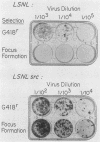
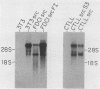
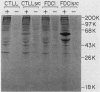
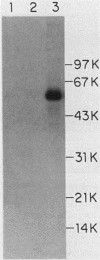
Abstract
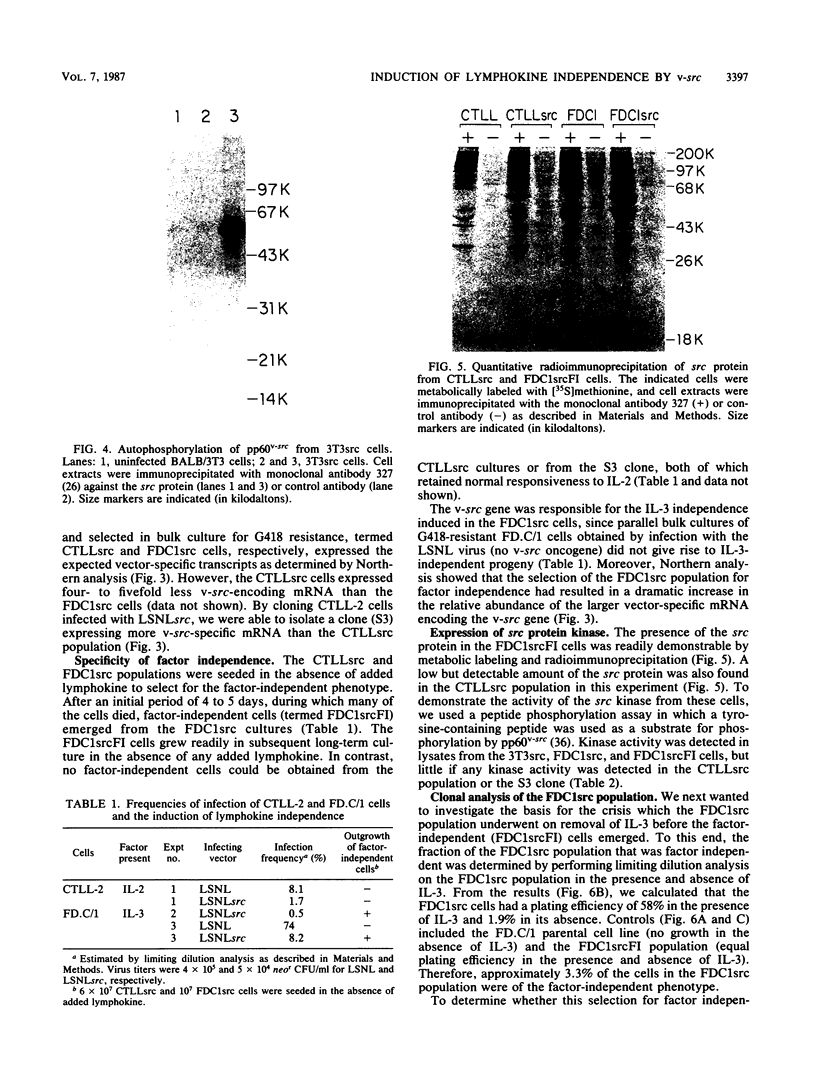
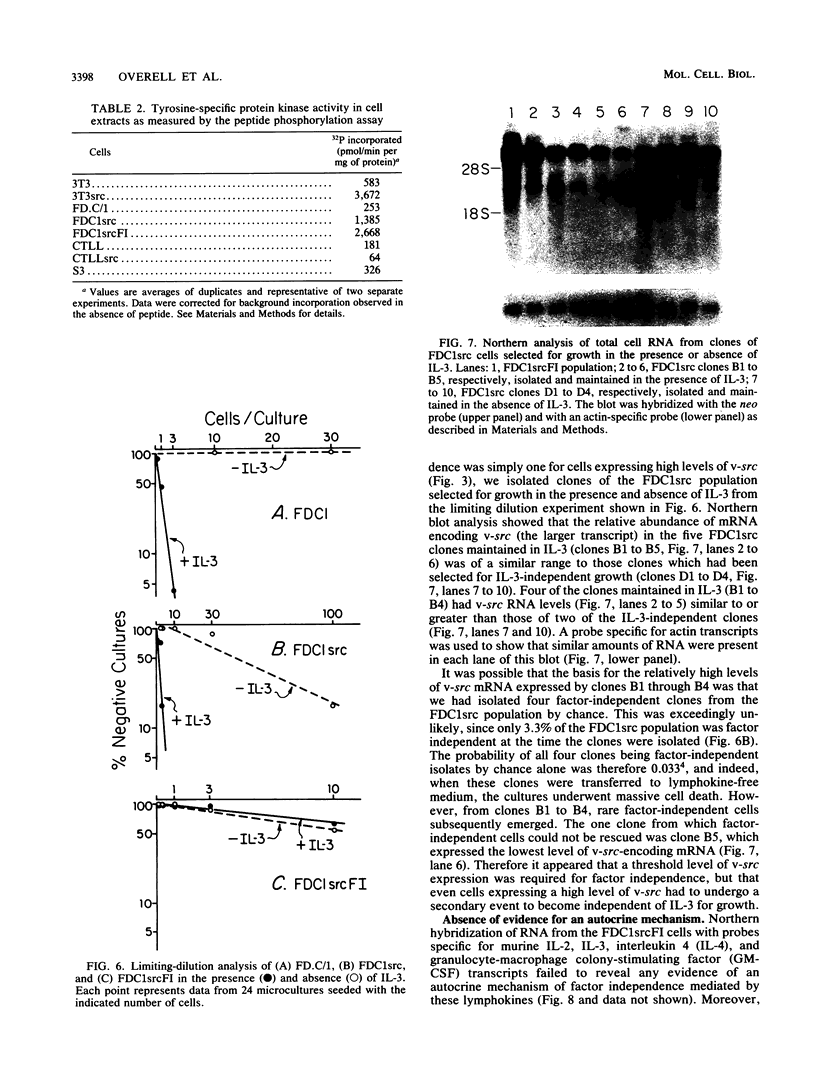
A murine retroviral vector, LSNLsrc, has been constructed and examined for its ability to induce growth factor independence in cells normally dependent on interleukin 2 (IL-2) or interleukin 3 (IL-3) for growth. The LSNLsrc vector coexpressed the v-src gene of Rous sarcoma virus and the neo gene from transposon Tn5, allowing infected cells to be selected on the basis of G418 resistance. The murine cell lines CTLL-2 and FD.C/1, which are dependent for growth on IL-2 and IL-3, respectively, were both readily infected with the LSNLsrc virus. LSNLsrc-infected, G418-resistant cultures of FD.C/1 cells were able to give rise to IL-3-independent progeny, but all G418-resistant CTLL-2 cells retained normal IL-2 dependence. The induction of IL-3 independence by v-src was not a direct event, since limiting dilution analysis of the LSNLsrc-infected FD.C/1 cells showed that most of them were IL-3 dependent, despite expression of v-src mRNA and active pp60v-src kinase. However, clones selected from this population in the presence of IL-3 were able to undergo a subsequent progression event and generate IL-3-independent progeny. The generation of factor-independent variants in the clonal cultures was a rare event, as witnessed by the death of most of the cells in each clone when IL-3 was withdrawn. Together, these data indicate that a secondary event, in addition to v-src expression, was required to generate IL-3-independent growth. No evidence was found for an autocrine mechanism of transformation involving IL-2, IL-3, interleukin 4, or granulocyte-macrophage colony-stimulating factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Anderson S. M., Klinken S. P., Hankins W. D. A murine recombinant retrovirus containing the src oncogene transforms erythroid precursor cells in vitro. Mol Cell Biol. 1985 Dec;5(12):3369–3375. doi: 10.1128/mcb.5.12.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Anderson S., Dexter T. M. Effect of src infection on long-term marrow cultures: increased self-renewal of hemopoietic progenitor cells without leukemia. Cell. 1984 Mar;36(3):763–773. doi: 10.1016/0092-8674(84)90356-8. [DOI] [PubMed] [Google Scholar]
