Abstract
Neurons of the hypothalamic paraventricular nucleus (PVN) are key controllers of sympathetic nerve activity and receive input from angiotensin II (ANG II)–containing neurons in the forebrain. This study determined the effect of ANG II on PVN neurons that innervate in the rostral ventrolateral medulla (RVLM)—a brain stem site critical for maintaining sympathetic outflow and arterial pressure. Using an in vitro brain slice preparation, whole cell patch-clamp recordings were made from PVN neurons retrogradely labeled from the ipsilateral RVLM of rats. Of 71 neurons tested, 62 (87%) responded to ANG II. In current-clamp mode, bath-applied ANG II (2 μM) significantly (P < 0.05) depolarized membrane potential from −58.5 ± 2.5 to −54.5 ± 2.0 mV and increased the frequency of action potential discharge from 0.7 ± 0.3 to 2.8 ± 0.8 Hz (n = 4). Local application of ANG II by low-pressure ejection from a glass pipette (2 pmol, 0.4 nl, 5 s) also elicited rapid and reproducible excitation in 17 of 20 cells. In this group, membrane potential depolarization averaged 21.5 ± 4.1 mV, and spike activity increased from 0.7 ± 0.4 to 21.3 ± 3.3 Hz. In voltage-clamp mode, 41 of 47 neurons responded to pressure-ejected ANG II with a dose-dependent inward current that averaged −54.7 ± 3.9 pA at a maximally effective dose of 2.0 pmol. Blockade of ANG II AT1 receptors significantly reduced discharge (P < 0.001, n = 5), depolarization (P < 0.05, n = 3), and inward current (P < 0.01, n = 11) responses to locally applied ANG II. In six of six cells tested, membrane input conductance increased (P < 0.001) during local application of ANG II (2 pmol), suggesting influx of cations. The ANG II current reversed polarity at +2.2 ± 2.2 mV (n = 9) and was blocked (P < 0.01) by bath perfusion with gadolinium (Gd3+, 100 μM, n = 8), suggesting that ANG II activates membrane channels that are nonselectively permeable to cations. These findings indicate that ANG II excites PVN neurons that innervate the ipsilateral RVLM by a mechanism that depends on activation of AT1 receptors and gating of one or more classes of ion channels that result in a mixed cation current.
INTRODUCTION
The hypothalamic paraventricular nucleus (PVN) subserves a variety of endocrine and autonomic functions (Armstrong et al. 1980; Hatton et al. 1976; Loewy 1981; Sawchenko and Swanson 1982; Toney et al. 2003). Regarding the latter, studies have established that stimulation of the PVN increases arterial pressure (AP) and sympathetic nerve activity (SNA) and causes significant renal vasoconstriction (Kannan et al. 1989; Porter and Brody 1985). These responses likely result from activation of one or more of the known PVN autonomic pathways, which terminate in the dorsomedial medulla (Loewy 1981; Sawchenko and Swanson 1982; Swanson and Kuypers 1980), the spinal intermediolateral cell column (IML) (Cechetto and Saper 1988; Sawchenko and Swanson 1982; Swanson and McKellar 1979; Tucker and Saper 1985), and the rostral ventrolateral medulla (RVLM) (Luiten et al. 1985; Pyner and Coote 1999; Tucker and Saper 1985). Although the latter pathway seems to excite reticulo-spinal vasomotor neurons whose activity is critical for maintenance of ongoing SNA and resting AP (Pyner and Coote 1999; Yang and Coote 1998), their electrophysiological properties and responses to transmitters/modulators known to target the PVN have not been fully explored. It should be noted, however, that a recent in vitro study by Li et al. (2003b) indicates that the discharge of PVN-RVLM neurons is tonically suppressed by NO-induced facilitation of GABAergic activity.
A principal source of afferent input to the PVN is the forebrain lamina terminalis (Camacho and Philips 1981; Johnson et al. 1996; McKinley et al. 1992). A substantial population of neurons in the subfornical organ, organum vasculosum, and median preoptic nucleus contain the peptide transmitter angiotensin II (ANG II) (Lind et al. 1985; Wright et al. 1993). There is widespread recognition that these cells convey both cardiovascular and body fluid regulatory information to the PVN (McKinley et al. 1992). Despite functional evidence that central actions of ANG II increase AP (Ferguson and Washburn 1998; Toney and Porter 1993) and renal SNA (Falcon et al. 1978; Ferguson and Washburn 1998), the neural pathways and mechanisms of action of ANG II in the CNS are not fully understood. What seems apparent, however, is that responses depend on the integrity of PVN neurons (Gutman et al. 1988; see also Mangiapani and Simpson 1980). More specifically, PVN neurons innervating the spinal IML may contribute to sympathetic and cardiovascular responses since in vivo electrophysiological studies have reported that these cells are activated by ANG II inputs from the forebrain (Bains and Ferguson 1995; Bains et al. 1992)—an effect that has been confirmed recently using patch-clamp electrophysiology in vitro (Li et al. 2003a). The goal of this study was to determine the ANG II responsiveness of PVN neurons that innervate the RVLM. Brain slices were prepared from rats and whole cell patch-clamp recordings were performed in vitro from PVN neurons retrogradely labeled from the ipsilateral RVLM. Results indicate that a vast majority (87%) of PVN neurons innervating the RVLM are excited by ANG II through an AT1 receptor-dependent mechanism and activation of a mixed cation current. The latter could reflect activation of multiple channel subtypes. Some of these results have been presented previously (Toney et al. 2002 2003).
METHODS
Experiments were performed using tissue from 42 male Sprague-Dawley rats (100–120 g, Charles River Labs, Wilmington, MA). All procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio.
Retrograde labeling of PVN neurons
Rats were anesthetized with pentobarbital sodium (65 mg/kg, ip) and placed in a stereotaxic head frame. A glass micropipette (tip: ~35 μm) was filled with rhodamine-labeled fluorescent microspheres (Lumafluor, Naples, FL) and lowered into the ventrolateral medulla using the following coordinates (in mm): −10.8 AP (bregma), 1.6 ML, and −9.5–9.7 DV (skull surface). Each injection consisted of a 50-nl volume delivered slowly (~2 min) by pressure ejection. The volume of each injection was determined by measuring the movement of the fluid meniscus in the pipette through a dissecting microscope (80×) equipped with an eyepiece reticule. After each injection, the pipette was left in place for 5 min to allow for diffusion of tracer away from the injector tip and to minimize reflux along the pipette tract. Each animal received a daily injection of penicillin G (30,000 units/100 g bw, sc) for 3 days after surgery. A period of 5–7 days was allowed for recovery and retrograde transport of the tracer to the PVN.
Hypothalamic slice preparation
The method used to prepare hypothalamic brain slices was adapted from Tasker and Dudek (1991). Briefly, rats were decapitated under halothane anesthesia, and the portion of brain containing the hypothalamus was quickly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) equilibrated with a gas mixture containing 95% CO2-5% O2. Coronal sections (300 μm thick) were cut on a vibratome (Series 1000, Technical Products, St. Louis, MO) and allowed to equilibrate in a holding chamber for 1–2 h at room temperature in continuously gassed ACSF.
