial quantity (copies/μL).
Standard preparation
The amplified DNA products of A. fumigatus were run on a 1.8% agarose gel and extracted using a QIAquick Gel Extraction Kit according to the manufacturer’s instructions (Qiagen). The purified target DNA was ligated into a pGEM-TEasy Vector System I (A1360; Promega), while the recombinant plasmid was transformed into Escherichia coli-competent DH5α cells (Beijing BioMedTech Co., Ltd., China) and extracted using a Wizard Plus SV Miniprep DNA Purification Kit (Promega) according to the manufacturer’s instructions. The recombinant plasmid DNA was run on an agarose gel with positive and negative controls and then amplified and identified. The predicted product size was 120 bp. The transformation was also confirmed by sequencing the target fragment using liquid cultures of E. coli. After identification, the recombinant plasmid DNA was diluted 10-fold from 1 × 109 copies/μL to 1 × 103 copies/μL with RNase-free water (Qiagen) for a series of standard qPCR preparations used to generate the standard curve. Each sample was processed in a set of triplicate counterparts.
Calculation of Aspergillus qPCR cut-off value
Both cases of proven and probable IA were assumed to be true. The other patients and the 10 healthy volunteers were considered non-IA in the analysis. The initial qPCR copies for every case were included in the calculation. The cut-off value of the qPCR was determined using receiver operating characteristic (ROC) analysis. Sensitivity and specificity were calculated using IA cases as the reference standard.
Statistical analysis
Continuous variables were compared using a nonparametric test (Mann–Whitney U test). Two-tailed P values < 0.05 were considered statistically significant. An appropriate qPCR cut-off value was estimated using ROC analysis. Statistical analyses were performed using SPSS software version 19.0 (IBM, USA).
Results
Real-time qPCR development
The qPCR protocol was optimized with respect to primer and probe concentrations, DNA template quantities, buffer composition, number of cycles, and cycle parameters (data not shown). The qPCR yielded positive results for all 15 tested isolates of Aspergillus: the mean Cq value was 21.66 (95% confidence interval [CI], 20.05–23.27), while the mean initial number of DNA copies was 2.47 × 106/μL (95% CI, 1.69 × 105–5.11 × 106/μL). None of the 24 Candida samples or the 10 bacterial and human DNA samples tested was amplified. Thus, the analytical specificity of this method was 100%. The use of serial 10-fold dilutions of A. fumigatus DNA in water showed that the qPCR limit of detection was one copy per microliter of DNA with the Cq value around 37, while the analytical sensitivity was six copies of DNA.
The recombinant plasmid DNA was identified by running on an agarose gel and sequencing. It was then diluted for a series of standard preparations and subjected to qPCR. The standard curve was Cq = −3.332 × log (initial quantity, copies/μL) + 40.75 (RSq = 0.991; Eff = 99.6%).
Characteristics of the study population
A total of 72 patients (30 women, 42 men) were included in the study (Table 1). The median age was 42.5 years (range, 3–76 years). Forty (55.6%) patients had acute leukemia, while the other 25 (34.7%) had undergone HSCT. According to the EORTC/MSG criteria [20], IA was diagnosed in 41 patients (four patients with proven IA confirmed via lung biopsy, 18 with probable IA, and 19 with possible IA), and the 28S-ITS2 qPCR results (median, range) for proven, probable, and possible IA were 123.47 (25.96–266.95), 43.65 (0–549.5), and 16.15 (0–98.76) copies/μL (Table 2). No amplification was seen in the controls (four patients with normal temperature and the 10 healthy volunteers). Febrile neutropenia was the most common in-host factor, while the halo sign (78%) was the most common clinical criterion. No patients were tested using the galactomannan (GM) test, and the mycological criteria were judged mainly according to sputum microscopic evaluation or culture results (Table 2).
Table 1.
