Abstract
Contingency management (CM) is a powerful behavioral intervention that has been shown to reduce the use of a wide variety of substances including tobacco. Use of CM techniques for smoking cessation has been restricted by the use of multiple daily measurements of breath CO as the objective indicator to reinforce abstinence. Cotinine, with its longer half-life, may be a better marker. We evaluated the use of urine cotinine (determined using once-daily semiquantitative Immunoassay Test Strips and verified using quantitative GC/HPLC techniques) as an abstinence indicator in treatment seeking adult and adolescent smokers participating in a CM-based intervention program. The results indicate that both techniques of determining urine cotinine were highly sensitive and moderately specific at detecting abstinence, and were highly concordant. However, specificity was somewhat lower during the first few days of a quit attempt and improved over time. The results were similar in adults and adolescent smokers and suggest that during the first few days of a quit attempt it would be advisable to continue to use daily multiple CO measurements to verify abstinence. However, once abstinence is achieved, once daily immunoassay test strips could be used for continued monitoring of urine cotinine levels. Immunoassay testing can identify individuals who relapse to smoking, though this study cannot evaluate whether the strips can identify resumption of abstinence. These results suggest that the use of cotinine as an abstinence indicator, by reducing the number of daily appointments, could significantly enhance the feasibility and utility of CM-based interventions for smoking cessation.
Introduction
Contingency management (CM) approaches have revolutionized the field of behavioral therapies for substance use disorders, as they have demonstrated the ability to retain patients in treatment and foster stable periods of abstinence (Carroll & Onken, 2005; Higgins & Silverman, 1999). CM interventions are based on two simple principles: first, substance use is an operant behavior, in as much as the reinforcing effects of the drug maintain substance use; and second, that substance use can be decreased by the availability of potent non-drug reinforcers (Higgins et al., 1994; Petry, 2000). CM interventions for substance use have three basic requirements: 1) Obtain objective (generally biochemical) evidence of abstinence from drugs, 2) Provide immediate and tangible reinforcers (e.g., money, goods, privileges) when abstinence is demonstrated and 3) Withhold reinforcement when the evidence indicates non-abstinence (see review in Petry, 2000).
CM techniques have been employed to reduce the use of substances including benzodiazepines (Stitzer, Bigelow, & Liebson, 1979), cocaine (Petry et al., 2001), opioids (Carroll et al., 2001; Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002) and alcohol (Petry, Martin, Cooney, & Kranzler, 2000; Petry et al., 2001). CM techniques have also been used to reduce smoking in non-treatment seeking adults (Alessi, Badger, & Higgins, 2004; Burling, Stitzer, Bigelow, & Russ, 1982; Roll, Higgins, & Badger, 1996; Schmitz, Rhoades, & Grabowski, 1995; Stitzer & Bigelow, 1983, 1985) for short time periods; also, CM has demonstrated efficacy in inducing smoking reductions in non-treatment seeing adolescents (Roll et al., 1996) and in aiding abstinence in adolescents attempting smoking cessation (Krishnan-Sarin et al., 2006; Roll, 2005).
Despite the demonstrated efficacy of CM techniques to achieve smoking abstinence, this approach is rarely used in smoking cessation programs. This is partly because of the need for biochemical confirmation of abstinence (Lando, 1989; Lichtenstein, 1982; West, Hajek, Stead, & Stapleton, 2005), as the efficacy of CM is undermined when non-abstinence is rewarded. Until recently, the best method for determining abstinence from cigarettes was measurement of breath Carbon Monoxide (CO) levels. CO has a relatively short half-life of 2 hours, which makes multiple daily appointments necessary to verify abstinence. While multiple assessments using CO conveys the advantage of detecting early abstinence, the feasibility of CM techniques for smoking cessation were limited considerably when only CO could be used to verify abstinence. A number of investigators have evaluated the use of less intensive CM-based programs in treatment-seeking adult smokers but have reported much lower rates of abstinence (Paxton, 1980; Rand, Stitzer, Bigelow, & Mead, 1989; Shoptaw, Jarvik, Ling, & Rawson, 1996) than those found with more intensive techniques (e.g., Stitzer & Bigelow, 1985).