Electrophysiology
Following equilibration, brain slices were transferred to a glass-bottomed recording chamber (Warner Instruments, Hamden, CT) and perfused with gassed ACSF at a rate of 3.0 ml/min. All recordings were performed at room temperature (24–26°C). Slices were visualized through a fixed-stage, upright microscope (E600FN, Nikon) equipped with DIC optics, epi-fluorescence, an infrared (IR) filter, and an IR-sensitive video camera (C2400, Hamamatsu, Bridgewater, NJ). An appropriate filter cube was used to visualize neurons retrogradely labeled with rhodamine.
Patch electrodes were pulled (Flaming/Brown P-97, Sutter Instrument, Novato, CA) from borosilicate glass (type 8250, Garner Glass, Claremont, CA) and polished to a tip resistance of 4–5 MΩ. Electrodes were positioned in the PVN under visual control and advanced with a piezoelectric micropositioner (Burleigh, PCS-5000, Fishers, NY) to contact a labeled neuron. Whole cell patch-clamp recordings were digitally acquired in current- and voltage-clamp mode using an Axopatch 200B amplifier, Digidata 1322A A-D converter and pCLAMP 8.1 software (Axon Instruments, Union City, CA). Whole cell capacitance was compensated 70–80%, and series resistance was corrected so that the residual did not exceed 10 MΩ. Leak current was subtracted on-line using the P/n = 3 method. Cell capacitance was determined in voltage-clamp mode using the “Membrane Test” function in ClampEx software (v 8.1). The capacitance was calculated from the current response averaged from two pulses, each of which had a duration of 1.0 s and an amplitude of +20 mV. Pulses were generated at a frequency of 0.5 Hz from a holding potential of −80 mV. All capacitance measures were performed with pipette capacitance fully compensated and after achieving whole cell configuration. In all cases, whole cell capacitance and series resistance compensation were turned off.
Intracellular and extracellular solutions
The intracellular solution consisted of (in mM) 130 K gluconate, 10 HEPES, 5 EGTA, 1 MgCl2, 1 NaCl, 1 CaCl2, 2 K2-ATP, and 0.5 Na-GTP. The pH and osmolarity were adjusted to 7.2 and 280 mosmol/l, respectively. Normal ACSF consisted of (in mM) 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, and 10 dextrose. Where indicated, TTX (0.5 μM) was added to the bath ACSF to block voltage-gated sodium channels. In some experiments, gadolinium (Gd3+, 100 μM) was added to ACSF with the following composition (in mM): 145 NaCl, 2 KCl, 2 MgCl2, 10 HEPES, 0.1 GdCl3, 2 CaCl2, and 10 dextrose. Each bath solution change was completed using a computer-actuated, flow-through valve system (Valco Instruments, Houston, TX). The bath perfusion rate was 3 ml/min, and the chamber volume was maintained at 350 μl.
Drug application
In current-clamp mode, ANG II (2 μM) was bath-applied for a period of 3 min, and recordings were maintained to observe the full time course of evoked responses and recovery. Effects of locally applied ANG II were recorded in voltage- and current-clamp mode. Precise shearing of a three-barreled glass micropipette (3B120F-4, World Precision Instruments, Sarasota, FL) produced two low-resistance barrels with the same diameter (12.5 μm OD). The shearing process was accomplished by viewing the pulled three-barreled pipette through a microscope (20×) fitted with an eyepiece micrometer. The pipette was positioned with two barrels in the same plane and in focus to allow their diameters to be accurately measured. The third barrel was positioned below. Under microscopic control, the three-barreled pipette was inserted into the lumen of an unpulled glass capillary (0.57 ID) until the taper reached the desired diameter. By moving the capillary tube downward with an anchored micromanipulator, the two upper barrels were sheared off at the predetermined diameter. The third barrel had a somewhat variable tip diameter/shape due to apparent asymmetries in the shearing process and was not used.
In all studies using local injections, two barrels of the injector pipette were positioned in the slice at the same depth as the recorded neurons at a distance of 25 μm. For dose-response studies, the two barrels were filled with different concentrations of ANG II (0.5–7.5 mM; see following text for details). For experiments testing effects of antagonists/blockers, one pipette barrel was filled with ANG II (5 mM) alone and the other with either vehicle (normal ACSF) or a cocktail containing ANG II (5 mM) and an AT1 receptor antagonist ([Sar, Ile]-ANG II or Losartan, 10 mM). ANG II doses of 0.2, 1.0, 2.0, and 3.0 pmol were delivered in an average volume of 0.4 nl over a period of 5 s using a custom-designed, low-pressure (8–10 inches H2O or 0.36 psi) picopump (Dagan, Minneapolis, MN). The picopump was equipped with a low-pressure Norgen R07-100-NR4A regulator equipped with sensitive 4-lb spring. In addition, it has a high-resolution output pressure gauge (1–60 inches H2O) so that delivery pressure could be consistently set to 8–10 inches H2O. Finally, a low-pressure relieving diaphragm ensured that pressure did not build between puffs. In preliminary studies, we determined that each ejection delivered a consistent volume by measuring the movement of the fluid meniscus in the ejector pipette. In each of six different pipettes, we determined that the fluid meniscus moved a nearly identical distance following 25 repeated ejections each lasting 5 s. The time between ejections was fixed at 60 s. Based on the total movement of the meniscus, we calculated that each ejection delivered an average volume of 0.4 nl.
Experimental protocols
Access and membrane resistance were monitored in voltage-clamp mode by recording current responses to 20-mV depolarizing test pulses generated from a holding potential of −80 mV. Total access resistance typically averaged 15–20 MΩ and was compensated to ≤10 MΩ. Membrane potential and current responses were digitized at 5 kHz and low-pass filtered at 1 kHz.
Responses to bath-applied ANG II were recorded in current-clamp mode at resting Vm. Responses to low-pressure local application of ANG II were recorded in voltage-clamp mode with membrane potential held at −60 mV and in current-clamp mode (with and without TTX) at resting Vm. The magnitude of the baseline response of each neuron to locally applied ANG II was determined as the average of at least two nearly identical responses spaced 1 min apart.
Dose-response effects of ANG II were determined by local pressure application in voltage-clamp mode. Membrane current responses to ANG II doses of 0.2, 1.0, 2.0, and 3.0 pmol were assessed using two barrels of the three-barreled ejector pipette. One barrel was always filled with 5.0 mM ANG II and was used to deliver a 2.0-pmol dose. The other barrel was filled with either 0.5, 2.5, or 7.5 mM ANG II and was used to test one of the other doses. In this way, the response to a 0.2-, 1.0-, or 3.0-pmol dose of ANG II was paired in each cell with a response to a 2.0-pmol dose. The two doses of ANG II were delivered in random order. Effects of AT1 receptor blockade were determined in current-clamp mode (with and without TTX) and in voltage-clamp mode. The ANG II AT1 receptor antagonist [Sar, Ile]-ANG II or Losartan (4 pmol) was applied concurrently with ANG II (2 pmol) as a cocktail. Parameters used for ejection of the ANG II-AT1 antagonist cocktail were identical to those used for delivery of ANG II alone (i.e., 8–10 inches H2O, 5 s). Responses to ANG II alone (2 pmol) were recorded at intervals of 1 min to assess recovery.