Clinical features of febrile patients with hematological malignancies
| Demographic/characteristic | No. patients (%) (n = 72) |
|---|---|
| Age (years) |
|
| Median |
42.5 |
| Range |
3–76 |
| Gender |
|
| Male |
42 (58.3) |
| Female |
30 (41.7) |
| Hematological malignancy |
|
| AML |
30 (41.7) |
| ALL |
10 (13.9) |
| CML |
3 (4.2) |
| CMML |
1 (1.4) |
| CLL |
2 (2.8) |
| NHL |
14 (19.4) |
| MDS |
7 (9.7) |
| MM |
5 (6.9) |
| HSCT |
25 (34.7) |
| Allo-HSCT |
21 (29.2) |
| Auto-HSCT |
4 (5.6) |
| IA |
41 (56.9) |
| Proven |
4 (5.6) |
| Probable |
18 (25) |
| Possible |
19 (26.4) |
| Sites |
|
| Pulmonary |
40 (55.6) |
| Systemic | 1 (1.4) |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CMML, chronic myelomonocytic leukemia; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; MDS, myelodysplastic syndromes; MM, multiple myeloma; HSCT, hematopoietic stem cell transplantation; IA, invasive aspergillosis.
Table 2.
Diagnostic criteria and 28S-ITS2 qPCR results for invasive aspergillosis
|
Diagnostic criteria |
IA category |
Total (%) (n=41) |
||
|---|---|---|---|---|
| Proven (%) (n=4) | Probable (%) (n=18) | Possible (%) (n=19) | ||
|
Host factors |
|
|
|
|
| Neutropenia |
4 (100) |
6 (33.3) |
3 (15.8) |
13 (31.7) |
| T > 38°C with |
|
|
|
|
| Prolonged neutropenia |
4 (100) |
11 (61.1) |
6 (31.6) |
21 (51.2) |
| Immunosuppressant |
1 (25) |
9 (50) |
9 (47.4) |
19 (46.3) |
| Previous IFI |
0 (0) |
4 (22.2) |
3 (15.8) |
7 (17.1) |
| With AIDS |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
| GVHD |
0 (0) |
3 (16.7) |
2 (10.5) |
5 (12.2) |
| Corticosteroids |
3 (75) |
4 (22.2) |
7 (36.8) |
14 (34.1) |
|
Clinical criteria |
|
|
|
|
| Halo sign |
3 (75) |
17 (94.4) |
12 (63.1) |
32 (78) |
| Air-crescent sign |
2 (50) |
1 (5.6) |
2 (10.5) |
5 (12.2) |
| Cavity |
1 (25) |
3 (16.7) |
0 (0) |
4 (9.8) |
| Symptoms of LRI |
3 (75) |
8 (44.4) |
13 (68.4) |
24 (58.5) |
| Permanent fever |
2 (50) |
7 (38.9) |
13 (68.4) |
22 (53.7) |
|
Mycological criteria |
|
|
|
|
| Positive sputum microscopy |
0(0) |
6 (33.3) |
0 (0) |
6 (14.6) |
| Positive sputum culture |
1 (25) |
5 (27.8) |
0 (0) |
6 (14.6) |
| G test positive |
1 (25) |
8 (44.4) |
1 (5.3) |
10 (24.4) |
| No bacterial positive |
0 (0) |
6 (33.3) |
3 (15.8) |
9 (22) |
|
Histology |
|
|
|
|
| Biopsy specimen of the lung |
4 (100) |
0 (0) |
0 (0) |
4 (9.8) |
|
qPCR results (copies/μL) (median, range) |
123.47 |
43.65 |
16.15 |
28.12 |
| (25.96–266.95) | (0–549.5) | (0–98.76) | (0–549.5) | |
qPCR, real-time qualitative polymerase chain reaction; IA, invasive aspergillosis; IFI, invasive fungal infections; AIDS, acquired immune deficiency syndrome; GVHD, graft versus host disease; LRI, lower respiratory infections.
Calculating the cut-off value for the Aspergillus 28S-ITS2 qPCR
Whole blood and plasma samples were simultaneously collected from the patients for Aspergillus DNA isolation and purification, and the qPCR assays were then performed using the hydrolysis probe method. Significantly greater numbers of DNA copies were amplified by qPCR from whole blood than from plasma (28.36 vs. 7.28, P < 0.001), and DNA was not amplified from most of the plasma samples. Therefore, the whole blood qPCR results were used in the subsequent analysis. A significantly higher median number of initial quantity was amplified from patients with proven and probable IA (57.87 copies/μL; range, 0–549.5) than patients without IA (P < 0.001).