- Conscience J. F., Verrier B., Martin G. Interleukin-3-dependent expression of the c-myc and c-fos proto-oncogenes in hemopoietic cell lines. EMBO J. 1986 Feb;5(2):317–323. doi: 10.1002/j.1460-2075.1986.tb04215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Cook W. D. Thymocyte subsets transformed by Abelson murine leukemia virus. Mol Cell Biol. 1985 Feb;5(2):390–397. doi: 10.1128/mcb.5.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Thomas T. P., Anderson W. B. Altered cytosol/membrane enzyme redistribution on interleukin-3 activation of protein kinase C. Nature. 1985 May 16;315(6016):235–237. doi: 10.1038/315235a0. [DOI] [PubMed] [Google Scholar]
- Gallis B., Lewis A., Wignall J., Alpert A., Mochizuki D. Y., Cosman D., Hopp T., Urdal D. Phosphorylation of the human interleukin-2 receptor and a synthetic peptide identical to its C-terminal, cytoplasmic domain. J Biol Chem. 1986 Apr 15;261(11):5075–5080. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Pierce J. H., Davidson W. F., Morse H. C., 3rd Murine hematopoietic cells with pre-B or pre-B/myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986 Aug 1;164(2):443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Majors J. E., Varmus H. E. Hormonal regulation of the Rous sarcoma virus src gene via a heterologous promoter defines a threshold dose for cellular transformation. Cell. 1984 Oct;38(3):757–765. doi: 10.1016/0092-8674(84)90271-x. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kahn P., Adkins B., Beug H., Graf T. src- and fps-containing avian sarcoma viruses transform chicken erythroid cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7122–7126. doi: 10.1073/pnas.81.22.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros G. S., Gillis S., Watson J. D. Induction of IL 2 responsiveness in a murine IL 3-dependent cell line. J Immunol. 