As an alternative to CO, biochemical verification of abstinence from cigarettes can be obtained through measurement of cotinine levels via urine, plasma or saliva. Cotinine is the principal metabolite of nicotine, and is has a longer half-life (18 hrs) than CO or nicotine (both 2 hours). Cotinine has superior sensitivity and specificity in measuring tobacco use when compared with CO levels (Gariti et al., 2002), and it is a reliable indicator of recent nicotine intake. Until recently, cotinine levels were measured using laboratory-based GC quantitative techniques which were expensive, required special equipment and made immediate reinforcement of abstinence impracticable. However, the recent availability of semiquantitative urine cotinine immunoassay test strips has provided the smoking cessation field with an alternative method of verifying abstinence. The semi-quantitative immunoassay test strips are easy to utilize, provide rapid (10–20 minutes) results and do not require special equipment. Investigations have found that the immunoassay test strips are highly effective at classifying smokers and non-smokers, when compared to quantitative cotinine assessment (Gariti et al., 2002; Parker et al., 2002). These studies, however, have not compared quantitative and immunoassay test strip cotinine levels to CO levels, which are regularly used as a biochemical marker for recent smoking. Furthermore, no studies have examined the utility of immunoassay test strip assessment in adolescent smokers.
In the present study, we evaluated the diagnostic validity of the immunoassay test strips in verifying abstinence in smokers participating in a CM-based smoking cessation program, and also confirmed rates of abstinence with quantitative cotinine levels. We examined both adult and adolescent smokers to determine differences in the sensitivity of using urine cotinine to verify abstinence. Thus, this investigation is unique in its cross-validation of immunoassay test strip cotinine measurement against quantitative urine cotinine levels and exhaled CO levels in both adults and in adolescent smokers seeking treatment for smoking cessation.
Methods
Procedures
The adult project used CM procedures in combination with a daily cognitive behavioral therapy (CBT)-based behavioral intervention for the first eight days of abstinence. The adolescent study used CM procedures in combination with weekly CBT sessions for four weeks. In order to present evidence from comparable time periods, we will present data from the first week of abstinence in both studies. The CM procedures for both projects involved reinforcement of abstinence on a progressively increasing schedule adapted from Roll et al (1996). Abstinence was verified twice or thrice daily using breath CO levels. Urine samples for determination of cotinine levels were obtained at one of the daily appointments and urine cotinine levels were immediately determined using immunoassay test strips (see below) and the samples were also sent out for confirmatory quantitative cotinine analyses.
CO was measured in parts per million using the Breath Vitalograph BreathCO, (Vitalograph Inc., Lenexa, KS), which detects CO in exhaled breath. This instrument produces no cross sensitivity to potential contaminants. Semiquantitative urine cotinine levels were measured using the “Nicometer” immunoassay strips (NIS) purchased from Jant Pharmacal Corporation (Enrico, CA). These immunoassay strips utilize antibodies specific to cotinine, and produce results indicating a range for the individual’s cotinine level. The test strips used in this investigation have 6 levels, which are as follows: 0 = 0–100ng/ml, 1 = 100–250 ng/ml, 2 = 250–1000 ng/ml, 3 = 1000–2000 ng/ml, 4 = 2000–5000 ng/ml, 5 = 5000–10000 ng/mL, 6 ≥10,000 ng/mL; according to the package insert, values of 0–2 indicate no use of tobacco products while values of 3 or greater indicate tobacco use (Jant Pharmacal Corporation, n.d.). Quantitative urine cotinine (QUC) levels in the adult study were analyzed in the Department of Laboratory Medicine at Yale using reverse-phased HPLC techniques modified from the procedures of Hariharan and collaborators (1988). Quantitative urine cotinine levels in the adolescent study were analyzed using Gas Chromatography techniques at Graham-Massey Analytical Labs (Shelton, CT).