Changes in membrane input conductance were determined in voltage-clamp recordings by delivering a 20-mV (50 ms) hyperpolarizing test pulse at a frequency of 4 Hz from a holding potential of −60 mV. Step changes were made during baseline, throughout the current response to locally applied ANG II (2 pmol, 5 s), and continued until membrane current returned to baseline. The reversal potential of the ANG II current was determined from a holding potential of −60 mV by stepping first to −40 mV for 100 ms (to minimize transient outward potassium currents) and imposing repeated ramp (0.14 mV/ms) depolarizations from −40 to +20 mV before, during, and after low-pressure delivery of vehicle (ACSF) or ANG II (2 pmol) for 5 s. The two current responses were subtracted, and the resulting current plotted as a function of voltage (I-V curve). The zero-current potential determined for each individual cell was averaged to determine the reversal potential for the group of cells tested.
A potential role for nonselective cation channels in mediating ANG II responses was assessed in voltage-clamp mode. Two reproducible responses to locally applied ANG II (2 pmol) were obtained before switching to bath perfusion with ACSF containing the trivalent cation Gd3+, which is known to block nonselective cation channels. Time-dependent effects of Gd3+ (100 μM) were assessed by testing responses to locally applied ANG II (2 pmol) at 1-min intervals. Recovery from effects of Gd3+ was determined at 5-min intervals following return to bath perfusion with normal ACSF.
Recovery of biocytin-filled cells
In the majority of experiments, biocytin (0.25%; Sigma, St. Louis, MO) was added to the intracellular solution to enable identification of recorded cells. Immediately after each recording, brain slices were fixed in 4% paraformaldehyde for 24 h, rinsed in 0.1 M PBS for 30 min, and incubated for 4 h in 0.3% H2O2 to quench endogenous peroxidase activity. Slices were rinsed for 30 min in PBS and incubated at room temperature for 24 h in PBS containing 0.5% Triton X-100 and avidin-peroxidase conjugate (1:2,000). Recorded neurons were stained dark blue-black by reacting slices for ~5 min in buffer containing 50 mM Tris, 0.5 mg/ml diaminobenzidine, 0.025% cobalt chloride, 0.02% nickel ammonium sulfate, and 0.006% H2O2. Slices were mounted on slides, coverslipped, and viewed through an Olympus BX60 microscope. Digital images were captured using a Spot camera (Diagnostic Instruments, Sterling Heights, MI) and Image Pro Plus software (Media Cybernetics, Silver Springs, MD).
Data analysis/statistics
A liquid junction potential of 7–10 mV was corrected off-line (Neher 1992). Whole cell current responses to locally applied ANG II were low-pass filtered (8-pole Bessel) with the −3-dB cutoff set at 500 Hz. A cell was considered to be responsive to ANG II when a 2 pmol dose elicited a consistent current response with an amplitude of ≥10 pA in voltage-clamp mode at a holding potential of −60 mV. Baseline discharge frequency (Hz) was determined from interval histograms constructed from ≥30 s of stable activity recorded prior to testing for the acute response to locally applied ANG II. Values are reported as the reciprocal of the average interval. Discharge responses to locally applied ANG II were determined using the same method, with histograms constructed from a data segment which began with the first spike and ended with the last spike of the induced burst. In voltage- and current-clamp recordings, the magnitude of the ANG II-induced response was determined as the difference between the baseline value and the maximal value that occurred during application of ANG II. Effects of ANG II on discharge frequency, membrane potential, membrane current, and input conductance were analyzed using a standard one-way ANOVA. ANG II dose-response curves were similarly analyzed. Significant interactions were analyzed for pair-wise differences using a Tukey post hoc test. Effects of AT1 receptor antagonists and Gd3+ were compared with control ANG II responses using the paired t-test. Values of summary data are reported as the means ± SE.
RESULTS
Distribution and morphology of PVN-RVLM neurons
Based on histological analysis of brain stem tissue, retrograde tracer injections were confined to the ventrolateral medulla at a location ventral to the nucleus ambiguous and ≤500 μm caudal to the facial nucleus. Tracer injections infrequently extended rostrally to include a portion of the paragigantocellular nucleus (Fig. 1A). Microinjection of the excitatory amino acid l-glutamate (1 nmol) at this site increased arterial blood pressure 25–30 mmHg (Fig. 1A, inset), indicating activation of neurons in the RVLM pressor region. Retrogradely labeled PVN cell bodies were located in the dorsal, ventrolateral, and posterior parvocellular subnuclei, with sparse/absent labeling in the magnocellular rich central subnucleus (Fig. 1B). Retrogradely labeled neurons in parvocellular subregions of PVN were recorded and labeled intracellularly (Fig. 1C). They typically had elliptical soma with one to three primary dendrites emerging from each pole. Some dendrites coursed medially and branched along the wall of the third ventricle, whereas others projected laterally/dorsally and rarely were observed to exit the PVN. In nearly every instance, the axon appeared to arise from the proximal portion of a primary dendrite. There were no differences in resting membrane potential, membrane resistance, spontaneous discharge frequency, or ANG II responsiveness between labeled neurons in different PVN subnuclei.
Fig. 1.
Experimental preparation. A: rhodamine-containing microspheres were microinjected (50 nl) into the rostral ventrolateral medulla (RVLM) at a site where prior microinjection of l-glutamate (1 nmol in 100 nl) elicited an increase in arterial pressure (inset). B: retrograde labeling of the ipsilateral paraventricular nucleus (PVN) was observed in the dorsal and ventrolateral subnuclei, but was effectively absent from the central magnocellular (CM) subnucleus. C: recorded neurons were filled with biocytin. Peroxidase staining revealed elliptical cell bodies with multiple primary dendrites. Note: each labeled cell was individually recorded and filled with biocytin. D: infrared-DIC image of PVN slice preparation showing the patch electrode positioned on a recorded neuron and the microinjector pipette positioned adjacent to the recorded neuron (left). Fluorescent image shows that the recorded (and a nearby) neuron contained retrograde tracer (right).