Using proven and probable IA cases as the reference standard, the area under the ROC curve (AUC) was 0.828 (95% CI, 0.733–0.923; P < 0.001). The optimal infection point was located between a qPCR index of 21.34 (sensitivity, 95.5%; specificity, 68.8%; Youden index, 0.643) and 25.24 (sensitivity, 90.9%; specificity, 73.4%; Youden index, 0.643) (Figure 2). According to the ROC results and considering the clinical situation in which the rate of missed diagnosis is very high compared to the misdiagnosis rate, and if the diagnosis is missed in patients with IA, the prognosis is very poor, so we chose a threshold with a high sensitivity and a suboptimal specificity. In this study, qPCR was considered positive when there were ≥25 copies/μL (Table 3). Based on this cut-off value, the diagnostic sensitivity and specificity for IA by 28S-ITS2 real-time PCR were 90.9% and 73.4%, respectively.
Figure 2.
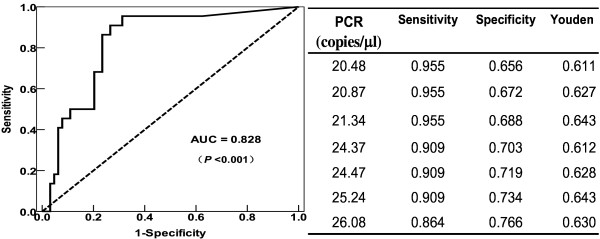
The receiver operating characteristic (ROC) analysis. The ROC curve and table of statistics for the cut-off value for the group of patients with proven and probable invasive aspergillosis were used as standards.
Table 3.
The diagnostic results of qPCR and EORTC/MSG criteria
|
Reference standard according to IA category |
qPCR (≥25copies/μL) |
Total |
|
|---|---|---|---|
| Positive | Negative | ||
| Positive |
|
|
22 |
| Proven |
4 |
0 |
|
| Probable |
16 |
2 |
|
| Negative |
|
|
64 |
| Possible |
7 |
12 |
|
| No IA |
10 |
35 |
|
| Total | 37 | 49 | 86 |
qPCR, real-time qualitative polymerase chain reaction; EORTC/MSG, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group; IA, invasive aspergillosis.
Discussion
The results of this study showed that the target region of 28S-ITS2 is specific and applicable to the genetic diagnosis of Aspergillus and that the whole blood qPCR assay may be a very useful and reliable method for the early diagnosis of IA in patients with hematological malignancies.
Over the past decade, many researchers have explored and evaluated PCR assays for the early diagnosis of IA [10,21-24], while the European Aspergillus PCR Initiative (EAPCRI) has performed a lot of meaningful work around PCR standardization [13,19,25]. However, PCR is still not included in the EORTC/MSG criteria [16]. Meanwhile, there is a major consensus in favor of qPCR technology due to its sensitivity and speed [13,16,24,26-28], especially after release of the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines, which has encouraged better experimental practice for more reliable and unequivocal interpretation of qPCR results [14,29]. A critical issue of qPCR is the choice of the target DNA, which is highly debated since it is directly associated with assay sensitivity and specificity. To date, several genes have been used to assess qPCR assay results in the diagnosis of IA [10,11,15], and the ribosomal DNA gene region has been used more and more frequently and shows the greatest potential [16-18,25,29,30]. Ribosomal DNA comprises transcriptional domains (5S, 5.8S, 18S, and 28S), which evolve relatively slowly and have higher conservation rates, and non-transcriptional domains (ITS1 and ITS2), which are more variable [31]. A multicenter study comparing the use of the 28S and 18S primer sets targeting the D1–D2 region of the large ribosomal subunit gene revealed that the former was more specific, probably because when the 18S region is targeted, a portion of the human rDNA gene needs to be amplified, especially in the absence of A. fumigatus DNA [18,32].