Data Analysis
For the purpose of data analyses, receiver operating characteristic (ROC) curves were plotted and utilized (for a review, please see either Eng, 2005; or Greenberg, Daniels, Flanders, Eley, & Boring, 2005). ROC curves calculate the sensitivity and specificity of the measure in question to a predefined “gold standard” for the presence or absence of a condition (here, recent smoking). ROC curves allow for continuous data to be analyzed, which is an advantage over traditional sensitivity and specificity analyses that require predetermined cut scores. Analysis includes the calculation of the area under the curve (AUC) for the ROC curve, with a .5 AUC value signifying a test that produces equal numbers of true and false positive test results. The closer a test’s AUC value comes to 1.0, the more valid it is as a measure, with great numbers of true positives and correspondingly few false positives. Non-parametric statistical significance is calculated by testing the null hypothesis that the true AUC value is equal to 0.5 for the measure being tested.
For this paper, we decided to use CO as the primary reference criterion since it is commonly used as the “gold standard” for abstinence in CM studies (e.g., Heil, Tidey, Holmes, Badger, & Higgins, 2003). While CO has a short half-life (2 hours), this standard can be used as a gold standard in CM trials that employ measurement many times per day. Nonetheless, there is no definitive test for recent smoking, so it should be noted that CO is a relative gold standard. For CO, abstinence was defined as a level ≤ 8ppm (SRNT Subcommittee on Biochemical Verification, 2002). Both QUS and NIC were compared to the CO gold standard value, and these comparisons were used to construct ROC curves for both measures of cotinine. We determined overall (i.e., all samples combined) as well as daily ROC curves over the eight-day abstinence period. In addition, given evidence that cotinine levels may need 3 or 4 days of no tobacco use to reach levels indicative of abstinence (Schepis et al., 2007), ROC curves were constructed for the assessment days past day 3 (i.e., day 4 until termination of the trial). Finally, correlations were conducted between the three biochemical measures (parametric between CO and QUC; non-parametric for those involving NIS) to investigate concordance between the measures.
Results
Participants
Subjects were 115 adult and 43 adolescent treatment-seeking smokers participating in two different studies. Both projects were approved by the Yale Institutional Review Board. The adult sample consisted of 56 males and 59 females, with an average age of 39.42 years (SD = 11.12), smoking an average of 22.28 (SD = 9.34) cigarettes/day with mean baseline intake urine cotinine levels of 1689.35 (SD = 893.43; range = 405–4486) ng/mL and average FTND scores of 6.49 (SD = 2.14). The adolescent sample consisted of 24 males and 19 females, with an average age of 16.20 years (SD = 1.23), smoking an average of 15.30 (SD = 6.93) cigarettes/day with baseline intake urine cotinine levels of 861.03 ng/mL (SD = 524.93; range = 268–2514) and a mean FTND score of 4.39 (SD = 1.39). Adult smokers were recruited from communities in New Haven County through newspaper and other advertising sources. Adolescent smokers were recruited from high schools in New Haven County. A total of 853 sets of urine cotinine and CO samples were available from the adult study a total of 640 sets were available from the adolescent study.
Attendance
The overall percentage of attended appointments in the adult participants was 81.7%, with the highest attendance rate on day 1 (92.2%) and the lowest on day 7 (73.9%). The attendance rate on days 1 through 8 in adults was: 92.2% (day 1), 87.0% (day 2), 85.2% (day 3), 82.6% (day 4), 80% (day 5), 76.5% (day 6), 73.9% (day 7), and 76.5% (day 8), respectively. In the adolescent participants, the overall attendance rate across the first 8 days of treatment was 75.9%. The attendance rate on days 1 through 8 in adolescents was: 86% (day 1), 83.7% (day 2), 83.7% (day 3), 81.4% (day 4), 67.4% (day 5), 69.8% (day 6), 58.1% (day 7), and 65.1% (day 8), respectively.