Effects of ANG II on discharge frequency, membrane potential, and membrane current
Neurons retrogradely labeled from the ipsilateral RVLM were selected for study (Fig. 1D). Of 71 cells recorded in current- and voltage-clamp mode, 62 responded to bath- or locally applied ANG II. Resting membrane potential and capacitance of ANG II responsive neurons averaged −56.3 ± 1.5 mV and 44.0 ± 2.1 pF, respectively. Membrane properties of neurons unresponsive to ANG II were not significantly different. Bath application of ANG II (2 μM, 3 min) in current-clamp recordings significantly (P < 0.05) depolarized membrane potential from −58.5 ± 2.5 to −54.5 ± 2.0 mV and increased discharge frequency from 0.7 ± 0.3 to 2.8 ± 0.8 Hz (n = 4). The increase in discharge typically began within 2–3 min and lasted for several minutes (range, 5–25 min) after returning to perfusion with normal ACSF (Fig. 2).
Fig. 2.
Effect of bath-applied angiotensin II (ANG II; 2 μM) on PVN-RVLM neuronal discharge. During bath perfusion with normal artificial cerebrospinal fluid (ACSF), the recorded neuron fired slowly (0.2 Hz) and irregularly at its resting membrane potential of −53 mV (Control: top sweep). Switching to ANG II-containing ACSF (2 μM) for 3 min gradually increased discharge (sweep 2), which reached a maximum of 3.0 Hz ~4 min after returning to normal ACSF (sweep 3). More than 20 min after initiating the ANG II washout, discharge had nearly returned to baseline (0.9 Hz; bottom sweep). Dashed line = 0 mV.
For local application of ANG II by low-pressure ejection, the recording electrode and multi-barreled application pipette were positioned on opposite sides of retrogradely labeled PVN neurons (Fig. 1D). Figure 3A shows representative traces of cell discharge (left column), membrane potential (middle column), and membrane current (right column) responses to locally applied ANG II (2 pmol). Discharge, membrane potential, and membrane current responses are from different cells. Individual sweeps in each recording mode were obtained 60 s apart. Note that in both current- and voltage-clamp modes, local application of ANG II (2 pmol) elicited highly reproducible responses (Fig. 3A, compare top 2 sweeps: left, middle, and right columns).
Fig. 3.
Effect of locally applied ANG II on PVN-RVLM neurons. A: example traces in each column show cell discharge (left), membrane potential (middle), and membrane current (right) responses to locally applied ANG II (2 pmol, 5 s). Bar above each sweep denotes the period of ANG II application. In each recording mode, low-pressure application of ANG II produced a rapid and reproducible response (top 2 sweeps). During each ANG II application, discharge increased to a peak of ~20 Hz and exhibited clear frequency adaptation. Each ANG II–induced burst was followed by slight membrane afterhyperpolarization (left). In the presence of 0.5 mM TTX, local application of ANG II induced an obvious membrane potential depolarization of ~23 mV. Note that current-clamp responses in the absence and presence of TTX (left and middle columns) are from different cells. In both cases, responses to ANG II were elicited at resting Vm (−54 and −53 mV, respectively). In voltage-clamp mode, local ANG II induced a rapid inward current that reached a peak of −65 pA, with Vm held at −60 mV. Note that Im decayed toward baseline even during the continued application of ANG II. Local application of vehicle (ACSF) for 5 s (sweep 3) was without effect on either membrane potential or current and did not alter the subsequent response to locally applied ANG II (sweep 4). The interval separating each application (ANG II or vehicle) was 60 s. B: summary voltage-clamp data for a group of 14 cells showing that the transient inward current response to low-pressure application of ANG II is dose-dependent. Current response was maximal at a dose of 2.0 pmol. *P < 0.001 compared with vehicle (data not shown).
In current-clamp mode without TTX (Fig. 3A, left column), the discharge response to ANG II reached a peak of ~20 Hz, underwent spike frequency adaptation, and was often followed by membrane after hyperpolarization. On average, the amplitude of afterhyperpolarization was −7.8 ± 0.8 mV and lasted for 19 ± 2 s (n = 7). Of 20 PVN-RVLM neurons tested for their response to locally applied ANG II (2 pmol), 17 increased their discharge frequency from a baseline of 0.7 ± 0.4 to a maximum of 21.3 ± 3.3 Hz (P < 0.001). Among these cells, eight were quiescent and nine were spontaneously active. The resting membrane potential of quiescent neurons (−60 ± 1.5 mV) was significantly (P < 0.001) hyperpolarized compared with that of spontaneously active neurons (−51 ± 1.5 mV). Cells with spontaneous discharge exhibited either a continuous basal discharge pattern that averaged 2.6 ± 1.2 spikes/s (n = 3) or a slower (P < 0.05) irregular pattern that averaged 0.58 ± 0.49 spikes/s (n = 6). Resting Vm was not different between continuously and irregularly firing neurons. In the presence of TTX, locally applied ANG II induced consistent membrane depolarization (Fig. 3A, top 2 sweeps, middle column). In 12 of 15 neurons tested, ANG II (2 pmol) depolarized membrane potential by an average of 21.5 ± 4.1 mV (P < 0.001). In voltage-clamp mode in the presence of TTX, 41 of 47 PVN-RVLM neurons responded to locally applied ANG II (2 pmol). With membrane potential held at −60 mV, the peak current amplitude averaged −54.7 ± 3.9 pA (range, −25.0 to −118.2 pA; P < 0.005).
Note that discharge, depolarization, and current responses were not due to mechanical artifacts since low-pressure local application of vehicle (ACSF) to the same cells was consistently without effect (Fig. 3A, sweep 3: left, middle, and right columns). Vehicle application also did not influence the subsequent responsiveness of cells since responses to ANG II recorded 60 s after ACSF (sweep 4) were not different from control (sweeps 1 and 2). It should also be noted that in current-and voltage-clamp recordings, responses to locally applied ANG II were transient—Vm and Im returned toward baseline even in the continued presence of locally applied ANG II (Fig. 3A, sweeps 1–3: left, middle, and right columns).
Figure 3B shows that, in a subgroup of 14 cells, the inward current response to locally applied ANG II was dose-dependent over a range of 0.2–3.0 pmol. An ANG II dose of 2.0 pmol elicited a maximal inward current that was significantly greater (P < 0.001) than the response to vehicle. Therefore the inward current response to locally applied ANG II was further characterized using the 2.0-pmol dose.
Mediation by AT1 receptors
Responses were mediated by activation of ANG II AT1 receptors. Figure 4A shows representative effect of local ANG II AT1 receptor blockade with [Sar,Ile]-ANG II (4 pmol) delivered as a cocktail with ANG II (2 pmol). Note that discharge, depolarization, and inward current responses were reproducible under control conditions (sweeps 1 and 2: left, middle, and right columns). Responses to ANG II in all recording modes were nearly prevented by treatment with the AT1 receptor antagonist (sweep 3: left, middle, and right columns) and were almost fully recovered within 2–3 min (sweep 4: left, middle, and right columns). Figure 4B depicts group response data and shows that blockade of AT1 receptors with [Sar,Ile]-ANG II (4 pmol; n = 4) or Losartan (4 pmol; n = 7) effectively abolished ANG II-induced discharge (P < 0.001, n = 5, Fig. 4B, left) and significantly reduced depolarization (middle) and inward current (right) responses by an average of 86.7 ± 7.5 (P < 0.001, n = 3) and 60.4 ± 5.4% (P < 0.01, n = 11), respectively.