Overall, use of the 28S primer set is more suitable than the 18S primer set for the diagnosis of Aspergillus. The primers in our study were derived from the 28S-ITS2 region in the GenBank database and targeted both the conservative and variable regions as described before, while the probe was modified by minor groove binder, which further confirmed its specificity. The target sequence was aligned using BLAST and tested for analytic sensitivity and specificity using 15 Aspergillus species, 24 Candida species, 10 bacterial species, and serial 10-fold dilutions of A. fumigatus DNA, with the results of 6 copies and 100%, respectively, indicating the applicability of the Aspergillus qPCR assay.
With regard to the best blood fraction to test, specimen choice had critical implications in the extraction methodology and PCR results. Historically, the pathogenesis of IA has been poorly understood, and knowledge regarding the source of Aspergillus nucleic acids in the blood is limited with no consensus having been achieved to date. Testing serum specimens using PCR is dependent on the detection of free circulating Aspergillus DNA, while the use of EDTA-anticoagulated whole blood samples allows for the detection of conidia, hyphal fragments, and freely circulating DNA [12,18]. In our study, plasma and whole blood were compared for Aspergillus DNA extraction, and the results showed greater numbers of initial DNA copies in whole blood than in plasma. Thus, whole blood was the better specimen for Aspergillus DNA extraction, a finding that was consistent with those of earlier reports and the EAPCRI recommendation [12,13,33].
To initially assess the clinical applicability of this Aspergillus qPCR, 86 blood samples from 86 individuals were tested: 72 of these individuals were febrile and had hematological malignancies, while 41 were diagnosed with IA according to clinical criteria. The qPCR results and clinical data were analyzed using ROC [27]. The main obstacle we encountered was that the number of proven cases of aspergillosis was too low to be used as the sole criterion for diagnosis since the blood cultures were almost always negative for Aspergillus species. The probability of confirming the diagnosis histopathologically was also low because patient status was not suitable for invasive diagnostic procedures. According to the qPCR results, the proven and probable cases were defined as being within the reference standard category on the basis of the EORTC/MSG criteria, the only accepted tool, although it has some limitations [20,34].
The choice of an optimal cut-off value depends on the test purpose. An ROC analysis was conducted that revealed a good AUC value (0.828). In clinical practice, there are few effective clinical approaches for the diagnosis of IA; once a diagnosis is missed, patient prognosis is very poor. Hence, we chose a qPCR index that yielded an adequately high sensitivity, an acceptable false-positive rate (1-specificity), and a better Youden index, which reflects integrated diagnostic ability. In the ROC analysis, a cut-off value of 25.24 seemed to yield the optimal trade-off (Figure 2). The qPCR cut-off value of ≥25 copies/μL was tentatively established as the most suitable for obtaining a high yield in clinical screening for IA, while the Cq value was 36, consistent with that of the EAPCRI report (mean, 35.3; upper limit, 38.8 cycles) [13]. According to this threshold value, the diagnostic sensitivity and specificity were 90.9% and 73.4%, respectively, while meta-analysis showed that these values would be 88% (95% CI, 75–94%) and 75% (95% CI, 63–84%) if only a single positive sample was required [35]. With this threshold, the specificity (73.4%) seemed suboptimal, and 41% (7/17) of the patients with false-positive results were diagnosed as possible IA and 59% (10/17) were diagnosed as no IA, which accounted for the possible IA and no IA rates of 37% (7/19) and 22% (10/45), respectively (Table 3). We analyzed these patients and speculated that their degraded diagnoses were mainly due to the limitations of the EORTC/MSG criteria [34] and the fact that high sensitivity can also cause false-positive results due to low-level contamination [36]. Due to the heterogeneity of the different studies, there is no way to compare them. Of course, increasing the size of the study population, especially the number of proven cases, may result in a better cut-off index and a more suitable guide for the presumptive diagnosis of IA in future studies.
Another limitation of this study was that we did not collect serial specimens for qPCR since we immediately administered empirical antifungal treatment to patients with neutropenic fever, which was thought to affect the diagnostic detection [37]. Otherwise, no association was found between Aspergillus DNA detection and treatment efficacy or patient outcome [38-41], the probable reason for this being the lower DNA loads in the blood, which made reliable quantification difficult due to the random dispersion of the few available DNA copies [39]. The current use of Aspergillus qPCR as a diagnostic screening tool is recommended instead of evaluation for therapy, and a single PCR-negative result is sufficient to exclude the diagnosis of IA because of this assay’s high sensitivity [10,16,42]. The GM test was not performed simultaneously with the one-time qPCR for each patient in this study since Aspergillus GM and DNA release do not occur in a parallel fashion due to IA pathogenesis and host factor changes that occur over time [43-45]. Additionally, several studies have indicated that qPCR was comparable or better to GM for IA diagnosis and that it is more scientific and appropriate to combine GM and qPCR for IA diagnosis [42,45-47].