Adult Outcomes
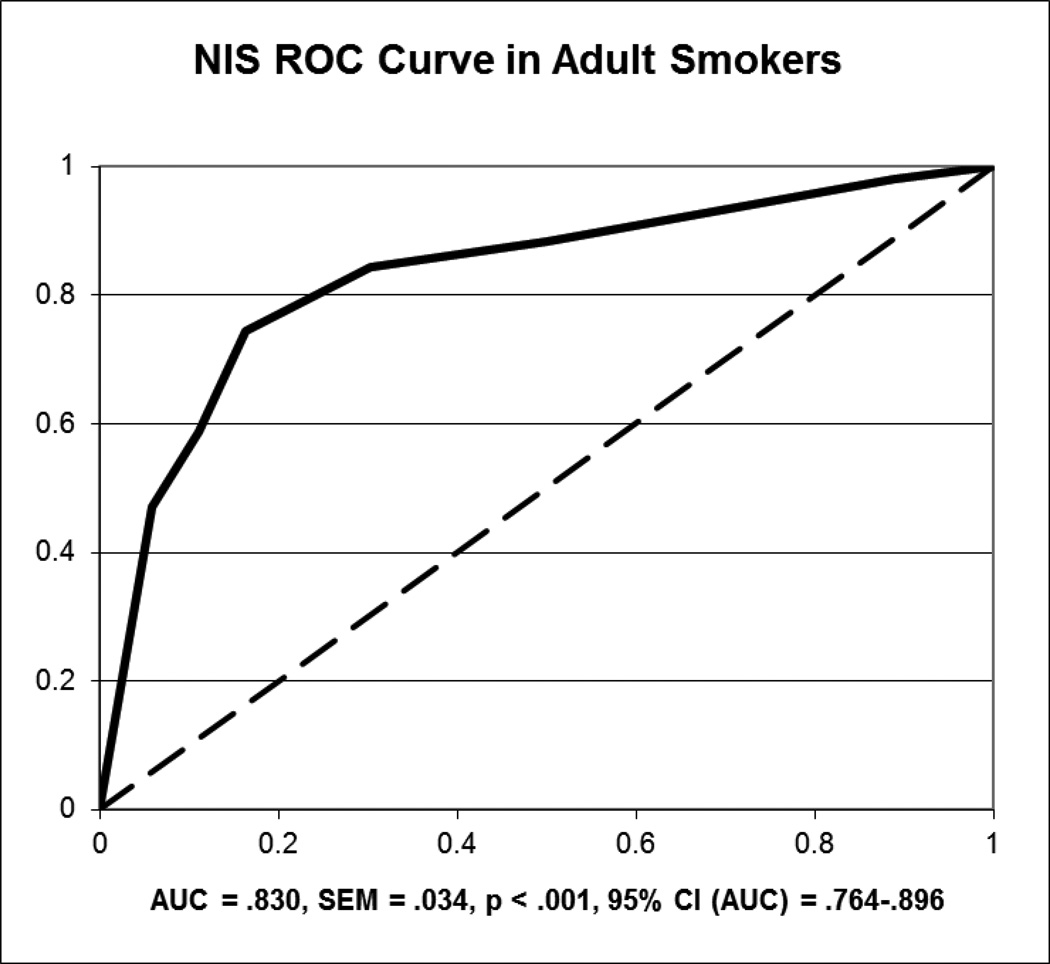
In adult smokers, the AUC values were .830 (95% CI: .764–.896; SEM = .034) for NIS (Figure 1) and .930 (95% CI: .909–.951; SEM = .011) for QUC for the duration of the 8 day trial. Both AUC values were significantly greater than .5 (ps < .001), indicating non-chance classification of abstinent and non-abstinent adults. All correlations between the biochemical measures for the entire sample were significant at a p-level below .001. Specifically, they were .665 between CO and QUC, .837 between QUC and NIS, and .338 between NIS and CO. The AUC values for both QUS (.833; 95% CI: .719–.947; SEM = .058; p < .001) and NIS (.611; 95% CI: .416–.805; SEM = .099; p = .234) were lower when examining days 4 through 8 of the trial; correlations over this period were .184 between CO and QUC (p < .001), .566 between QUC and NIS (p < .001), and .131 between NIS and CO (p = .03).
Figure 1.