Fig. 4.
Effects of locally applied ANG II depend on AT1 receptors. A: locally applied ANG II (2 pmol, 5 s) increased cell discharge (left), depolarized membrane potential (middle), and induced an inward membrane current (right). Bar above each sweep denotes the period of ANG II application. Top 2 sweeps in each column show the rapid and reproducible response induced by local ANG II. The 3rd sweep shows that effects of ANG II are effectively prevented when ANG II (2 pmol) and the peptide AT1 receptor antagonist [Sar,Ile]-ANG II (4 pmol) are delivered concurrently for 5 s as a cocktail. Responses to local ANG II recovered within ~3 min of antagonist application (bottom sweeps). B: summary graphs showing that cell discharge (n = 5, left), membrane potential (n = 3, middle), and membrane current (n = 11, right) responses to a cocktail containing ANG II (2 pmol) and the AT1 receptor antagonist [Sar,Ile]-ANG II (4 pmol; gray bars) were significantly reduced compared with prior responses induced by ANG II (2 pmol) alone (open bars). Responses to ANG II recovered within 2–3 min following exposure to the ANG II-AT1 receptor antagonist cocktail (black bars). **P < 0.001 and *P < 0.01 vs. ANG II alone.
ANG II increases membrane input conductance
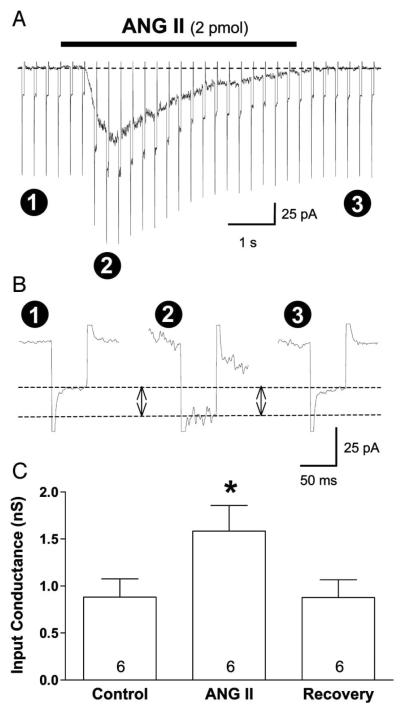
Effects of locally applied ANG II (2 pmol) on membrane input conductance was determined in six cells whose inward current response averaged −75.8 ± 15.4 pA. In voltage-clamp mode, potential was stepped from −60 to −80 mV for 50 ms at a frequency of 4 Hz (Fig. 5A). The resulting current response was compared before, during, and after local application of ANG II (Fig. 5B). The current response to each voltage step reached a maximum at the peak of the ANG II-induced inward current and returned to baseline upon completion of the ANG II response. Summary data show that ANG II application significantly increased (P < 0.001) membrane input conductance by an average of 0.70 ± 0.09 nS (range, 0.37–0.93 nS) from 0.88 ± 0.19 to 1.58 ± 0.27 nS (Fig. 5C), indicating that the ANG II current is carried by a net influx of cations.
Fig. 5.
Effect of locally applied ANG II on membrane input conductance. A: holding potential was repeatedly stepped (50 ms, 4 Hz) from −60 to −80 mV, and the inward current response was determined before (1), during (2), and after (3) locally applied ANG II (2 pmol). Note: initial current response to each voltage step is a capacitive current transient. Positive-going transients have been truncated for clarity. B: current response to step hyperpolarization increased during application of ANG II, indicating that membrane input conductance increased. Note that sweeps in B are taken from A. Baselines are aligned, and time and amplitude scales are expanded to emphasize the response, which is denoted by the 2 horizontal dashed lines. Capacitive artifacts have been truncated to emphasize the change in ionic current. C: summary data indicate that ANG II significantly increased input conductance of PVN-RVLM neurons by an average of ~80%. *P < 0.001 compared with control.
Reversal potential of ANG II current
Reversal potential of the ANG II–induced current was determined in voltage-clamp mode from I-V curves. The ANG II current was determined by subtracting whole cell currents responses to depolarizing ramps (0.14 mV/ms) from −40 to +20 mV generated prior to and at the peak response to locally applied ANG II (2 pmol, 5 s). In a group of nine cells, the summary I-V relationship for the ANG II current was consistently nonrectifying and reversed polarity at +2.2 ± 2.2 mV (Fig. 6A). With membrane potential held at −60 mV, these cells had an ANG II-induced inward current that averaged −48.6 ± 9.1 pA. These data indicate that the inward current response to locally applied ANG II is likely due to activation of a voltage-insensitive, nonselective cation current.
Fig. 6.
Local ANG II induces a nonselective inward cation current in PVN-RVLM neurons. A: summary I-V relationship of the ANG II current from a group of 9 PVN-RVLM neurons. Holding potential was ramped (0.14 mV/ms) from −40 to +20 mV in the absence and presence of ANG II (2 pmol). The ANG II current was determined as the difference of the 2 current responses and was plotted as a function of applied potential. The I-V relationship revealed that the ANG II current was voltage-insensitive (nonrectifying) and had an average reversal potential of +2.2 ± 2.2 mV. Value of the current response to local ANG II at −60 mV averaged −48 ± 16 pA in these cells. B: traces (top) show that the response of an individual PVN-RVLM to locally applied ANG II (2 pmol; left sweep) was reduced in a time-dependent manner during perfusion with ACSF containing Gd3+ (100 μM; middle 3 sweeps). Current response was effectively eliminated 8 min after switching to Gd3+-containing ACSF and recovered toward the control amplitude ~20 min after returning to normal ACSF (right sweep). Summary graph (bottom) shows that bath application of 100 μM Gd3+ significantly reduced the amplitude of the ANG II-induced inward current in a group of 8 PVN-RVLM neurons. *P < 0.01 compared with control.
Effects of Gd3+ on the ANG II current
Consistent with ANG II activation of a nonselective cation current, we found that bath application of the trivalent cation Gd3+ (100 μM), a known blocker of nonselective cation currents, significantly reduced (P < 0.01) the ANG II current in a time-dependent manner (6–10 min) by an average of 78.7 ± 4.5% (n = 8; Fig. 6B). Responses to ANG II recovered to 60–70% of baseline within 30 min of returning to perfusion with normal bath ACSF. The holding current for the eight cells tested averaged −2.6 ± 7.2 pA before switching to the Gd3+-containing ACSF and −0.3 ± 3.2 pA after the maximal effect of Gd3+ had been achieved. The difference was not statistically significant (P = 0.9322; paired t-test).