Conclusions
Here we found a specific sequence of Aspergillus 28S-ITS2 for qPCR that offers good sensitivity and specificity. This assay was initially assessed in patients with hematological malignancies, and a tentative cut-off value for the diagnosis of IA was obtained. Future clinical studies are necessary, especially diagnostic randomized controlled trials for this assay using the novel superior DNA extraction method and ultraclean production according to the MIQE guidelines [14,36,43]. We expect that this qPCR assay will be useful for early diagnosing to enable the initiation of preemptive IA treatment.
Abbreviations
28S-ITS2: Partial sequence of the ribosomal DNA including the 28S domain and internal transcribed space 2 domain; IA: Invasive aspergillosis; qPCR: Real-time quantitative PCR; EORTC/MSG: European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group; Cq: Quantification cycle; ROC: Receiver operating characteristic; GM: Galactomannan; AUC: Area under the ROC curve.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YL, LG, and LY designed this study. YD and YX performed the PCR assays and data collection. YL and LG performed the data analysis, calculated the statistics, and drafted the article, while LW and LY critically revised the article. WH, YJ, and HL provided valuable advice, supported the clinical protocol, and edited the article. Study funding was secured by LY. All of the authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Yan Li, Email: liyan0411346@163.com.
Li Gao, Email: gaorui1583@163.com.
Yi Ding, Email: 275448230@163.com.
Yuanyuan Xu, Email: yuanyuanxu@163.com.
Minhang Zhou, Email: sulfur1986@126.com.
Wenrong Huang, Email: huangwr301@yahoo.com.cn.
Yu Jing, Email: jingy@163.com.
Honghua Li, Email: liyanly301@126.com.
Lili Wang, Email: daughter126@126.com.
Li Yu, Email: chunhuiliyu@yahoo.com.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 30971297 and 90919044), Major State Basic Research Development Program of China (No. 2005CB522400), Key Research Program for Clinical High-Tech Research of People's Liberation Army (No. 2010gxjs091), and Key Program of the Capital Development Foundation (No. 2007–2040).
The authors appreciate Prof. Jijiang Suo, Prof. Yunxi Liu, and Prof. Dingxia Shen (all of the Chinese PLA General Hospital) for their help with preparing the strains as well as all of the physicians, nurses, and supporting personnel for their dedicated care of the patients in this study.
References
- Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, Walsh TJ, Fridkin SK, Pappas PG, Warnock DW. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(Suppl 1):S49–S58. doi: 10.1080/13693780400020113. [DOI] [PubMed] [Google Scholar]
- Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- Meyer MH, Letscher-Bru V, Jaulhac B, Waller J, Candolfi E. Comparison of mycosis IC/F and plus aerobic/F media for diagnosis of fungemia by the bactec 9240 system. J Clin Microbiol. 2004;42:773–777. doi: 10.1128/JCM.42.2.773-777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, Kaneko M, Sato H, Watanabe T, Hosoya N, Izutsu K, Asai T, Hangaishi A, Motokura T, Chiba S, Kurokawa M. False-positive Aspergillus galactomannan antigenaemia after haematopoietic stem cell transplantation. J Antimicrob Chemother. 2008;61:411–416. doi: 10.1093/jac/dkm463. [DOI] [PubMed] [Google Scholar]
- Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med. 2006;173:707–717. doi: 10.1164/rccm.200505-727SO. [DOI] [PubMed] [Google Scholar]