ROC Curve for Nicometer immunoassay test strip sensitivity and specificity in adult smokers over the eight-day abstinence period, using CO as the “gold standard”. The red line in the figure depicts the recommended cutoff of
In examining the data from the ROC curve using CO as the reference standard, the sensitivity and specificity values for NIS were .882 and .500, respectively, when the cutoff was ≤ 1. The positive predictive value (PPV) for NIS was 17.5% and the negative predictive value (NPV) for NIS was 97.2%. For QUS ≥ 100 ng/ml, the sensitivity was .982, specificity was .554, the PPV was 34.3% and the NPV was 99.2%. In examining only days 4 through 8, the sensitivity and specificity values were .400 and .743 for NIS and .818 and .792 for QUS, using the predetermined cutoff values. Over this time period, the PPV and NPV were 20% and 95.2% for NIS and 38% and 98% for QUC.
Figure 2 presents the changes in NIS levels in adult smokers who were either abstinent (n = 47) or in those who quit smoking for a minimum of one day but relapsed to smoking during the 7-day period (n = 33) as determined using CO levels ≤ 8ppm. As can be observed, cotinine levels steadily decline in both groups of smokers, reaching levels below 100 ng/mL by day 6 in abstinent smokers. In contrast, non-abstinent smokers NIS values tended to rise after day 5, and the mean value did not reach 100 ng/mL or below during the 8 day period. Importantly, this figure appears to indicate the ability to detect “relapse” to smoking using the NIS, as levels never fell below the 100 ng/mL cutoff in “relapsed” participants.
Figure 2.
Change in Nicometer immunoassay test strip levels over the eight-day abstinence period in adult smokers.
Adolescent Outcomes
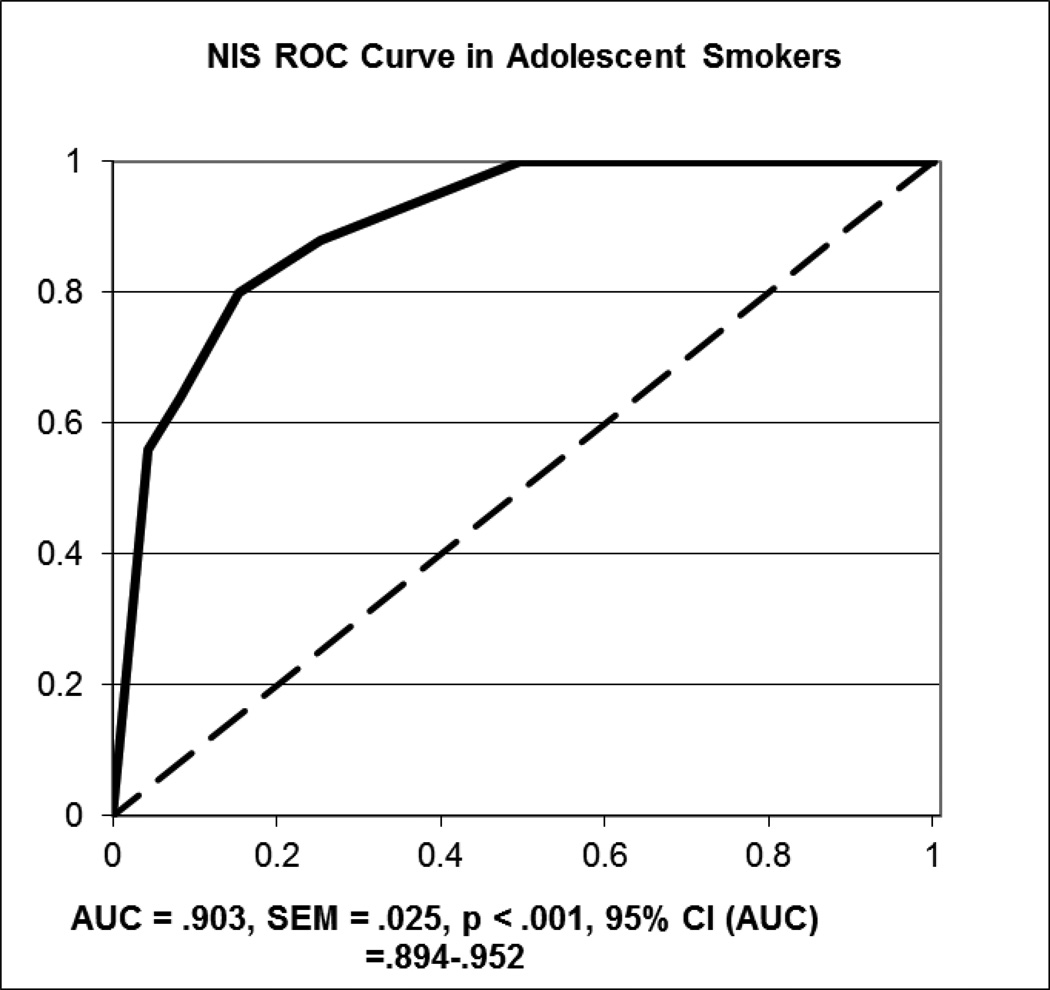
In adolescent smokers, the AUC values were .903 (95% CI: .894–.952; SEM = .025) for NIS (Figure 3) and .868 (95% CI: .776–.960; SEM = .047) for QUC for the duration of the 8 day trial; both values demonstrated classification that was significantly better than chance (ps < .001). The correlations between the biochemical measures of recent smoking were .518 between QUC and CO, .344 between NIS and CO, and .738 between NIS and QUC (all ps < .001). In examining data from day 4 of the trial onward, the AUC values were generally comparable to the values for the entire set: .892 for NIS (95% CI: .815–.970; SEM = .040; p < .001) and .919 for QUC (95% CI: .796–1.042; SEM = .063; p = .044).
Figure 3.
ROC Curve for Nicometer immunoassay test strip sensitivity and specificity in adolescent smokers over the initial fourteen-day treatment period, using CO as the “gold standard”.