DISCUSSION
This study showed that ANG II excited the majority (87%) of PVN neurons retrogradely labeled from the brain stem RVLM. Given evidence that PVN neurons target a relatively wide rostro-caudal area of the ventrolateral medulla (Hardy 2001; Krukoff et al. 1997; Yang and Coote 1999), it should be acknowledged that some labeling could have resulted from diffusion of tracer to regions adjacent to the RVLM. Nevertheless, it seems likely that most of the labeling arose from terminals in the RVLM pressor area, since relatively small volumes (50 nl) of tracer were used and injections were centered on a site from which the excitatory amino acid l-glutamate (1 nmol) elicited a significant increase in arterial pressure. That the majority of labeled cells responded to ANG II suggests that a considerable fraction of PVN neurons that target the RVLM pressor region are ANG II responsive. For reasons indicated above, we cannot be certain whether PVN neurons unresponsive to ANG II target the RVLM pressor region, an adjacent area, or both. Additional studies in which tracer is microinjected in a volume even smaller than that used here (i.e., <50 nl) may help to resolve these issues.
In this study, bath application of ANG II significantly depolarized membrane potential and increased the discharge of PVN-RVLM neurons. The magnitude of the ANG II–induced discharge response was similar to that of other central neurons for comparable bath concentrations (Bai and Renaud 1998; Li and Guyenet 1996; Okuya et al. 1987; Ono et al. 2001). However, it should be noted that the mechanism by which ANG II induces neuronal excitation seems to differ in different populations of cells. For example, ANG II has been reported to increase the discharge of spinally projecting neurons of the PVN through presynaptic inhibition of GABA release (Li et al. 2003a). It remains to be determined whether ANG II-induced excitation of PVN-RVLM neurons involves GABA disinhibition. Within the median preoptic nucleus (Bai and Renaud 1998) and rostral ventrolateral medulla (Li and Guyenet 1996) and in some cells of the subfornical organ (Ferguson and Li 1996), ANG II excitation involves postsynaptic actions that inhibit a resting potassium current. This is clearly not the case for PVN-RVLM neurons since ANG II–induced inward current recorded in this study was accompanied by a significant increase in membrane input conductance. Moreover, the ANG II current typically reversed polarity between ±10 mV, far from the potassium equilibrium potential. Together, these results indicate that among PVN-RVLM neurons, ANG II activates a nonselective cation current. This is highly reminiscent of responses reported among a subgroup of subfornical organ neurons (Ferguson et al. 1997; Ono et al. 2001) and among magnocellular neurons of the PVN (Latchford and Ferguson 2003).
It should be emphasized that the identity of the channels activated by ANG II in this study is not known. Indeed, the mixed cation current could be produced by gating of more than a single class of channels. Moreover, effects of ANG II could be mediated presynaptically or postsynaptically or both. With respect to possible postsynaptic effects of ANG II, channels capable of mediating a response similar to that reported in this study are mammalian homologues of Drosophila transient receptor potential (TRP) channels. TRP channels have received considerable attention as candidate mediators of a variety of excitatory responses in the nervous system (for review, see Clapham 2002). In this regard, it is interesting to note that TRP channels are permeable to Na+ and Ca2+ and are activated by Gαq/11-coupled receptors (Clapham 2002; Harteneck et al. 2000), like the ANG II AT1 receptor (Guo et al. 2001). Evidence that ANG II induces a transient inward current in Chinese hamster ovary (CHO) cells expressing TRPC3 channels (Zitt et al. 1997) is consistent with a possible role for TRP channels in mediating ANG II responses.
In this study, the transient inward current induced by ANG II was effectively blocked by bath application of Gd3+, a blocker of nonselective cation channels, including TRPC1 (Brereton et al. 2001) and TRPC3 (Oh et al. 2003) channels. Still, it should be stressed that additional studies will be necessary to precisely define the channel types that mediate ANG II responses among PVN-RVLM neurons. This is the case because Gd3+ can also block voltage-activated calcium channels (Lacampagne et al. 1994) and the Na+-Ca2+ exchanger (Zhang and Hancox 2000). This is important because the actions of ANG II reported here could involve activation/modulation of Gd3+-sensitive currents located at presynaptic terminals. In this regard, it is interesting to note that ANG II has been recently reported to excite PVN magnocellular neurons by recruiting a local glutamatergic circuit (Latchford and Ferguson 2004). That glutamate can act at ionotropic receptors to induce a nonselective cation current raises the possibility that responses to ANG II in this study could involve a glutamatergic mechanism. However, because we found that excitatory effects of ANG II persisted in the presence of TTX, excitation of PVN-RVLM neurons by glutamate would seem to require that ANG II directly facilitate terminal release of glutamate. To our knowledge, such an effect in the PVN has not been reported. Still, there is recent evidence that AT1 receptors can be located at presynatic terminals that target spinally projecting neurons of the PVN (Li et al. 2003a). Moreover, Barnes et al. (2003) have reported that excitation of neurons in the nucleus tractus solitarius by locally applied (micro-drop) ANG II involves a presynaptic action to facilitate terminal release of glutamate with subsequent activation of a non–N-methyl-d-aspartate (NMDA) ionotropic current. Taken together, available evidence raises the possibility that ANG II-induced excitation of PVN neurons could involve actions at synaptic terminals. From the foregoing discussion it is apparent that further study will be necessary to identify the channel types and synaptic mechanisms that mediate the response of PVN-RVLM neurons to locally applied ANG II.
Whatever the underlying mechanism may be, a consistent finding is that neuronal responses to bath applied ANG II are typically long—lasting for 5–30 min (Bai and Renaud 1998; Li and Guyenet 1996; Li et al. 2003a; Ono et al. 2001). A similar response was observed in this study. Another characteristic of the response to repeated bath application of ANG II is tachyphylaxis (Bai and Renaud 1998; Okuya et al. 1987; Ono et al. 2001). This study indicates that such an effect is largely absent when responses to ANG II are induced by delivering the peptide locally by low-pressure ejection. Indeed, using this approach, we found that responses of individual PVN-RVLM neurons to ANG II were highly reproducible at intervals of 40–60 s (see Figs. 3 and 4A). Because bath and local application of ANG II in this study induced clearly distinct responses, it would seem that different mechanisms may be involved. It is possible, for example, that bath and local application of ANG II could access AT1 receptors localized to different regions of PVN-RVLM neurons (soma vs. distal dendrites). In this regard, it is important to emphasize that bath and local application provide different, but highly complimentary, information.
One concern that arises when compounds are delivered by pressure ejection is that mechanical artifacts may occur. This was not the case in this study since holding current and resting Vm were unaffected by local application of vehicle (see Fig. 3). The fact that responses to ANG II were effectively blocked when the peptide and an AT1 receptor antagonist were delivered as a cocktail (Fig. 4A) supports the conclusion that mechanical artifacts did not accompany the response. Another issue for studies in which compounds are delivered locally in brain slices is that the concentration of compounds at the cell membrane is not known with certainty. Here, our standard test dose of ANG II (2 pmol) was delivered from a pipette containing a 5 mM concentration of ANG II. Given the volume and time course of each ejection, it seems clear that considerable dilution would have occurred during delivery of the peptide and its diffusion to the cells surface. Consequently, the concentration of ANG II at the neuronal membrane would likely be considerable less than the barrel concentration—although the actual concentration is not known. Never the less, it is important to mention that a principle advantage of local delivery is that studies can be readily conducted using a paired design. In the present study, effects of ANG II were recorded for each cell before, during, and after administration of each antagonist/blocker. Thus pharmacological effects are likely to be specific even though the concentration of drugs at the neuronal membrane is not precisely known.