- Horger M, Einsele H, Schumacher U, Wehrmann M, Hebart H, Lengerke C, Vonthein R, Claussen CD, Pfannenberg C. Invasive pulmonary aspergillosis: frequency and meaning of the "hypodense sign" on unenhanced CT. Br J Radiol. 2005;78:697–703. doi: 10.1259/bjr/49174919. [DOI] [PubMed] [Google Scholar]
- Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- Robenshtok E, Gafter-Gvili A, Goldberg E, Weinberger M, Yeshurun M, Leibovici L, Paul M. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471–5489. doi: 10.1200/JCO.2007.12.3851. [DOI] [PubMed] [Google Scholar]
- Hot A, Maunoury C, Poiree S, Lanternier F, Viard JP, Loulergue P, Coignard H, Bougnoux ME, Suarez F, Rubio MT, Mahlaoui N, Dupont B, Lecuit M, Faraggi M, Lortholary O. Diagnostic contribution of positron emission tomography with [18F] fluorodeoxyglucose for invasive fungal infections. Clin Microbiol Infect. 2011;17:409–417. doi: 10.1111/j.1469-0691.2010.03301.x. [DOI] [PubMed] [Google Scholar]
- Schabereiter-Gurtner C, Selitsch B, Rotter ML, Hirschl AM, Willinger B. Development of novel real-time PCR assays for detection and differentiation of eleven medically important aspergillus and candida species in clinical specimens. J Clin Microbiol. 2007;45:906–914. doi: 10.1128/JCM.01344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PL, Barnes RA. Aspergillus PCR-platforms, strengths and weaknesses. Med Mycol. 2006;44:191–198. doi: 10.1080/13693780600898003. [DOI] [PubMed] [Google Scholar]
- Klingspor L, Loeffler J. Aspergillus PCR formidable challenges and progress. Med Mycol. 2009;47(Suppl 1):S241–S247. doi: 10.1080/13693780802616823. [DOI] [PubMed] [Google Scholar]
- White PL, Bretagne S, Klingspor L, Melchers WJ, McCulloch E, Schulz B, Finnstrom N, Mengoli C, Barnes RA, Donnelly JP, Loeffler J. Aspergillus PCR: One step closer to standardization. J Clin Microbiol. 2010;48:1231–1240. doi: 10.1128/JCM.01767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Klingspor L, Jalal S. Molecular detection and identification of Candida and aspergillus spp. From clinical samples using real-time PCR. Clin Microbiol Infect. 2006;12:745–753. doi: 10.1111/j.1469-0691.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- White PL, Linton CJ, Perry MD, Johnson EM, Barnes RA. The evolution and evaluation of a whole blood polymerase chain reaction assay for the detection of invasive aspergillosis in hematology patients in a routine clinical setting. Clin Infect Dis. 2006;42:479–486. doi: 10.1086/499949. [DOI] [PubMed] [Google Scholar]
- Cesaro S, Stenghele C, Calore E, Franchin E, Cerbaro I, Cusinato R, Tridello G, Manganelli R, Carlli M, Palu G. Assessment of the lightcycler PCR assay for diagnosis of invasive aspergillosis in paediatric patients with onco-haematological diseases. Mycoses. 2008;51:497–504. doi: 10.1111/j.1439-0507.2008.01512.x. [DOI] [PubMed] [Google Scholar]
- White PL, Barton R, Guiver M, Linton CJ, Wilson S, Smith M, Gomez BL, Carr MJ, Kimmitt PT, Seaton S, Rajakumar K, Holyoake T, Kibbler CC, Johnson E, Hobson RP, Jones B, Barnes RA. A consensus on fungal polymerase chain reaction diagnosis?: a United Kingdom-Ireland evaluation of polymerase chain reaction methods for detection of systemic fungal infections. J Mol Diagn. 2006;8:376–384. doi: 10.2353/jmoldx.2006.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PL, Perry MD, Loeffler J, Melchers W, Klingspor L, Bretagne S, McCulloch E, Cuenca-Estrella M, Finnstrom N, Donnelly JP, Barnes RA. European Aspergillus PCR Initiative. Critical stages of extracting DNA from Aspergillus fumigatus in whole-blood specimens. J Clin Microbiol. 2010;48:3753–3755. doi: 10.1128/JCM.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano-Mori Y. Fungal infections after hematopoietic stem cell transplantation. Int J Hematol. 2010;91:576–587. doi: 10.1007/s12185-010-0574-0. [DOI] [PubMed] [Google Scholar]