1985 Dec;135(6):4009–4014. [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Agranovsky O., McKinney M. D., Murty V. V., Bauchwitz R. Friend murine leukemia virus-immortalized myeloid cells are converted into tumorigenic cell lines by Abelson leukemia virus. Proc Natl Acad Sci U S A. 1985 May;82(10):3306–3310. doi: 10.1073/pnas.82.10.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A., Anderson S. M. Hematopoietic cell transformation by a murine recombinant retrovirus containing the src gene of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2374–2378. doi: 10.1073/pnas.81.8.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Epidermal growth factor stimulates the phosphorylation of synthetic tyrosine-containing peptides by A431 cell membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1443–1447. doi: 10.1073/pnas.79.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Sabath D. E., Hoover R. G., Prystowsky M. B. Recombinant interleukin 2 regulates levels of c-myc mRNA in a cloned murine T lymphocyte. Mol Cell Biol. 1985 Dec;5(12):3361–3368. doi: 10.1128/mcb.5.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Clark-Lewis I., Crapper R. M., Wong G. W. P-cell stimulating factor: characterization, action on multiple lineages of bone-marrow-derived cells and role in oncogenesis. Immunol Rev. 1983;76:79–104. doi: 10.1111/j.1600-065x.1983.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Segal G. M., McCall E., Stueve T., Bagby G. C., Jr Interleukin 1 stimulates endothelial cells to release multilineage human colony-stimulating activity. J Immunol. 1987 Mar 15;138(6):1772–1778. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Vogt M., Lesley J., Bogenberger J., Volkman S., Haas M. Coinfection with viruses carrying the v-Ha-ras and v-myc oncogenes leads to growth factor independence by an indirect mechanism. Mol Cell Biol. 1986 Oct;6(10):3545–3549. doi: 10.1128/mcb.6.10.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D., Eszes M., Overell R., Conlon P., Widmer M., Gillis S. Effect of infection with murine recombinant retroviruses containing the v-src oncogene on interleukin 2- and interleukin 3-dependent growth states. J Immunol. 1987 Jul 1;139(1):123–129. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]