An additional concern that can arise when compounds are delivered through low-resistance pipettes is leakage. Given that the majority of our recordings were performed at a depth of 50–125 μm below the tissue surface, there is not a substantial bulk flow of ACSF. Therefore we believe that any ANG II that may have leaked from the pipette diffused away before having a significant impact on the recorded neurons. This conclusion is supported by the following observations. 1) Treatment with AT1 receptor antagonists alone had no effect on resting membrane potential or holding current (data not shown). 2) Bath-applied ANG II caused depolarization and spiking, but placement of the ANG II containing pipette near the cell had neither effect—although it is possible that bath-applied ANG II could have acted at distant neurons. 3) The response to puffed ANG II decayed rapidly, suggesting the occurrence of desensitization (see following text). If leak was substantial, it might have been expected that desensitization would eventually reduce the amplitude of the local ANG II response: this did not occur (see Figs. 3A and 4A).
In this study, responses to locally applied ANG II were transient—membrane potential and current returned toward baseline prior to termination of ANG II application. The mechanism that underlies this response is presently unknown, but, as mentioned above, is consistent with either presynaptic actions and release of a transmitter with transient actions (e.g., glutamate), with desensitization of AT1 receptors (Ferguson 2001), or both. Regarding AT1 receptor desensitization, it is known that AT1 receptors can internalize during ANG II stimulation (Smith et al. 1998). The fact that responses in this study lasted only seconds and were reproducible within ~60 s suggests a mechanism other than receptor internalization. One possibility might be AT1 receptor–G protein uncoupling, which has been reported to occur rapidly in response to receptor phosphorylation by second messenger-dependent kinases (e.g., protein kinase A, protein kinase C) or G protein–coupled receptor kinases (e.g., GRK2–6) (Ferguson 2001).
These findings have potentially important bearing on cardiovascular and body fluid homeostasis. Functional studies have established that elevated plasma ANG II and central hyperosmolality acutely increase sympathetic nerve discharge, presumably through activation of neurons in the forebrain subfornical organ and organum vasculosum of the lamina terminalis. Because these brain regions lack an effective blood brain barrier, ANG II and other blood-borne factors can gain direct access to the CNS. On activation, both mono- and polysynaptic pathways are thought to influence the activity PVN sympathetic-regulatory neurons (for review, see McKinley et al. 1992). A major chemical mediator of this excitation seems to be ANG II (Bains et al. 1992; Chen and Toney 2001). This study shows that RVLM-projecting neurons of the PVN are excited by direct application of ANG II. The possibility that PVN-RVLM neurons are a potential target of ANG II inputs from the forebrain has important implication for homeostatic responses to body fluid perturbations such as hemorrhage and dehydration that increase plasma ANG II and stimulate neurons of the forebrain (Tanaka et al. 1993; Toney et al. 2003). ANG II activation of PVN-RVLM neurons also has implications for cardiovascular disease. In certain forms of arterial hypertension, for example, sympathetic hyperactivity has been linked to exaggerated actions of ANG II within the PVN (Li et al. 1996) and the RVLM (Ito et al. 2002, 2003). Of particular interest in this regard is evidence implicating ANG II as a mediator of RVLM excitation following PVN neuronal activation (Tagawa and Dampney 1999). Collectively, available evidence raises the possibility that a neurochemically conserved ANG II–containing neural pathway from the forebrain, through the PVN, to the RVLM could mediate sympathoexcitation in specific disease conditions. However, this possibility requires a great deal more investigation. Furthermore, it must be reconciled with in vivo electrophysiological data, which indicate that stimulation of the PVN with an excitatory amino acid increases RVLM neuronal activity through activation of vasopressin (V1) and ionotropic excitatory amino acid receptors (Yang and Coote 2001). Thus there is a clear need to define the neurochemical composition of ANG II responsive PVN-RVLM neurons.
In conclusion, the majority of PVN neurons with axonal projections to the pressor region of the RVLM are excited by the peptide ANG II. The mechanism of excitation seems to involve activation of a nonselective cation current. The identity of the channel or channels that mediate this response remains to be determined. That local delivery of ANG II by low-pressure ejection induced a transient excitation that was highly reproducible suggests that this approach may provide a useful method to study neuronal effects of ANG II. When combined with results of previous in vivo studies, these findings suggest that PVN-RVLM neurons are a potentially important substrate through which forebrain ANG II inputs regulate the activity of sympathetic nerves.
ACKNOWLEDGMENTS
We thank K. J. Keith for assistance with histology, Drs. S. D. Stocker, J. T. Cunningham, and S. W. Mifflin for useful discussions about this work, and Drs. L. P. LaGrange and L. C. Daws for critically reviewing drafts of this manuscript.
GRANTS This project was supported by National Heart, Lung, and Blood Institute Grant HL-56834. We are grateful for additional support from the Advanced Research Program of the Texas Higher Education Coordinating Board. This project was conducted during the tenure of an American Heart Association Established Investigator Award (0140161N) to G. M. Toney.