- Halliday C, Hoile R, Sorrell T, James G, Yadav S, Shaw P, Bleakley M, Bradstock K, Chen S. Role of prospective screening of blood for invasive aspergillosis by polymerase chain reaction in febrile neutropenic recipients of haematopoietic stem cell transplants and patients with acute leukaemia. Br J Haematol. 2006;132:478–486. doi: 10.1111/j.1365-2141.2005.05887.x. [DOI] [PubMed] [Google Scholar]
- Loffler J, Hebart H, Sepe S, Schumcher U, Klingebiel T, Einsele H. Detection of PCR-amplified fungal DNA by using a PCR-ELISA system. Med Mycol. 1998;36:275–279. doi: 10.1080/02681219880000441. [DOI] [PubMed] [Google Scholar]
- Somogyvari F, Horvath A, Serly J, Majoros H, Vagvolgyi C, Peto Z. Detection of invasive fungal pathogens by real-time PCR and high-resolution melting analysis. In Vivo. 2012;26:979–983. [PubMed] [Google Scholar]
- White PL, Mengoli C, Bretagne S, Cuenca-Estrella M, Finnstrom N, Klingspor L, Melchers WJ, McCulloch E, Barnes RA, Donnelly JP, Loeffler J. European Aspergillus PCR Initiative. Evaluation of Aspergillus PCR protocols for testing serum specimens. J Clin Microbiol. 2011;49:3843–3848. [Google Scholar]
- Millon L, Grenouillet F, Legrand F, Loewert S, Bellanger AP, Gbaguidi-Haore H, Scherer E, Henon T, Rohrlich P, Deconinck E. Ribosomal and mitochondrial DNA target for real-time PCR diagnosis of invasive Aspergillosis. J Clin Microbiol. 2011;49:1058–1063. doi: 10.1128/JCM.01904-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Choi JH, Choi SM, Lee DG, Shin WS, Min WS, Kim CC. Application of nucleic acid sequence-based amplification for diagnosis of and monitoring the clinical course of invasive aspergillosis in patients with hematologic diseases. Clin Infect Dis. 2005;40:392–398. doi: 10.1086/427284. [DOI] [PubMed] [Google Scholar]
- Bašková L, Buchta V. Laboratory diagnostics of invasive fungal infections: an overview with emphasis on molecular approach. Folia Microbiol. 2012;57:421–430. doi: 10.1007/s12223-012-0152-3. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Bibby DF, Wong S, Agrawal SG, Bustin SA. MIQE-compliant real-time PCR assay for Aspergillus detection. PLoS One. 2012;7:e40022. doi: 10.1371/journal.pone.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Sugita S, Watanabe K, Shimizu N, Mochizuki M. Novel diagnosis of fungal endophthalmitis by broad-range real-time PCR detection of fungal 28S ribosomal DNA. Graefes Arch Clin Exp Ophthalmol. 2012;250:1877–1883. doi: 10.1007/s00417-012-2015-7. [DOI] [PubMed] [Google Scholar]
- Einsele H, Hebart H, Roller G, Loffler J, Rothenhofer I, Muller CA, Bowden RA, van Burik J, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EC, Leeming JP, Palmer HM, Steward CG, Warnock D, Marks DI, Millar MR. Diagnosis of invasive aspergillosis in bone marrow transplant recipients by polymerase chain reaction. Br J Haematol. 2000;108:132–139. doi: 10.1046/j.1365-2141.2000.01795.x. [DOI] [PubMed] [Google Scholar]
- Loeffler J, Hebart H, Brauchle U, Schumacher U, Einsele H. Comparison between plasma and whole blood specimens for detection of Aspergillus DNA by PCR. J Clin Microbiol. 2000;38:3830–3833. doi: 10.1128/jcm.38.10.3830-3833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsikas DA, Morin A, Araf S, Murtagh B, Johnson G, Vinnicombe S, Ellis S, Suaris T, Wilks M, Doffman S, Agrawal SG. Impact of the revised (2008), EORTC/MSG definitions for invasive fungal disease on the rates of diagnosis of invasive aspergillosis. Med Mycol. 2012;50:538–542. doi: 10.3109/13693786.2011.630040. [DOI] [PubMed] [Google Scholar]