In examining the data from the ROC curve using CO as the reference standard, the sensitivity and specificity values for NIS were 1.0 and .504, respectively, when the cutoff was ≤ 1. For NIS, the PPV was 100% and the NPV was 50.4%. For QUS ≥ 100 ng/mL, the sensitivity was 1.0, the specificity was .562, the PPV was 15.5% and the NPV was 100%. In examining only days 4 through 8, the sensitivity and specificity values were 1.0 and .593, and the PPV and NPV were 100% and 1.6% for NIS; for QUS, the sensitivity and specificity were 1.0 and .737, and the PPV and NPV were 18.4% and 100%.
Figure 4 displays the changes in NIS values over the first 14 days cessation treatment, with 29 participants abstinent throughout and 8 non-abstinent. As with the adult sample, non-abstinence was defined as a CO level > 8 ppm following at least one abstinent sample (CO ≤ 8 ppm). As with the adult sample, the mean NIS value in the abstinent sample fell relatively steadily throughout the trial and the mean NIS value in the non-abstinent sample rose following day 7, never descending below 100 ng/mL.
Figure 4.
Change in Nicometer immunoassay test strip levels over the initial fourteen-day treatment period in adolescent smokers.
Discussion
The results of this study indicate that immunoassay strips like “Nicometer” can be used to verify abstinence in CM trials for smoking cessation. The test strips displayed high sensitivity and moderate specificity in identifying smoking status verified by CO levels and were in high agreement with quantitative urine cotinine levels determined using more time-consuming GC and HPLC techniques. Sensitivity and specificity scores were similar in both adolescent and adult smokers.
However, there are some caveats to the use of these test strips. Specifically, the specificity of these strips in correctly identifying abstinence may be impacted during the first 4–5 days of abstinence. Given the relatively long half-life of cotinine (18 hours), abstinent participants may appear to be currently smoking until cessation has stretched multiple days. These results are consistent with the recent findings of Acosta and colleagues (2004) who examined the utility of the urine immunoassay test strips as an index of smoking status in temporarily abstaining smokers during 96-hours of reinforced abstinence. They reported that the NIS had high sensitivity but weak specificity in identifying abstinence, a similar finding to those here. It is especially notable that the findings of this study and Acosta and collaborators (2004) were generally analogous, as they had relatively low levels of abstinence while we had very high levels of abstinence since all participants were treatment-seeking individuals participating in a smoking cessation program. In all, both studies reveal limitations to the use of the NIS during short-term abstinence.