REFERENCES
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Bai D, Renaud LP. ANG II AT1 receptors induce depolarization and inward current in rat median preoptic neurons in vitro. Am J Physiol. 1998;275:R632–R639. doi: 10.1152/ajpregu.1998.275.2.R632. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol. 2003;284:R1340–R1353. doi: 10.1152/ajpregu.00505.2002. [DOI] [PubMed] [Google Scholar]
- Brereton HM, Chen J, Rychkov G, Harland ML, Barritt GJ. Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochim Biophys Acta. 2001;1540:107–126. doi: 10.1016/s0167-4889(01)00124-0. [DOI] [PubMed] [Google Scholar]
- Camacho A, Philips MI. Horseradish peroxidase study in rat of the neural connections of the organum vasculosum of the lamina terminalis. Neurosci Lett. 1981;25:201–204. doi: 10.1016/0304-3940(81)90391-8. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol. 2001;281:R1844–R1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Sorting out MIC, TRP, and CRAC ion channels. J Gen Physiol. 2002;120:217–220. doi: 10.1085/jgp.20028618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon JC, Phillips MI, Hoffman WE, Brody MJ. Effects of intraventricular angiotensin II mediated by the sympathetic nervous system. Am J Physiol. 1978;235:H392–H399. doi: 10.1152/ajpheart.1978.235.4.H392. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Bicknell RJ, Carew MA, Mason WT. Dissociated adult rat subfornical organ neurons maintain membrane properties and angiotensin responsiveness for up to 6 days. Neuroendocrinol. 1997;66:409–415. doi: 10.1159/000127266. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Li Z. Whole cell patch recordings from forebrain slices demonstrate angiotensin II inhibits potassium currents in subfornical organ neurons. Regul Pept. 1996;66:55–58. doi: 10.1016/0167-0115(96)00049-3. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DL. Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol. 1998;54:169–192. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–180. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- Gutman MB, Jones DL, Ciriello J. Effect of paraventricular nucleus lesions on drinking and pressor responses to ANG II. Am J Physiol. 1988;255:R882–R887. doi: 10.1152/ajpregu.1988.255.6.R882. [DOI] [PubMed] [Google Scholar]
- Hardy SG. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/s0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Hutton UE, Hoblitzell ER, Armstrong WE. Morphological evidence for two populations of magnocellular elements in the rat paraventricular nucleus. Brain Res. 1976;108:187–193. doi: 10.1016/0006-8993(76)90176-1. [DOI] [PubMed] [Google Scholar]
- Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension. 2002;40:552–559. doi: 10.1161/01.hyp.0000033812.99089.92. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Cunningham JT, Thunhorst RL. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 1996;23:183–191. doi: 10.1111/j.1440-1681.1996.tb02594.x. [DOI] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Mactavish D, Jhamandas JH. Activation by hypotension of neurons in the hypothalamic paraventricular nucleus that project to the brainstem. J Comp Neurol. 1997;385:285–296. doi: 10.1002/(sici)1096-9861(19970825)385:2<285::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lacampagne A, Gannier F, Argibay J, Garnier D, LeGuennec JY. The stretch-activated ion channel blocker gadolinium also blocks L-type calcium channels in isolated ventricular myocytes of the guinea-pig. Biochim Biophys Acta. 1994;1191:205–208. doi: 10.1016/0005-2736(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Latchford KJ, Ferguson AV. Angiotensin II activates a nitric-oxide-driven inhibitory feedback in the rat paraventricular nucleus. J Neurophysiol. 2003;89:1238–1244. doi: 10.1152/jn.00914.2002. [DOI] [PubMed] [Google Scholar]
- Latchford KJ, Ferguson AV. ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol. 2004;286:R894–R902. doi: 10.1152/ajpregu.00603.2003. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003a;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Morris M, Diz DI, Ferrario CM, Ganten D, Callahan MF. Role of paraventricular angiotensin AT1 recepotrs in salt-sensitive hypertension in mRen-2 transgenic rats. Am J Physiol. 1996;270:R1178–R1181. doi: 10.1152/ajpregu.1996.270.5.R1178. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circ Res. 1996;78:274–282. doi: 10.1161/01.res.78.2.274. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience. 2003b;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985;1:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Descending pathways to sympathetic and parasympathetic preganglionic neurons. J Auton Nerv Syst. 1981;3:265–275. doi: 10.1016/0165-1838(81)90068-0. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in the medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Simpson JB. Subfornical organ lesions reduce the pressor effect of systemic angiotensin II. Neuroendocrinol. 1980;31:380–384. doi: 10.1159/000123107. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Bicknell RJ, Hards D, McAllen RM, Vivas L, Weisinger RS, Oldfield BJ. Efferent neural pathways of the lamina terminalis subserving osmoregulation. Prog Brain Res. 1992;91:395–402. doi: 10.1016/s0079-6123(08)62358-4. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch-clamp experiments. Methods Enzymol. 1992;207:123–31. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Oh EJ, Gover TD, Cordoba-Rodriguez R, Weinreich D. Substance P evokes cation currents through TRP channels in HEK293 cells. J Neurophysiol. 2003;90:2069–2073. doi: 10.1152/jn.00026.2003. [DOI] [PubMed] [Google Scholar]
- Okuya S, Inenaga K, Kaneko T, Yamashita H. Angiotensin II sensitive neurons in the supraoptic nucleus, subfornical organ and anteroventral third ventricle of rats in vitro. Brain Res. 1987;402:58–67. doi: 10.1016/0006-8993(87)91047-x. [DOI] [PubMed] [Google Scholar]
- Ono K, Honda E, Inenaga K. Angiotensin II induces inward currents in subfornical organ neurons of rats. J Neuroendocrinol. 2001;13:517–523. doi: 10.1046/j.1365-2826.2001.00663.x. [DOI] [PubMed] [Google Scholar]
- Porter JP, Brody MJ. Neural projections from paraventricular nucleus that subserve vasomotor functions. Am J Physiol. 1985;248:R271–R281. doi: 10.1152/ajpregu.1985.248.3.R271. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of efferent projections from the paraventricular nucleus of the hypothalamus terminating closely to spinally projecting rostral ventrolateral medullary neurons. Neuroscience. 1999;8:949–957. doi: 10.1016/s0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Smith RD, Hunyady L, Olivares-Reyes JA, Mihalik B, Jayadev S, Catt KJ. Agonist-induced phosphorylation of the angiotensin AT1a receptor is localized to a serine/threonine-rich region of its cytoplasmic tail. Mol Pharmacol. 1998;54:935–941. doi: 10.1124/mol.54.6.935. [DOI] [PubMed] [Google Scholar]
- Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Dampney RAL. AT1 receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Nojima K, Yamamuro Y, Saito H, Nomura M. Responses of subfornical organ neurons projecting to the hypothalamic paraventricular nucleus to hemorrhage. Brain Res. 1993;608:141–144. doi: 10.1016/0006-8993(93)90785-l. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney GM, Chen QH, Cato JM. Angiotensin II AT1 receptor activation evokes a transient inward current in RVLM-projecting neurons of the hypothalamic PVN. FASEB J. 2002;A652:407.7. [Google Scholar]
- Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- Toney GM, Porter JP. Functional role of brain AT1 and AT2 receptors in the central angiotensin II pressor response. Brain Res. 1993;603:57–63. doi: 10.1016/0006-8993(93)91299-8. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Saper CB. Specificity of spinal projections from hypothalamic and brainstem areas which innervate sympathetic preganglionic neurons. Brain Res. 1985;360:159–164. doi: 10.1016/0006-8993(85)91231-4. [DOI] [PubMed] [Google Scholar]
- Wright JW, Roberts KA, Stubley LA, Hanesworth JM, Harding JW. Hypothalamic angiotensin release to angiotensin II or glutamic acid stimulation of the SFO in rats. Brain Res Bull. 1993;31:649–654. doi: 10.1016/0361-9230(93)90136-y. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurons by paraventricular neurons. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Coote JH. The influence of the paraventricular nucleus on baroreceptor dependent caudal ventrolateral medullary neuornes of the rat. Pfluegers. 1999;438:47–52. doi: 10.1007/s004240050878. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Hancox JC. Gadolinium inhibits Na+-Ca2+ exchanger current in guinea-pig isolated ventricular myocytes. Br J Pharmacol. 2000;130:485–488. doi: 10.1038/sj.bjp.0703353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitt C, Obukhov AG, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]