- Mengoli C, Cruciani M, Barnes RA, Loeffler J, Donnelly JP. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:89–96. doi: 10.1016/S1473-3099(09)70019-2. [DOI] [PubMed] [Google Scholar]
- Springer J, Schloßnagel H, Heinz W, Doedt T, Soeller R, Einsele H, Loeffler J. A novel extraction method combining plasma with whole blood fraction shows excellent sensitivity and reproducibility in patients at high risk for invasive aspergillosis. J Clin Microbiol. 2012;50:2585–2591. doi: 10.1128/JCM.00523-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch E, Ramage G, Rajendran R, Lappin DF, Jones B, Warn P, Shrief R, Kirkpatrick WR, Patterson TF, Williams C. Antifungal treatment affects the laboratory diagnosis of invasive aspergillosis. J Clin Pathol. 2012;65:83–86. doi: 10.1136/jcp.2011.090464. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Porcher R, Menotti J, Poirot JL, Chagnon K, Vekhoff A, Cornet M, Isnard F, Raffoux E, Brethon B, Lacroix C, Touratier S, Latgé JP, Bouges-Michel C, Tazi A, Derouin F, Ribaud P, Sulahian A. Prospective evaluation of clinical and biological markers to predict the outcome of invasive pulmonary Aspergillosis in hematological patients. J Clin Microbiol. 2012;50:823–830. doi: 10.1128/JCM.00750-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretagne S. Primary diagnostic approaches of invasive Aspergillosis -molecular testing. Medical Mycology Month. 2011;49:S48–S53. doi: 10.3109/13693786.2010.508186. [DOI] [PubMed] [Google Scholar]
- Xu MZ. Antigen and molecular biology research on early diagnosis of invasive Aspergillosis in patient with hematologic malignancie. 2009. http://d.wanfangdata.com.cn/Thesis_Y1483956.aspx.
- Cuenca-Estrella M, Meije Y, Diaz-Pedroche C, Gomez-Lopez A, Buitrago MJ, Bernal-Martinez L, Grande C, Juan RS, Lizasoain M, Rodriguez-Tudela JL, Aguado JM. Value of serial quantification of fungal DNA by a real-time PCR-based technique for early diagnosis of invasive Aspergillosis in patients with febrile neutropenia. J Clin Microbiol. 2009;47:379–384. doi: 10.1128/JCM.01716-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive Aspergillosis. Lancet Infect Dis. 2004;4:349–357. doi: 10.1016/S1473-3099(04)01045-X. [DOI] [PubMed] [Google Scholar]
- Morrissey CO, Chen SC, Sorrell TC, Bradstock KF, Szer J, Halliday CL, Gilroy NM, Schwarer AP, Slavin MA. Design issues in a randomized controlled trial of a pre-emptive versus empiric antifungal strategy for invasive Aspergillosis in patients with high-risk hematologic malignancies. Leuk Lymphoma. 2011;52:179–193. doi: 10.3109/10428194.2010.542600. [DOI] [PubMed] [Google Scholar]
- Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary Aspergillosis. Infect Immun. 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino VR, Nagel F, Andreolla HF, de-Paris F, Xavier MO, Goldani LZ, Denning DW, Pasqualotto AC. The performance of real-time PCR, Galactomannan, and fungal culture in the diagnosis of invasive Aspergillosis in ventilated patients with chronic obstructive pulmonary disease (COPD) Mycopathologia. 2012;174:163–169. doi: 10.1007/s11046-012-9531-1. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L. Invasive mycoses: diagnostic challenges. Am J Med. 2012;125(Suppl):S14–S24. doi: 10.1016/j.amjmed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Springer J, Morton CO, Perry M, Heinz WJ, Paholcsek M, Alzheimer M, Rogers TR, Barnes RA, Einsele H, Loeffler J, White PL. Comparison of serum and whole blood specimens for the detection of Aspergillus DNA in high-risk haematological patients: a multicenter evaluation. J Clin Microbiol. 2013. Epub ahead of print. [DOI] [PMC free article] [PubMed]