Because of the long half-life of cotinine, it is likely best to avoid relying solely on urine cotinine levels to verify abstinence during the first 3–5 days of an abstinent period. It would be advisable to continue to use a more intensive approach using multiple daily CO levels to confirm and reinforce abstinence in CM trials during the first few days of a cessation attempt. If an individual remains abstinent during the first 3 or 4 days, urine cotinine levels should consistently decline and reach levels indicating abstinence using NIS. After day 4, abstinence could be determined using once daily urine dipstick levels. Our results indicate that the test strips are able to pick up increases in levels due to “lapse” in smoking. However, we did not have sufficient data to evaluate the ability of the NIS to pick up on resumption of abstinence after a “lapse” in smoking and therefore cannot provide guidance on how long it will take for the strips to detect abstinence again. However, a conservative approach may be to re-institute frequent CO monitoring upon a lapse for the purposes of abstinence determination.
Two limitations of the current study should be noted. First, the CO criterion for abstinence was set at a level of less than or equal to 8 ppm, which is consistent with the recommendations of the SRNT Subcommittee on Biochemical Verification (SRNT Subcommittee on Biochemical Verification, 2002). Nonetheless, this cut-off could allow for some individuals to smoke sporadically and avoid detection using CO, given the short half-life of CO. If this were to occur, it might appear that NIS was incorrectly identifying individuals as smokers when, in actuality, CO was producing a false negative. Second, the research protocol employed here required individuals to attend two or three daily clinic appointments for abstinence verification. This design, while ideal for collecting data to evaluate the utility of NIS, would be harder for clinical settings to carry out. Furthermore, many motivated individuals who want to attempt cessation may not be willing or able to attend multiple daily visits to assess abstinence. However, despite the requirement that participants attend multiple daily assessment sessions, adult participants in this investigation attended 81.7% of visits and adolescent participants attended 75.9%, indicating that it is possible to have high clinic attendance in a program requiring daily attendance. Reducing the number of daily visits to one by using NIS assessment may further increase the feasibility of such a CM-based smoking cessation program.
In summary, by reducing the number of contacts required to verify abstinence, the use of immunoassay test strips to determine urine cotinine levels could significantly increase the feasibility of conducting trials evaluating the use of CM techniques for smoking cessation in both adult and adolescent smokers. However, it is recommended that during the initiation of the quit period, multiple CO measurements (in conjunction with test strips) might still be the best measure of abstinence to use for the purposes of reinforcement in CM trials. Given the efficacy of CM techniques in fostering abstinence, it is hoped that these results will improve the feasibility of using CM as an intervention for smoking cessation and encourage further investigations into the use of CM techniques for smoking cessation in both adults and adolescents.
Acknowledgements
This research supported by NIH grants P50DA13334, P50DA09421 and T32DA007238.
Footnotes
No conflicts of interest were noted by any of the authors.
REFERENCES
- Acosta MC, Buchhalter AR, Breland AB, Hamilton DCP, Eissenberg T. Urine cotinine as an index of smoking status in smokers during 96-hr abstinence: Comparison between gas chromatography/mass spectrometry and immunoassay test strips. Nicotine & Tobacco Research. 2004;6(4):615–620. doi: 10.1080/14622200410001727867. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Experimental and Clinical Psychopharmacology. 2004;12(4):276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- Burling TA, Stitzer ML, Bigelow GE, Russ NW. Techniques used by smokers during contingency motivated smoking reduction. Addictive Behaviors. 1982;7(4):397–401. doi: 10.1016/0306-4603(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence - Efficacy of contingency management and significant other involvement. Archives of General Psychiatry. 2001;58(8):755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. American Journal of Psychiatry. 2005;162(8):1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10(1):54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Eng J. Receiver operating characteristic analysis: a primer. Academic Radiology. 2005;12(7):909–916. doi: 10.1016/j.acra.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gariti P, Rosenthal DI, Lindell K, Hansen-Flaschen J, Shrager J, Lipkin C, et al. Validating a dipstick method for detecting recent smoking. Cancer Epidemiology Biomarkers & Prevention. 2002;11(10):1123–1125. [PubMed] [Google Scholar]
- Greenberg RS, Daniels SR, Flanders WD, Eley JW, Boring JR., III . Medical Epidemiology. New York: McGraw-Hill; 2005. [Google Scholar]
- Hariharan M, Vannoord T, Greden JF. A High-Performance Liquid-Chromatographic Method for Routine Simultaneous Determination of Nicotine and Cotinine in Plasma. Clinical Chemistry. 1988;34(4):724–729. [PubMed] [Google Scholar]
- Heil SH, Tidey JW, Holmes HW, Badger GJ, Higgins ST. A contingent payment model of smoking cessation: Effects on abstinence and withdrawal. Nicotine & Tobacco Research. 2003;5(2):205–213. doi: 10.1080/1462220031000074864. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives Improve Outcome in Outpatient Behavioral Treatment of Cocaine Dependence. Archives of General Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K. Motivating behavior change among illicit-drug abusers. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Jant Pharmacal Corporation. Accutest® NicAlert™. [Retrieved March 6, 2006]; (n.d.). from http://www.accutest.net/products/pdf/ds47ny150.pdf.
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, et al. Contingency management for smoking cessation in adolescent smokers. Experimental and Clinical Psychopharmacology. 2006;14(3):306–310. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Lando HA. Treatment Outcome Evaluation Methodology in Smoking Cessation - Strengths and Key Issues. Advances in Behaviour Research and Therapy. 1989;11(3):201–214. [Google Scholar]
- Lichtenstein E. The Smoking Problem - a Behavioral-Perspective. Journal of Consulting and Clinical Psychology. 1982;50(6):804–819. doi: 10.1037//0022-006x.50.6.804. [DOI] [PubMed] [Google Scholar]
- Parker DR, Lasater TM, Windsor R, Wilkins J, Upegui DI, Heimdal J. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine & Tobacco Research. 2002;4(3):305–309. doi: 10.1080/14622200210142715. [DOI] [PubMed] [Google Scholar]
- Paxton R. Effects of a Deposit Contract as a Component in a Behavioral-Program for Stopping Smoking. Behaviour Research and Therapy. 1980;18(1):45–50. doi: 10.1016/0005-7967(80)90068-6. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58(1–2):9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68(2):250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Petrakis I, Trevisan L, Wiredu G, Boutros NN, Martin B, et al. Contingency management interventions: From research to practice. American Journal of Psychiatry. 2001;158(5):694–702. doi: 10.1176/appi.ajp.158.5.694. [DOI] [PubMed] [Google Scholar]
- Rand CS, Stitzer ML, Bigelow GE, Mead AM. The Effects of Contingent Payment and Frequent Workplace Monitoring on Smoking Abstinence. Addictive Behaviors. 1989;14(2):121–128. doi: 10.1016/0306-4603(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Roll JM. Assessing the feasibility of using contingency management to modify cigarette smoking by adolescents. Journal of Applied Behavior Analysis. 2005;38(4):463–467. doi: 10.1901/jaba.2005.114-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29(4):495–504. doi: 10.1901/jaba.1996.29-495. quiz 504-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Liss T, McFetridge A, Cavallo D, Smith A, Cooney JL, et al. Comparison of salivary and urine cotinine to expired CO as biomarkers of abstinence from smoking; Paper presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco.2007. [Google Scholar]
- Schmitz JM, Rhoades H, Grabowski J. Contingent Reinforcement for Reduced Carbon-Monoxide Levels in Methadone-Maintenance Patients. Addictive Behaviors. 1995;20(2):171–179. doi: 10.1016/0306-4603(94)00059-x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors. 1996;21(3):409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent Payment for Carbon-Monoxide Reduction - Effects of Pay Amount. Behavior Therapy. 1983;14(5):647–656. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced breath carbon monoxide levels: target-specific effects on cigarette smoking. Addictive Behaviors. 1985;10(4):345–349. doi: 10.1016/0306-4603(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE, Liebson I. Reducing Benzodiazepine Self-Administration with Contingent Reinforcement. Addictive Behaviors. 1979;4(3):245–252. doi: 10.1016/0306-4603(79)90034-0. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]